GASES Kinetic Molecular Theory A gas consists of









- Slides: 20

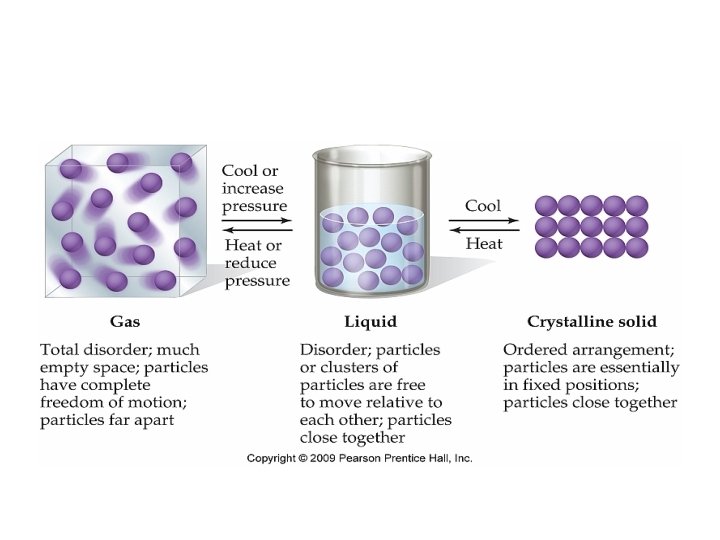
GASES


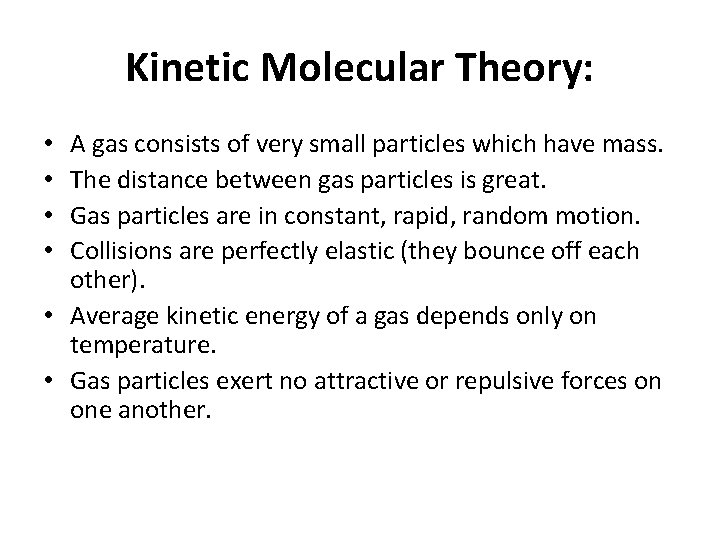
Kinetic Molecular Theory: A gas consists of very small particles which have mass. The distance between gas particles is great. Gas particles are in constant, rapid, random motion. Collisions are perfectly elastic (they bounce off each other). • Average kinetic energy of a gas depends only on temperature. • Gas particles exert no attractive or repulsive forces on one another. • •

Special Conversion Factors for Gases: • Temperature – We always measure temp in Kelvin when dealing with gases! – Equation: K = ºC + 273

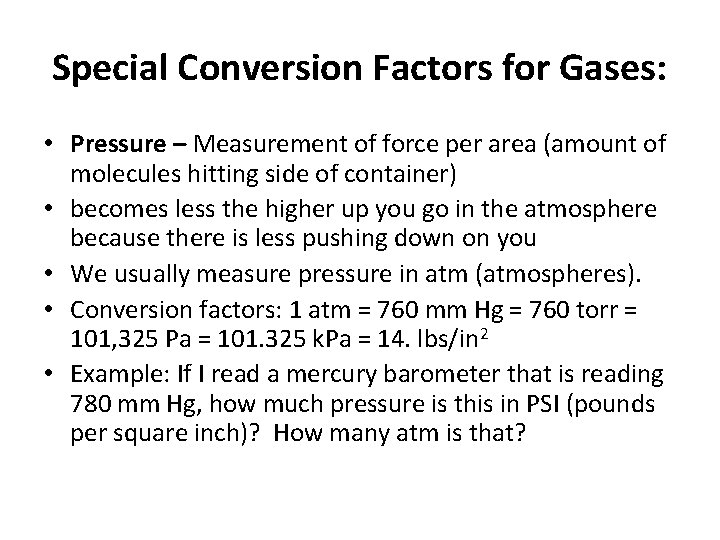
Special Conversion Factors for Gases: • Pressure – Measurement of force per area (amount of molecules hitting side of container) • becomes less the higher up you go in the atmosphere because there is less pushing down on you • We usually measure pressure in atm (atmospheres). • Conversion factors: 1 atm = 760 mm Hg = 760 torr = 101, 325 Pa = 101. 325 k. Pa = 14. lbs/in 2 • Example: If I read a mercury barometer that is reading 780 mm Hg, how much pressure is this in PSI (pounds per square inch)? How many atm is that?

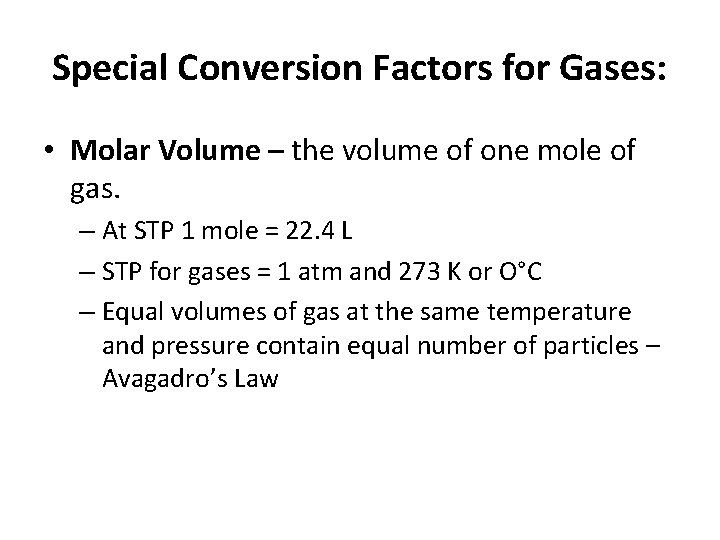
Special Conversion Factors for Gases: • Molar Volume – the volume of one mole of gas. – At STP 1 mole = 22. 4 L – STP for gases = 1 atm and 273 K or O°C – Equal volumes of gas at the same temperature and pressure contain equal number of particles – Avagadro’s Law

• http: //ed. ted. com/lessons/daniel-dulek-howbig-is-a-mole-not-the-animal-the-other-one

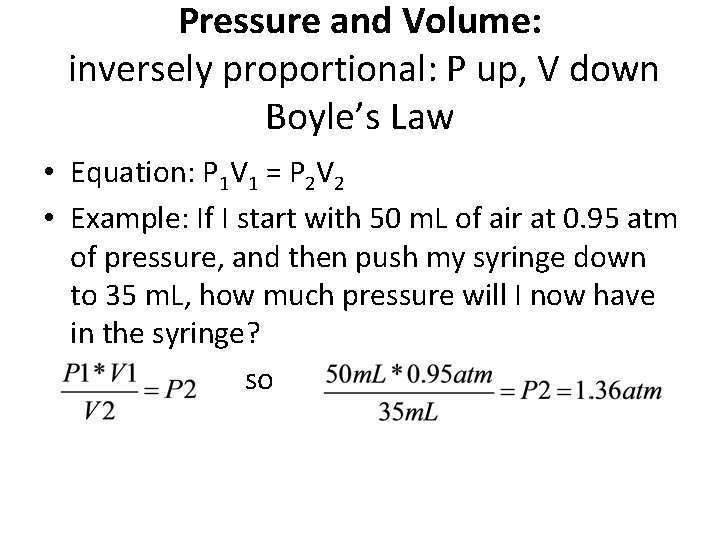
Pressure and Volume: inversely proportional: P up, V down Boyle’s Law • Equation: P 1 V 1 = P 2 V 2 • Example: If I start with 50 m. L of air at 0. 95 atm of pressure, and then push my syringe down to 35 m. L, how much pressure will I now have in the syringe? so

Temperature and Volume: directly proportional T up, V up Charles’s Law • Equation: • Example: balloon in N 2… If a balloon initially had a volume of 1. 3 L at room temperature (298 K), what would the new volume of the balloon be once I dunked it in liquid nitrogen which has a temperature of -196 ºC. (Don’t forget to convert to KELVIN!) cross multiply and you get • The Kelvin unit cancels out and your left with your final volume which is much lower than your initial volume of 1. 3 L…congrats your answer makes sense and you are done!

Temperature and Pressure: directly proportional T up, P up Gay-Lussac Law • Equation: • Example: balloon on a hot day… If we start with a balloon that is filled inside the cold grocery store, temp of 278 K, to 0. 016 atm of pressure, and then taken outside in the middle of summer where the temp is 309 K, what would be the final pressure on the balloon? If a regular balloon can withstand only. 017 atm of pressure, would this particular balloon pop once outside? (so the balloon would POP!) • Other example: spray can gets colder when used long

• MOVIE: http: //ed. ted. com/lessons/1207 -1 -abennet-brianh 264

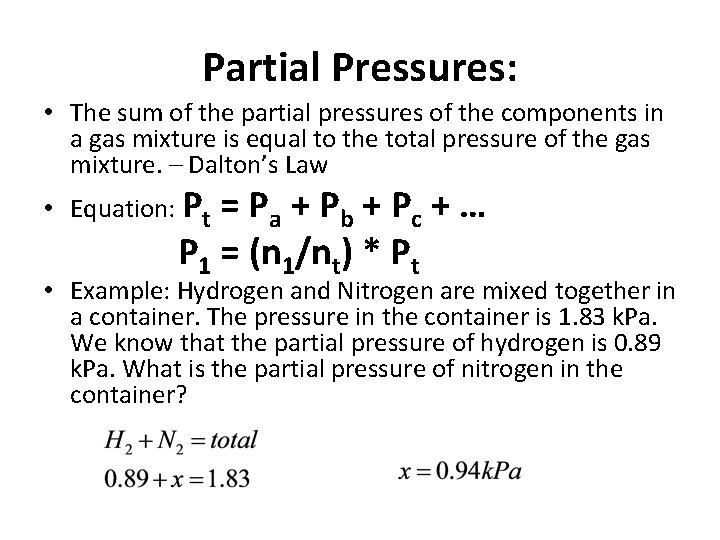
Partial Pressures: • The sum of the partial pressures of the components in a gas mixture is equal to the total pressure of the gas mixture. – Dalton’s Law • Equation: Pt = Pa + Pb + Pc + … P 1 = (n 1/nt) * Pt • Example: Hydrogen and Nitrogen are mixed together in a container. The pressure in the container is 1. 83 k. Pa. We know that the partial pressure of hydrogen is 0. 89 k. Pa. What is the partial pressure of nitrogen in the container?

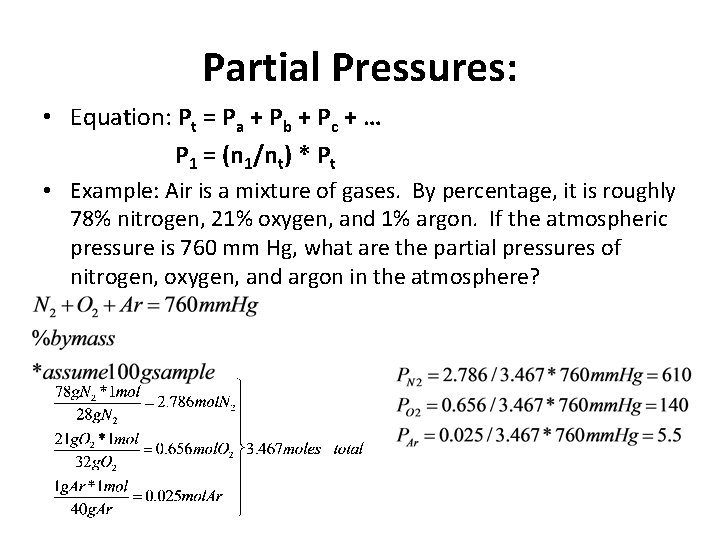
Partial Pressures: • Equation: Pt = Pa + Pb + Pc + … P 1 = (n 1/nt) * Pt • Example: Air is a mixture of gases. By percentage, it is roughly 78% nitrogen, 21% oxygen, and 1% argon. If the atmospheric pressure is 760 mm Hg, what are the partial pressures of nitrogen, oxygen, and argon in the atmosphere?

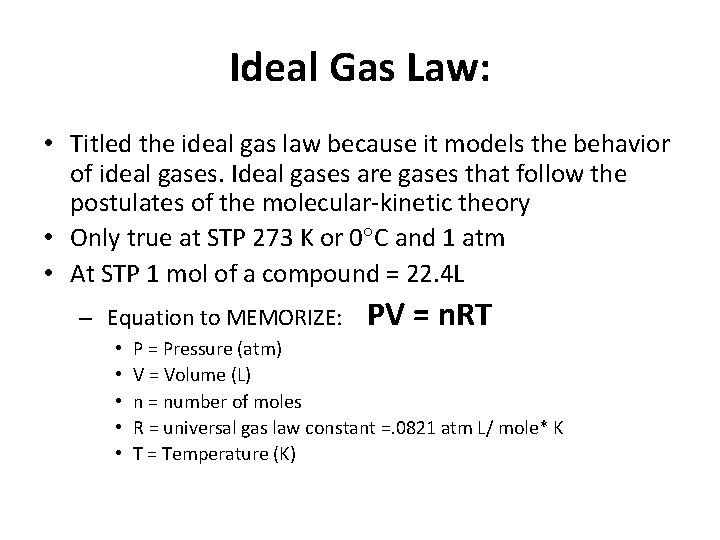
Ideal Gas Law: • Titled the ideal gas law because it models the behavior of ideal gases. Ideal gases are gases that follow the postulates of the molecular-kinetic theory • Only true at STP 273 K or 0 C and 1 atm • At STP 1 mol of a compound = 22. 4 L – Equation to MEMORIZE: • • • PV = n. RT P = Pressure (atm) V = Volume (L) n = number of moles R = universal gas law constant =. 0821 atm L/ mole* K T = Temperature (K)

Real Gases: • There are no true ideal gases most gases are real gases. Real gases behave like ideal gases under normal conditions but diverge at low temperature and high pressure. • Extreme (high) Pressure – molecules do take up space • Extreme (low) Temperature – polarity of molecules plays a factor

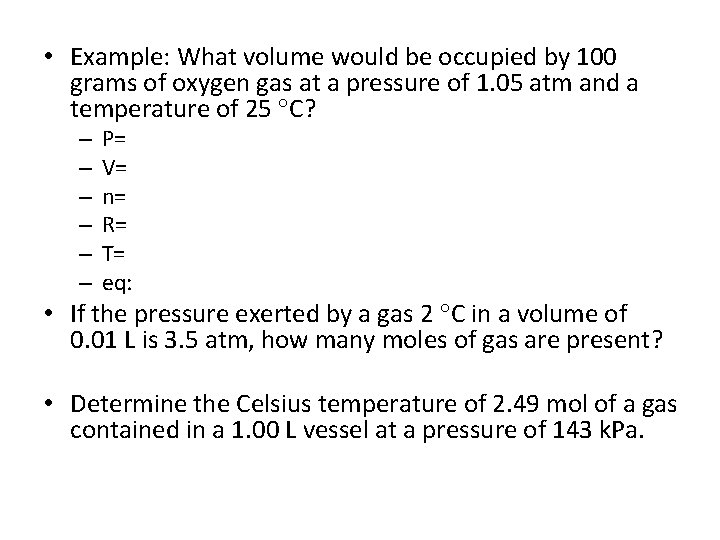
• Example: What volume would be occupied by 100 grams of oxygen gas at a pressure of 1. 05 atm and a temperature of 25 C? – – – P= V= n= R= T= eq: • If the pressure exerted by a gas 2 C in a volume of 0. 01 L is 3. 5 atm, how many moles of gas are present? • Determine the Celsius temperature of 2. 49 mol of a gas contained in a 1. 00 L vessel at a pressure of 143 k. Pa.

• Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl 2 gas is 9. 22 L at 1124 torr and 24°C. – How many grams of Cl 2 are there in the sample? – What volume will the Cl 2 occupy at STP? – At what temperature will the volume be 15. 00 L if the pressure is 8. 76*102 torr? – At what pressure will the volume equal 6. 00 L if the temperature is 58°C?

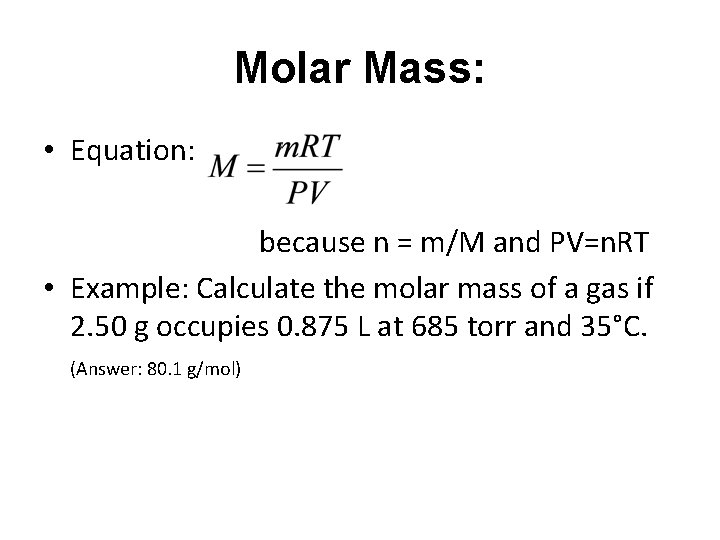
Molar Mass: • Equation: because n = m/M and PV=n. RT • Example: Calculate the molar mass of a gas if 2. 50 g occupies 0. 875 L at 685 torr and 35°C. (Answer: 80. 1 g/mol)

Density: • Equation: because n=m/M and D=m/V and PV=n. RT • Example: Calculate the density of NO 2 gas at 0. 97 atm and 35°C. (Answer: 1. 77 g/L)

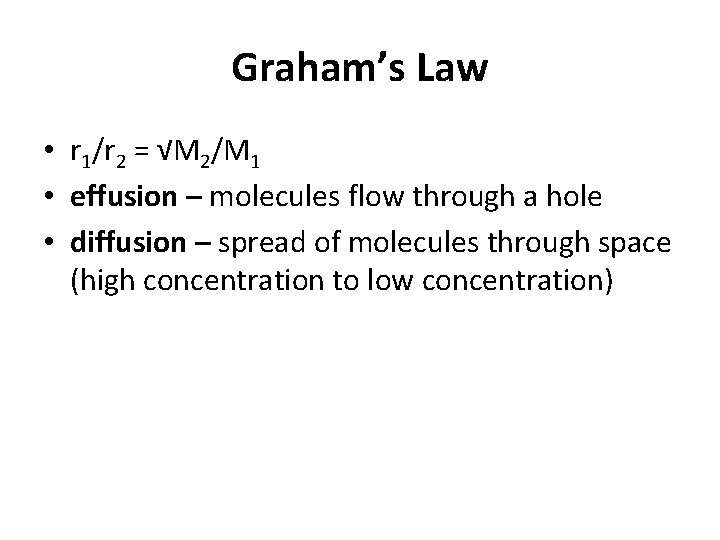
Graham’s Law • r 1/r 2 = √M 2/M 1 • effusion – molecules flow through a hole • diffusion – spread of molecules through space (high concentration to low concentration)
 Kinetic molecular model of gases
Kinetic molecular model of gases Kinetic particle theory of matter
Kinetic particle theory of matter Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic theory of gases
Kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases General gas equation is
General gas equation is Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic theory def
Kinetic theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Ideal gas law examples
Ideal gas law examples Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory







































