SPECIFIC HEAT CALORIMETRY HEAT Heat energy that is



- Slides: 10

SPECIFIC HEAT & CALORIMETRY

HEAT • Heat – energy that is flowing from a warmer object to a cooler object • Represented by the variable q • Measured in the following units: • calorie – energy required to raise the temperature of one gram of water one degree Celsius • Joule – SI unit of energy and heat • 1 J = 0. 2390 cal • 1 cal = 4. 184 J 1

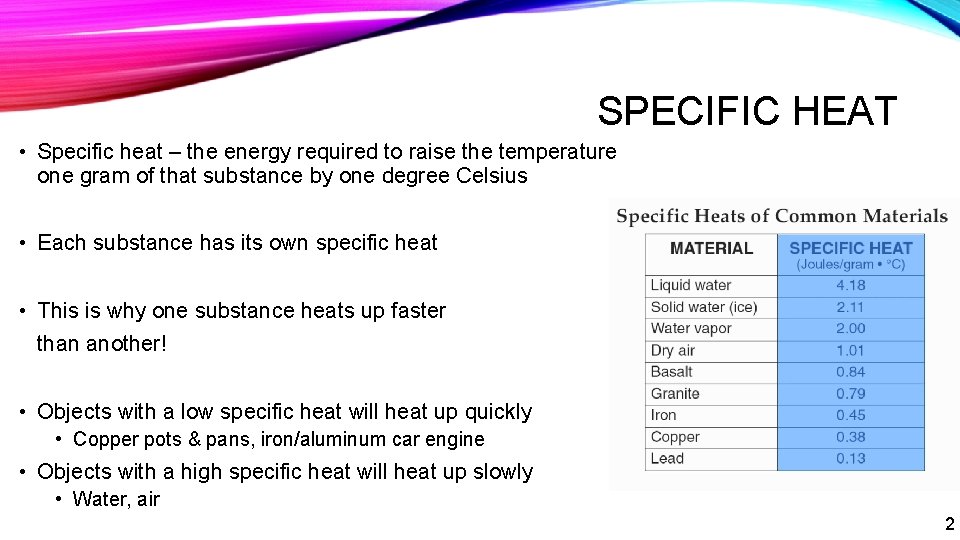
SPECIFIC HEAT • Specific heat – the energy required to raise the temperature one gram of that substance by one degree Celsius • Each substance has its own specific heat • This is why one substance heats up faster than another! • Objects with a low specific heat will heat up quickly • Copper pots & pans, iron/aluminum car engine • Objects with a high specific heat will heat up slowly • Water, air 2

CALCULATING HEAT • We can calculate the amount of heat that an object has gained or lost if we know a few pieces of information: • • Heat (q) – heat lost/absorbed, Joules (J) Specific heat (c) – specific heat of the substance, J/g·°C Mass (m) – mass of the substance, grams (g) Change in temperature (ΔT) – change in temperature of the substance, °C • ΔT = Tfinal - Tinitial • q = m · c · ΔT 3

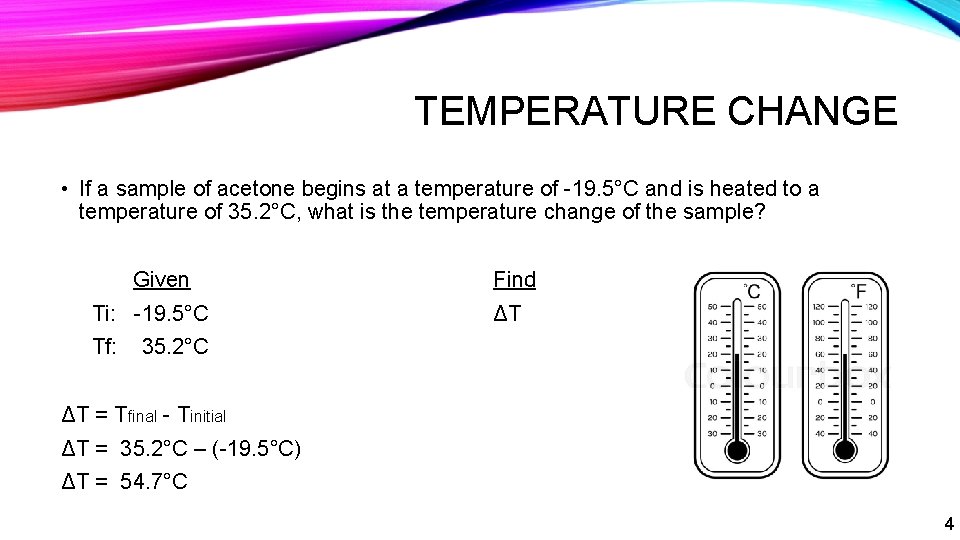
TEMPERATURE CHANGE • If a sample of acetone begins at a temperature of -19. 5°C and is heated to a temperature of 35. 2°C, what is the temperature change of the sample? Given Ti: -19. 5°C Find ΔT Tf: 35. 2°C ΔT = Tfinal - Tinitial ΔT = 35. 2°C – (-19. 5°C) ΔT = 54. 7°C 4

HEAT CALCULATIONS • If the previous sample of acetone had a mass of 114. 2 g has a specific heat of 2. 15 J/(g· °C), what amount of heat was absorbed by the sample? Given m – 114. 2 g c – 2. 15 J/(g· °C) ΔT - 54. 7°C Find q q = m · c · ΔT q =114. 2 g x 2. 15 J/(g· °C) x 54. 7°C q = 13430. 49 J or 13. 43 k. J 5

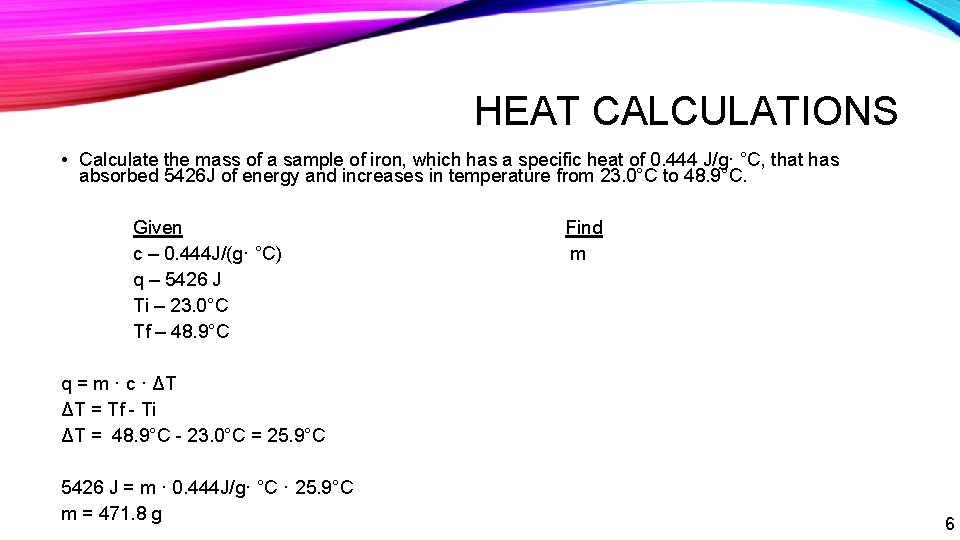
HEAT CALCULATIONS • Calculate the mass of a sample of iron, which has a specific heat of 0. 444 J/g· °C, that has absorbed 5426 J of energy and increases in temperature from 23. 0°C to 48. 9°C. Given c – 0. 444 J/(g· °C) q – 5426 J Ti – 23. 0°C Tf – 48. 9°C Find m q = m · c · ΔT ΔT = Tf - Ti ΔT = 48. 9°C - 23. 0°C = 25. 9°C 5426 J = m · 0. 444 J/g· °C · 25. 9°C m = 471. 8 g 6

CALORIMETRY • A known mass of water is placed into an insulated chamber (a calorimeter) to absorb the energy released from the reacting system or to provide the energy absorbed by the system. • We record the change in temperature of the water in the calorimeter. • In a calorimeter: • Heat gained by the water is equal to the heal lost from the reacting system. • Or the metal, like in our lab! • -qmetal = qwater 7

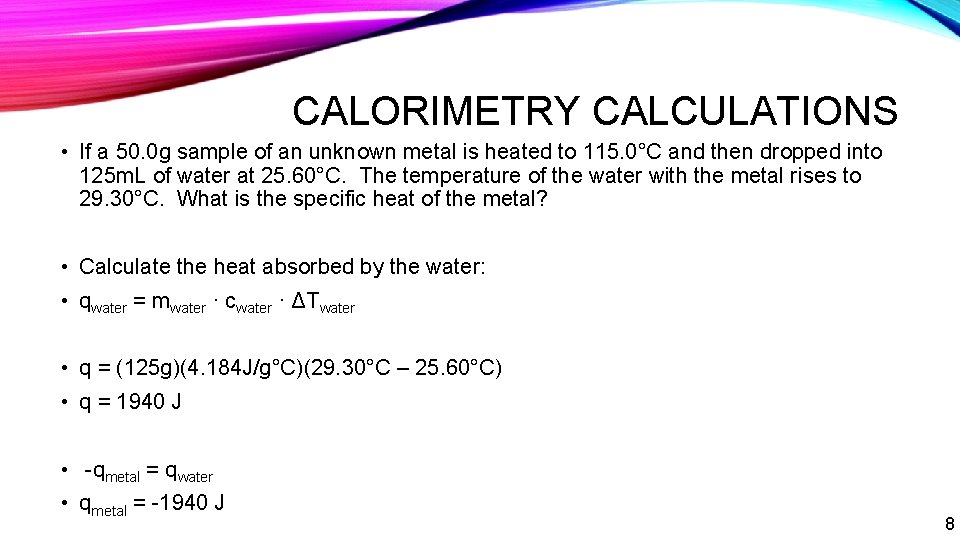
CALORIMETRY CALCULATIONS • If a 50. 0 g sample of an unknown metal is heated to 115. 0°C and then dropped into 125 m. L of water at 25. 60°C. The temperature of the water with the metal rises to 29. 30°C. What is the specific heat of the metal? • Calculate the heat absorbed by the water: • qwater = mwater · cwater · ΔTwater • q = (125 g)(4. 184 J/g°C)(29. 30°C – 25. 60°C) • q = 1940 J • -qmetal = qwater • qmetal = -1940 J 8

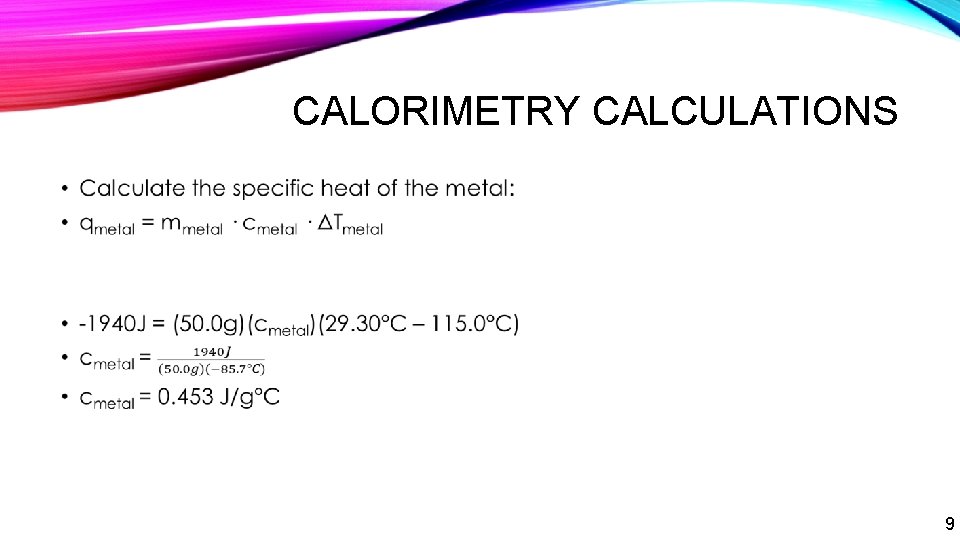
CALORIMETRY CALCULATIONS • 9