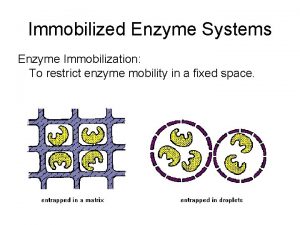
Enzyme Kinetics and Catalysis 3192003 Serine proteases Diverse




![For the reaction ‡ ‡ ‡ Where [X] is the concentration of the transition For the reaction ‡ ‡ ‡ Where [X] is the concentration of the transition](https://slidetodoc.com/presentation_image/1554a0bce7d187ba57b4d5b1f447af3c/image-31.jpg)
- Slides: 36

Enzyme Kinetics and Catalysis 3/19/2003

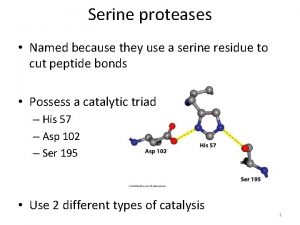
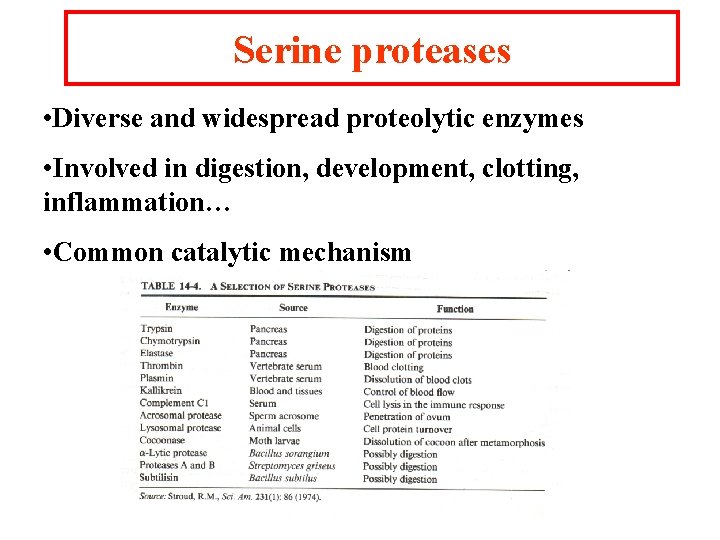
Serine proteases • Diverse and widespread proteolytic enzymes • Involved in digestion, development, clotting, inflammation… • Common catalytic mechanism

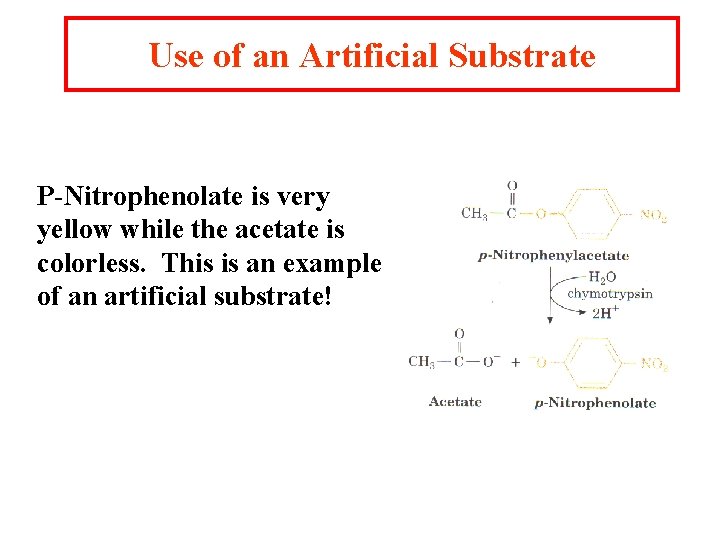
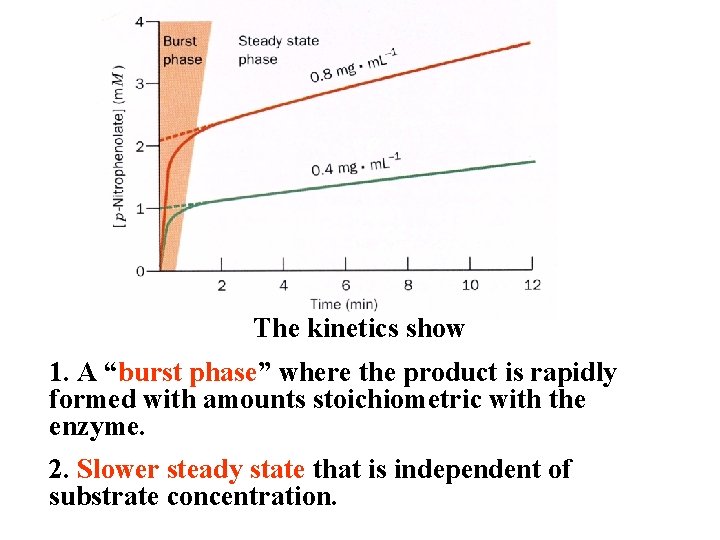
Use of an Artificial Substrate P-Nitrophenolate is very yellow while the acetate is colorless. This is an example of an artificial substrate!

The kinetics show 1. A “burst phase” where the product is rapidly formed with amounts stoichiometric with the enzyme. 2. Slower steady state that is independent of substrate concentration.

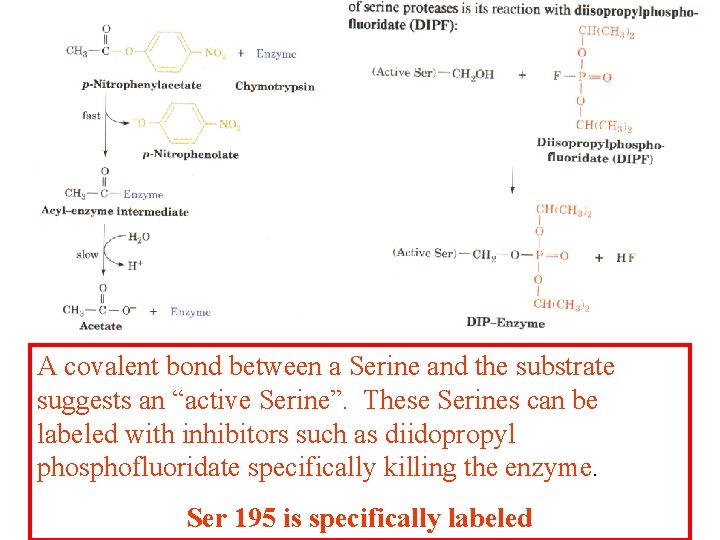
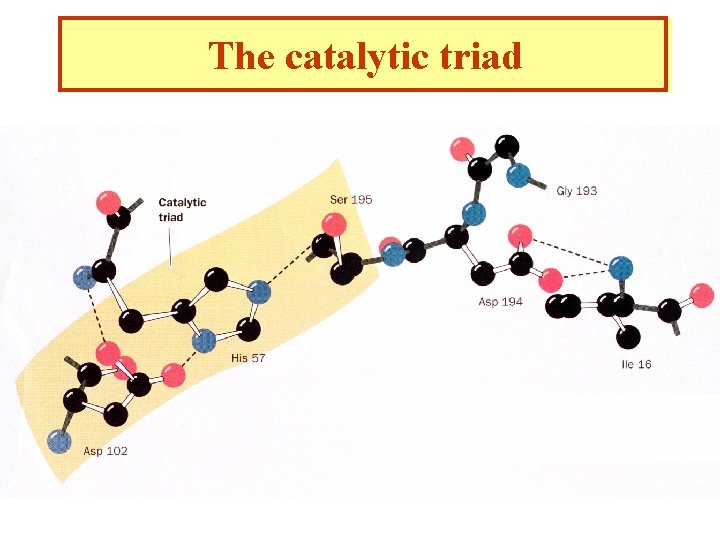
A covalent bond between a Serine and the substrate suggests an “active Serine”. These Serines can be labeled with inhibitors such as diidopropyl phosphofluoridate specifically killing the enzyme. Ser 195 is specifically labeled

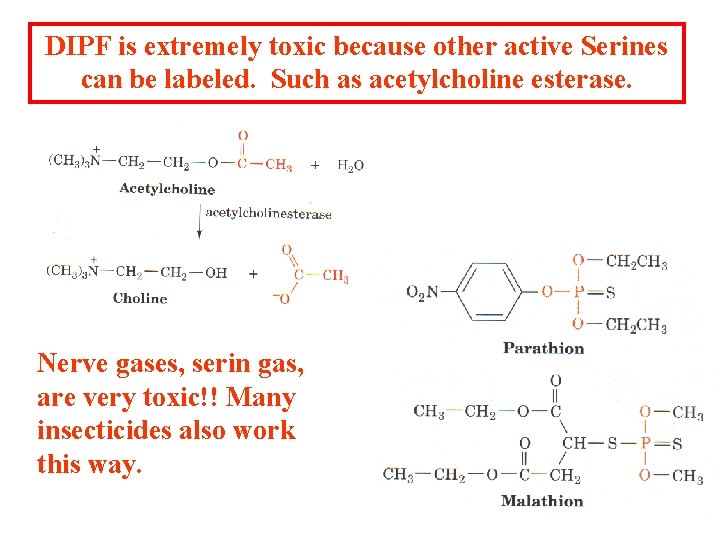
DIPF is extremely toxic because other active Serines can be labeled. Such as acetylcholine esterase. Nerve gases, serin gas, are very toxic!! Many insecticides also work this way.

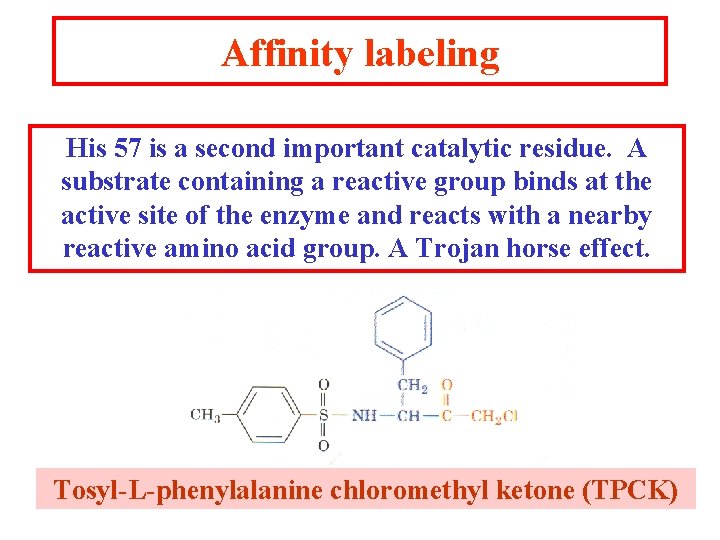
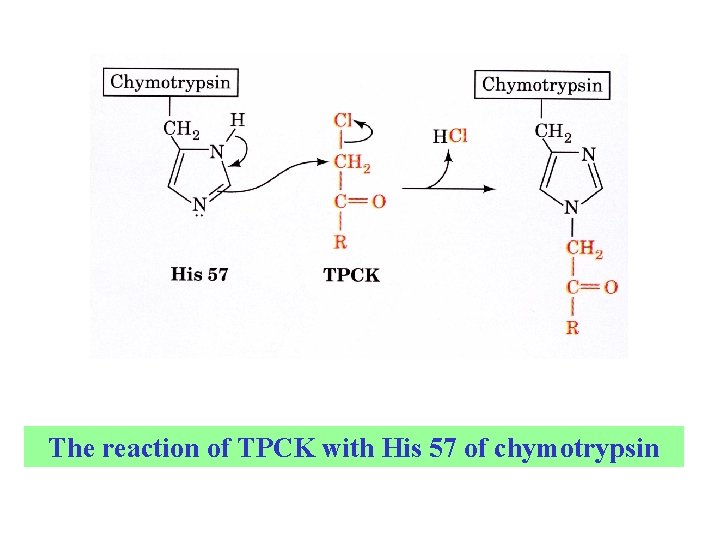
Affinity labeling His 57 is a second important catalytic residue. A substrate containing a reactive group binds at the active site of the enzyme and reacts with a nearby reactive amino acid group. A Trojan horse effect. Tosyl-L-phenylalanine chloromethyl ketone (TPCK)

The reaction of TPCK with His 57 of chymotrypsin

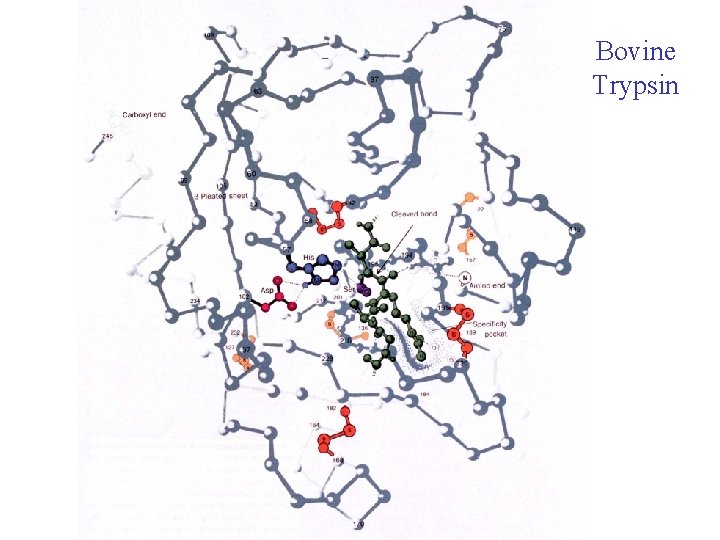
Bovine Trypsin

The catalytic triad

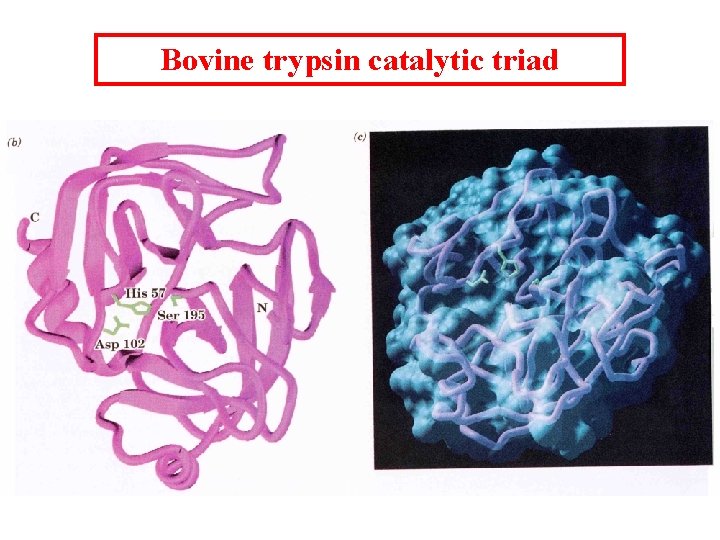
Bovine trypsin catalytic triad


Catalytic mechanism 1. After the substrate binds Ser 195 nucleophilically attacks the scissile peptide bond to form a transition state complex called the tetrahedral intermediate (covalent catalysis) the imidazole His 52 takes up the proton Asp 102 is hydrogen bonded to His 57. Without Asp 102 the rate of catalysis is only 0. 05% of wild-type. 2. Tetrahedral intermediate decomposes to the acylenzyme intermediate. His 57 acts as an acid donating a proton. 3. The enzyme is deacylated by the reverse of step 1 with water the attacking nucleophile and Ser 195 as the leaving group.

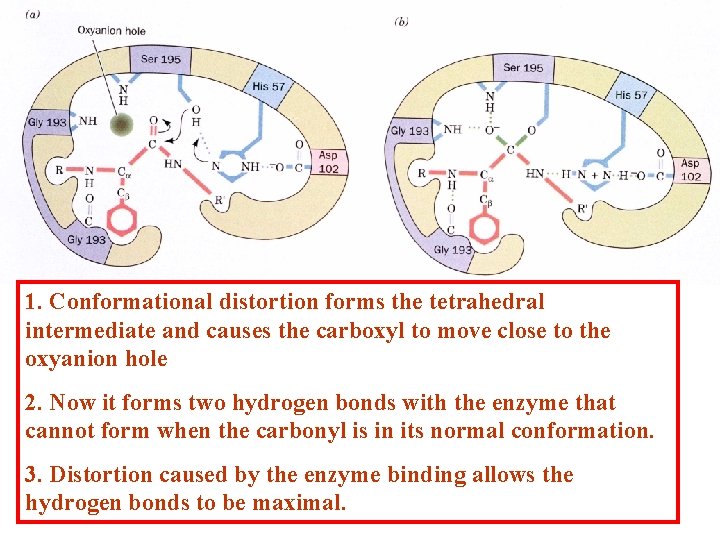
1. Conformational distortion forms the tetrahedral intermediate and causes the carboxyl to move close to the oxyanion hole 2. Now it forms two hydrogen bonds with the enzyme that cannot form when the carbonyl is in its normal conformation. 3. Distortion caused by the enzyme binding allows the hydrogen bonds to be maximal.

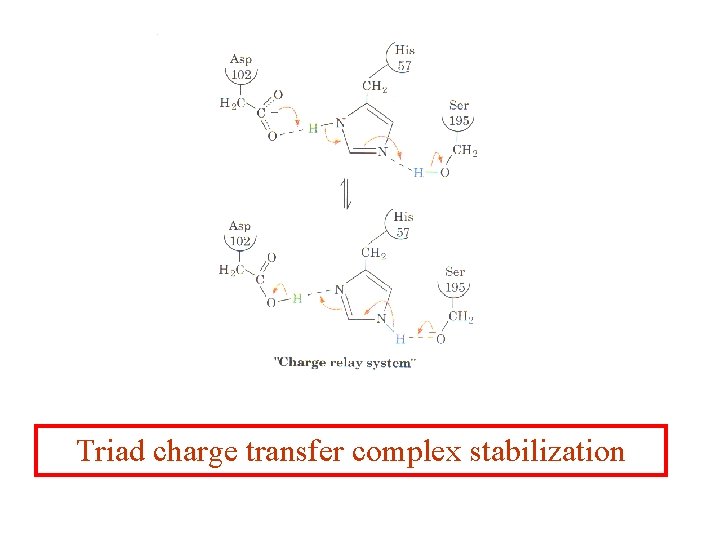
Triad charge transfer complex stabilization

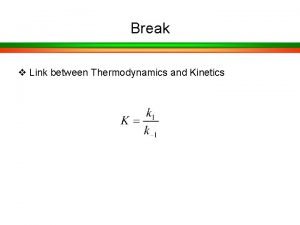
Enzyme Kinetics Rates of Enzyme Reactions How fast do reactions take place • Reaction rates Thermodynamics says I know the difference between state 1 and state 2 and DG = (Gf - Gi) But Changes in reaction rates in response to differing conditions is related to path followed by the reaction and is indicative of the reaction mechanism!!

Enzyme kinetics are important for many reasons 1. Substrate binding constants can be measured as well as inhibitor strengths and maximum catalytic rates. 2. Kinetics alone will not give a chemical mechanism but combined with chemical and structural data mechanisms can be elucidated. 3. Kinetics help understand the enzymes role in metabolic pathways. 4. Under “proper” conditions rates are proportional to enzyme concentrations and these can be determine “ metabolic problems”.

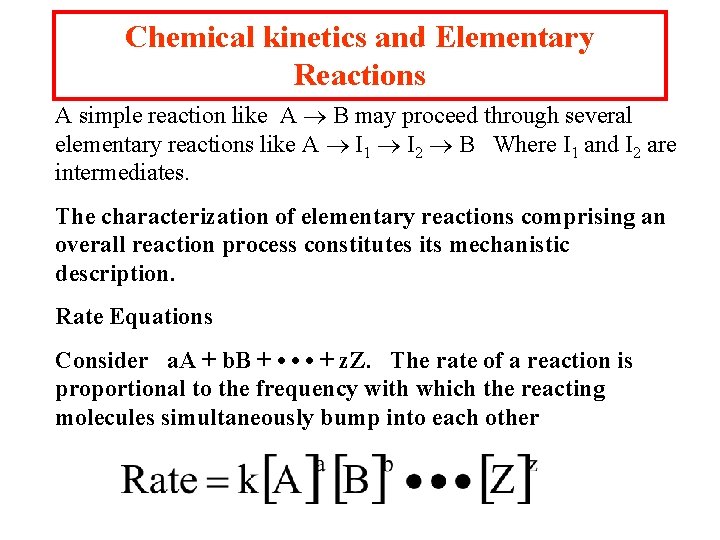
Chemical kinetics and Elementary Reactions A simple reaction like A B may proceed through several elementary reactions like A I 1 I 2 B Where I 1 and I 2 are intermediates. The characterization of elementary reactions comprising an overall reaction process constitutes its mechanistic description. Rate Equations Consider a. A + b. B + • • • + z. Z. The rate of a reaction is proportional to the frequency with which the reacting molecules simultaneously bump into each other

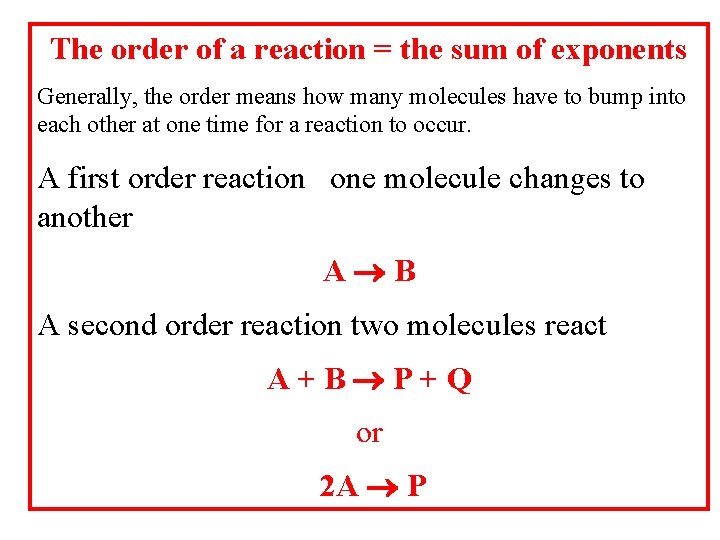
The order of a reaction = the sum of exponents Generally, the order means how many molecules have to bump into each other at one time for a reaction to occur. A first order reaction one molecule changes to another A B A second order reaction two molecules react A+B P+Q or 2 A P

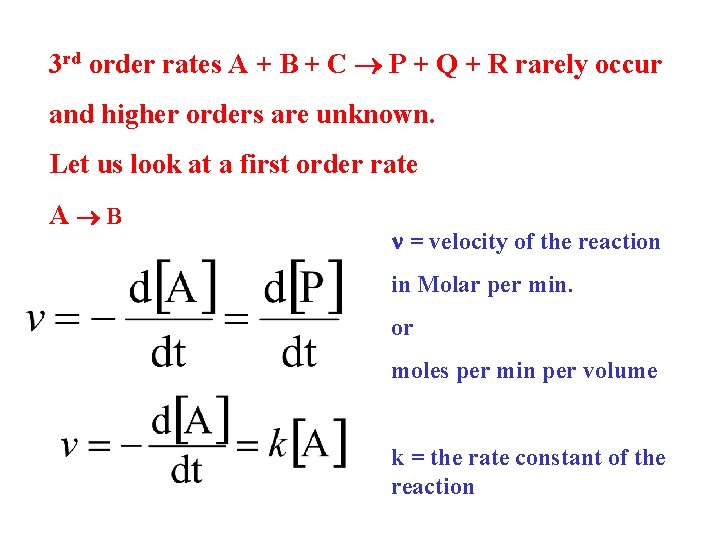
3 rd order rates A + B + C P + Q + R rarely occur and higher orders are unknown. Let us look at a first order rate A B n = velocity of the reaction in Molar per min. or moles per min per volume k = the rate constant of the reaction

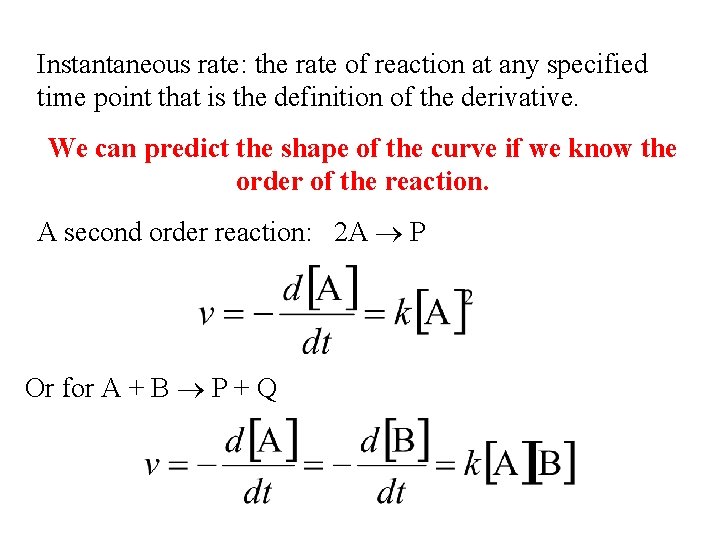
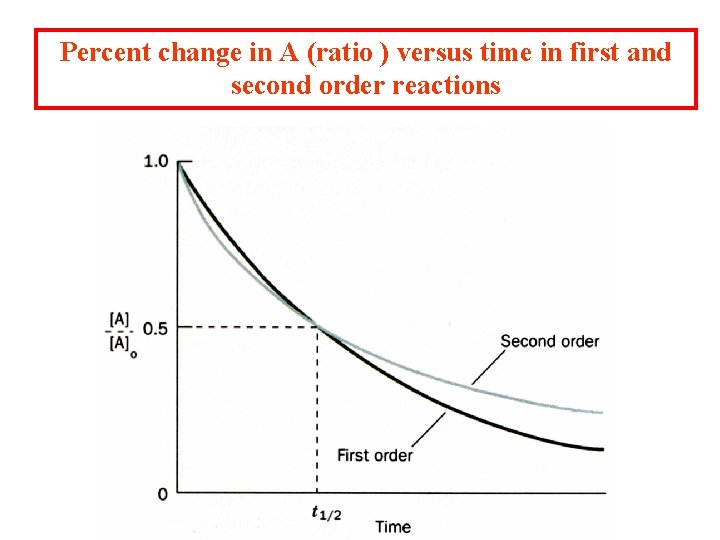
Instantaneous rate: the rate of reaction at any specified time point that is the definition of the derivative. We can predict the shape of the curve if we know the order of the reaction. A second order reaction: 2 A P Or for A + B P + Q

Percent change in A (ratio ) versus time in first and second order reactions

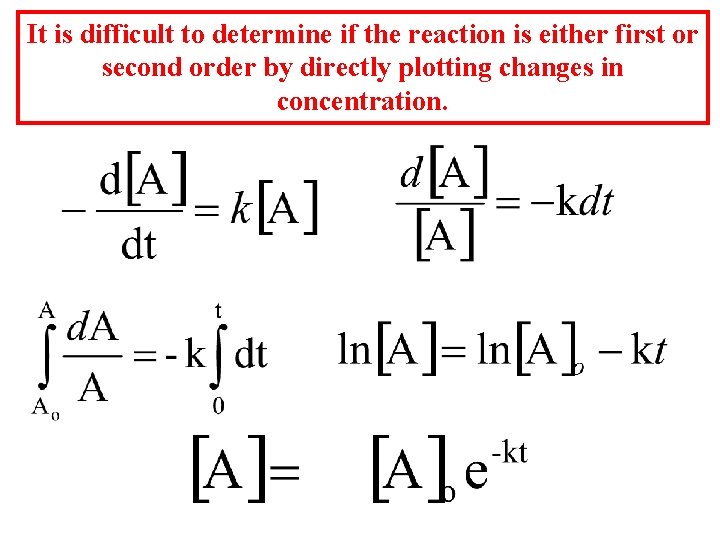
It is difficult to determine if the reaction is either first or second order by directly plotting changes in concentration.

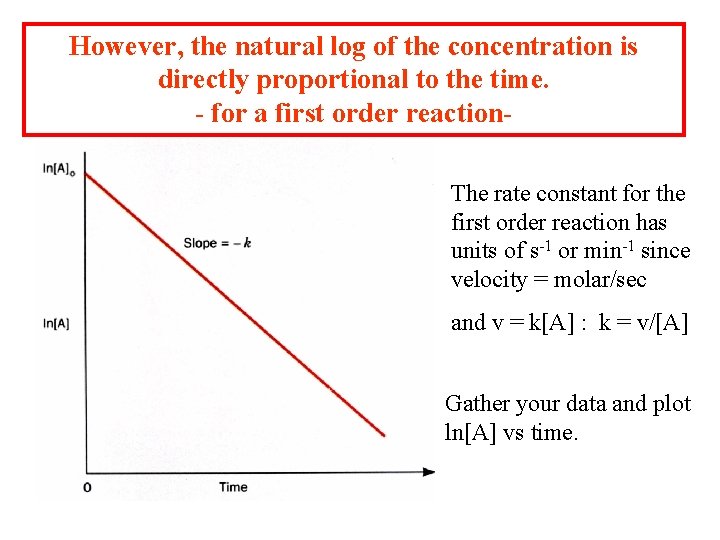
However, the natural log of the concentration is directly proportional to the time. - for a first order reaction. The rate constant for the first order reaction has units of s-1 or min-1 since velocity = molar/sec and v = k[A] : k = v/[A] Gather your data and plot ln[A] vs time.

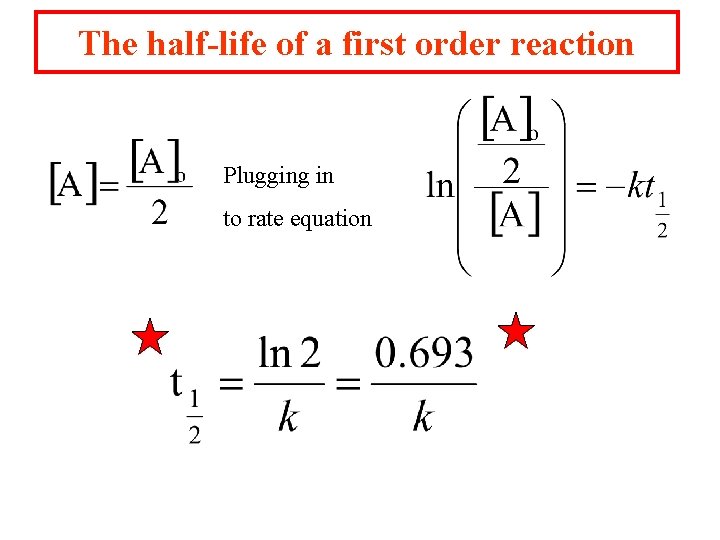
The half-life of a first order reaction Plugging in to rate equation

The half-life of a first order reaction can be used to determine the amount of material left after a length of time. The time for half of the reactant which is initially present to decompose or change. 32 P, a common radioactive isotope, emits an energetic b particle and has a half-life of 14 days. 14 C has a half life of 5715 years.

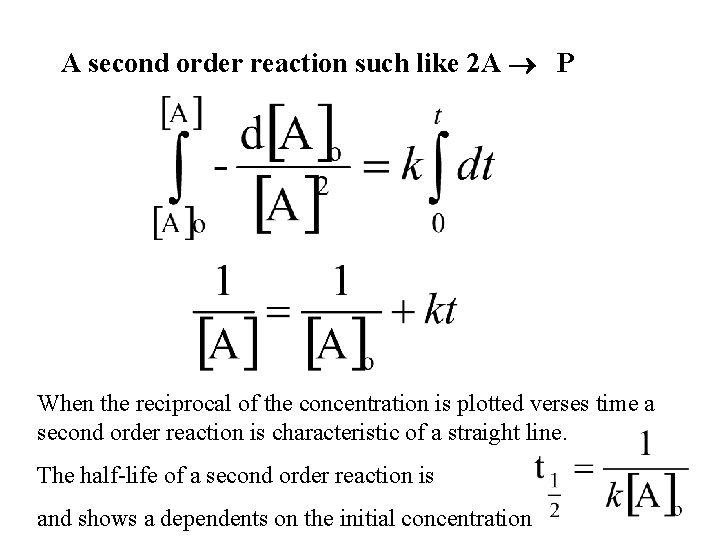
A second order reaction such like 2 A P When the reciprocal of the concentration is plotted verses time a second order reaction is characteristic of a straight line. The half-life of a second order reaction is and shows a dependents on the initial concentration

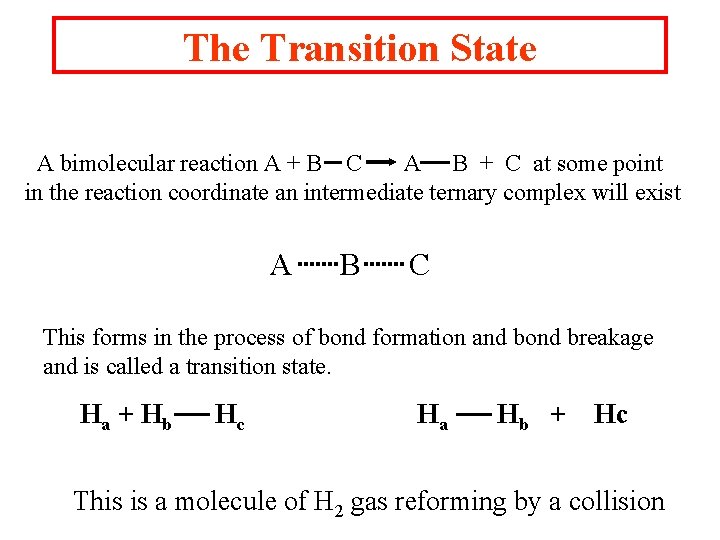
The Transition State A bimolecular reaction A + B C A B + C at some point in the reaction coordinate an intermediate ternary complex will exist A B C This forms in the process of bond formation and bond breakage and is called a transition state. Ha + Hb Hc Ha Hb + Hc This is a molecule of H 2 gas reforming by a collision

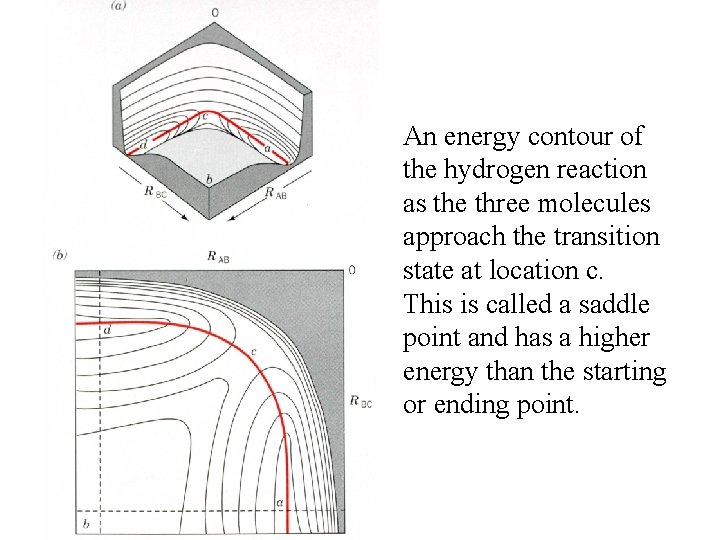
An energy contour of the hydrogen reaction as the three molecules approach the transition state at location c. This is called a saddle point and has a higher energy than the starting or ending point.

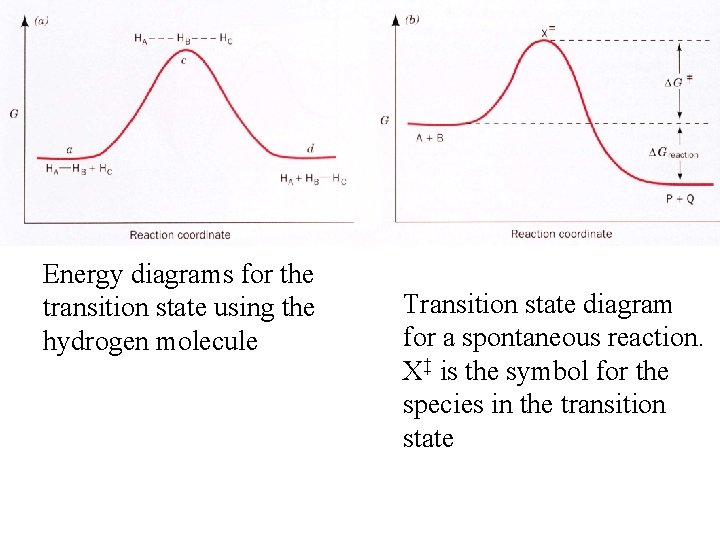
Energy diagrams for the transition state using the hydrogen molecule Transition state diagram for a spontaneous reaction. X‡ is the symbol for the species in the transition state
![For the reaction Where X is the concentration of the transition For the reaction ‡ ‡ ‡ Where [X] is the concentration of the transition](https://slidetodoc.com/presentation_image/1554a0bce7d187ba57b4d5b1f447af3c/image-31.jpg)
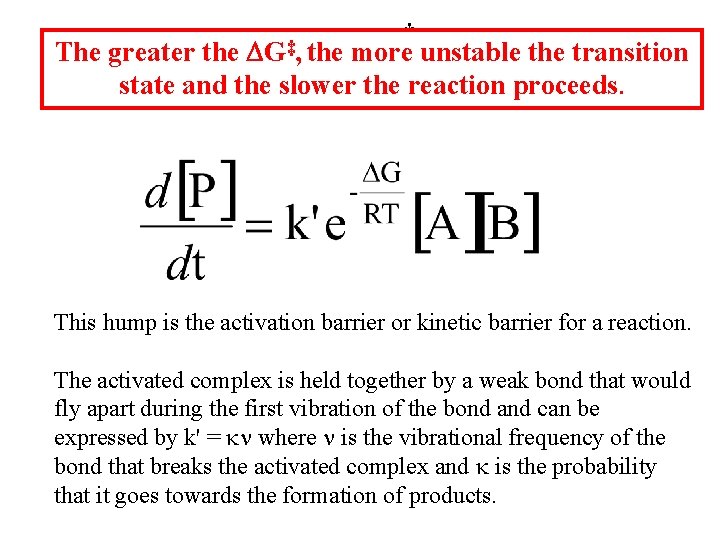
For the reaction ‡ ‡ ‡ Where [X] is the concentration of the transition state species k' = rate constant for the decomposition of the activated complex ‡ ‡ DG‡ is the Gibbs free energy of the activated complex.

‡ The greater the DG‡, the more unstable the transition state and the slower the reaction proceeds. This hump is the activation barrier or kinetic barrier for a reaction. The activated complex is held together by a weak bond that would fly apart during the first vibration of the bond and can be expressed by k' = kn where n is the vibrational frequency of the bond that breaks the activated complex and k is the probability that it goes towards the formation of products.

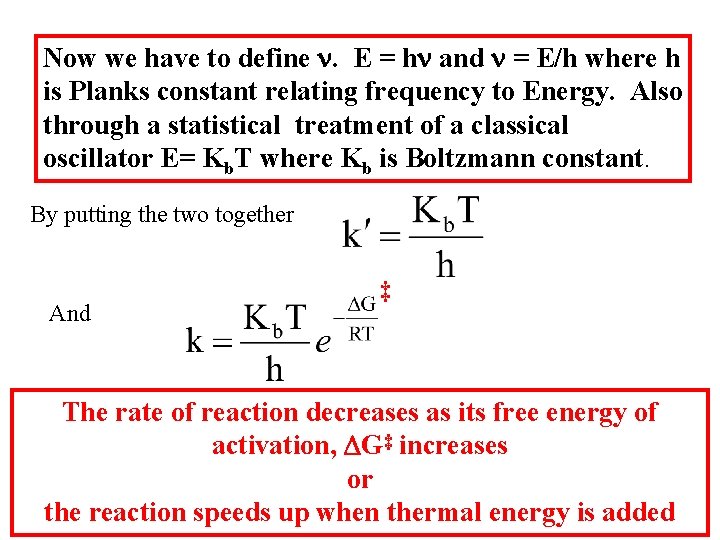
Now we have to define n. E = hn and n = E/h where h is Planks constant relating frequency to Energy. Also through a statistical treatment of a classical oscillator E= Kb. T where Kb is Boltzmann constant. By putting the two together And ‡ The rate of reaction decreases as its free energy of activation, DG‡ increases or the reaction speeds up when thermal energy is added

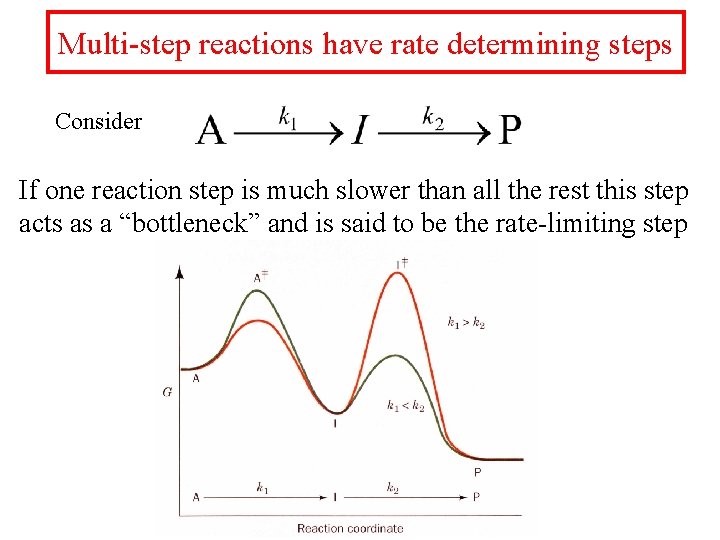
Multi-step reactions have rate determining steps Consider If one reaction step is much slower than all the rest this step acts as a “bottleneck” and is said to be the rate-limiting step

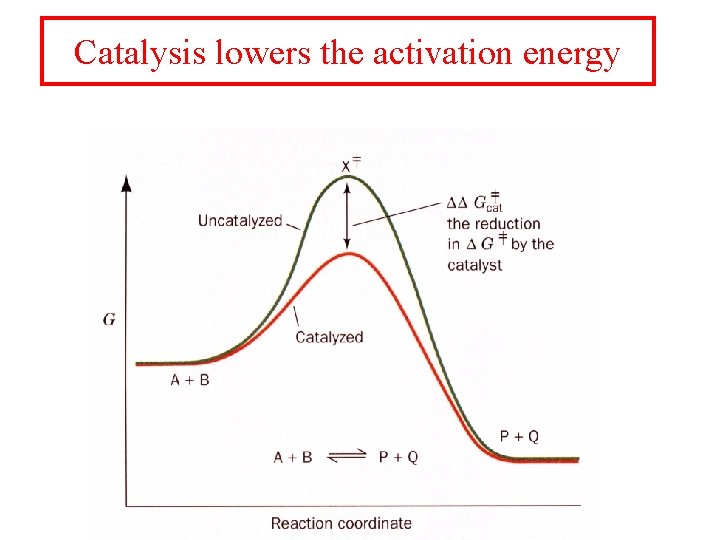
Catalysis lowers the activation energy

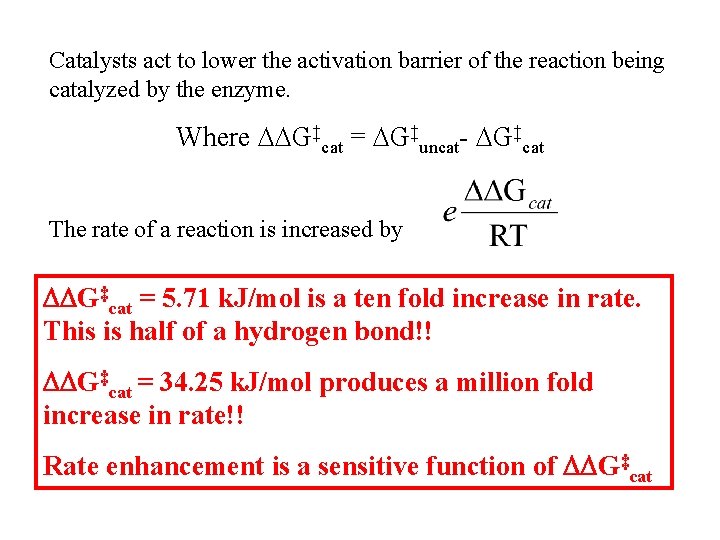
Catalysts act to lower the activation barrier of the reaction being catalyzed by the enzyme. Where DDG‡cat = DG‡uncat- DG‡cat The rate of a reaction is increased by DDG‡cat = 5. 71 k. J/mol is a ten fold increase in rate. This is half of a hydrogen bond!! DDG‡cat = 34. 25 k. J/mol produces a million fold increase in rate!! Rate enhancement is a sensitive function of DDG‡cat
 Diversity among protoctista
Diversity among protoctista Mixed inhibitor km and vmax
Mixed inhibitor km and vmax Lineweaver-burk equation
Lineweaver-burk equation Enzyme kinetics
Enzyme kinetics Lydia seems to be a kind considerate girl
Lydia seems to be a kind considerate girl Bioplek
Bioplek Quaternaire structuur
Quaternaire structuur Glycine serine
Glycine serine The park was serine at twilight
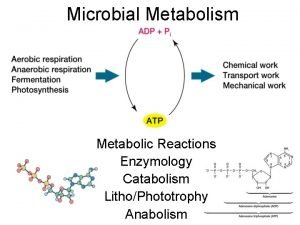
The park was serine at twilight Energy catalysis and biosynthesis
Energy catalysis and biosynthesis Decao
Decao Apoenzyme
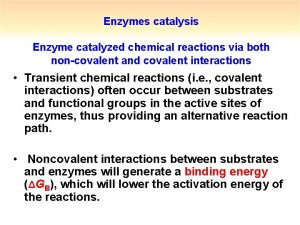
Apoenzyme What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
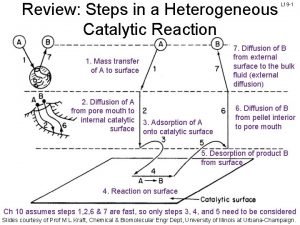
Specific acid base catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Boreskov institute of catalysis
Boreskov institute of catalysis Honh2 dissociation equation
Honh2 dissociation equation Kinetics and equilibrium
Kinetics and equilibrium Antagonistic effect
Antagonistic effect Difference between zero and first order kinetics
Difference between zero and first order kinetics Kinetics of rigid bodies
Kinetics of rigid bodies Kinetics of a particle: force and acceleration
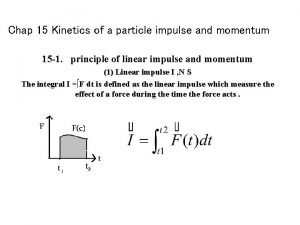
Kinetics of a particle: force and acceleration Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Leukocyte development kinetics and functions
Leukocyte development kinetics and functions Kinematics of rigid bodies
Kinematics of rigid bodies Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Cell kinetics and fermenter design
Cell kinetics and fermenter design One syllable adjectives
One syllable adjectives Working with culturally and linguistically diverse families
Working with culturally and linguistically diverse families Individuals and families diverse perspectives
Individuals and families diverse perspectives What is deep level diversity
What is deep level diversity Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry