Disorders of the Pituitary and Hypothalamus Hasan AYDIN

















































- Slides: 76

Disorders of the Pituitary and Hypothalamus Hasan AYDIN, MD Yeditepe University Medical faculty Department of Endocrinology and Metabolism


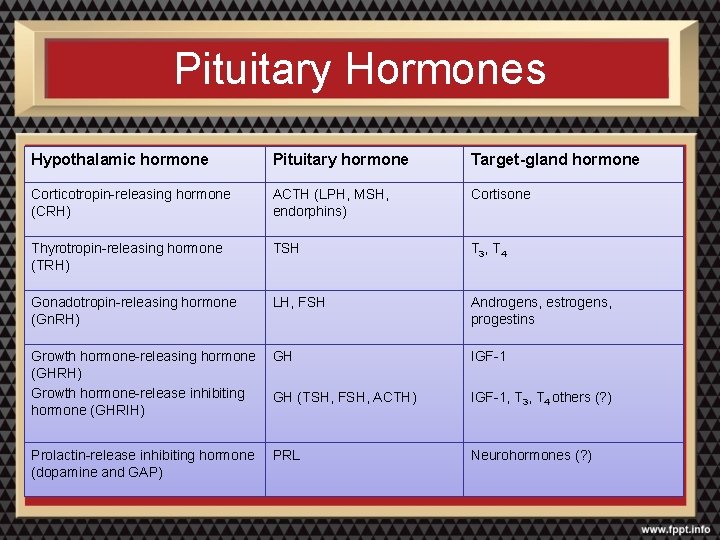
Pituitary Hormones Hypothalamic hormone Pituitary hormone Target-gland hormone Corticotropin-releasing hormone (CRH) ACTH (LPH, MSH, endorphins) Cortisone Thyrotropin-releasing hormone (TRH) TSH T 3, T 4 Gonadotropin-releasing hormone (Gn. RH) LH, FSH Androgens, estrogens, progestins Growth hormone-releasing hormone GH (GHRH) Growth hormone-release inhibiting GH (TSH, FSH, ACTH) hormone (GHRIH) IGF-1 Prolactin-release inhibiting hormone (dopamine and GAP) Neurohormones (? ) PRL IGF-1, T 3, T 4 others (? )

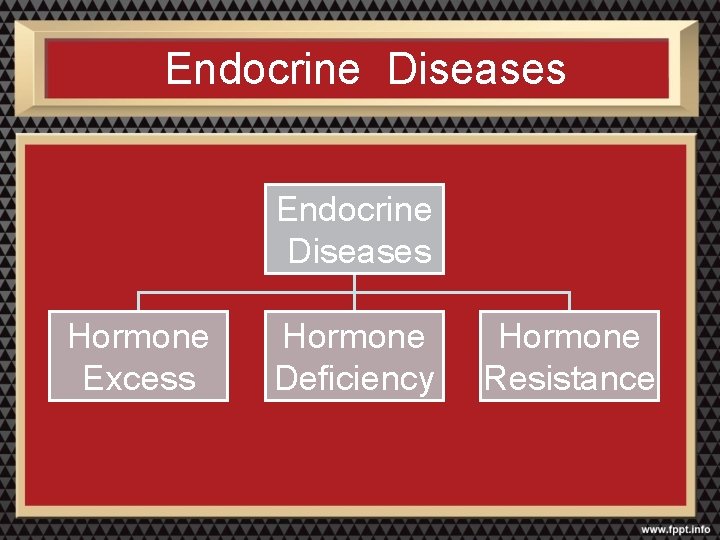
Endocrine Diseases Hormone Excess Hormone Deficiency Hormone Resistance

Pituitary Diseases • Diabetes Insipidus • Inappropriate ADH Syndrome • Empty Sella Syndrome • Hyperpituitarism • Hypopituitarism • Pituitary Apoplexy • Pituitary Neoplasms

Pituitary Tumors

Pituitary Adenomas • • Account for 10 % of all intracranial tumors Almost always benign Frequently seen after the third decade Should be differentiated from – – Hyperplasia Craniopharyngiomas (from Rathke’s pouch) Meningiomas, malignant tumors, cysts, abscess Lymphocytic hypophysitis • Microadenoma < 1 cm • Macroadenoma > 1 cm

Pituitary Tumors: Clinical Presentation – Mass effect • Superior extension – May compromise optic pathways – leading to impaired visual acuity and visual field defects – May produce hypothalamic syndrome – disturbed thirst, satiety, sleep, and temperature regulation • Lateral extension – May compress cranial nerves III, IV, V, and VI – leaning to diplopia • Inferior extension – May lead to cerebrospinal fluid rhinorrhea – Hormone defiency • Hypopituitarism • Diabetes insipitus – Hormone excess • Hyperpituitarism • Inappropriate secretion of ADH

Etiology of Pituitary Adenomas • Non-Functioning Pituitary Adenomas • Endocrine active pituitary adenomas – – – Prolactinoma Somatotropinoma Corticotropinoma Thyrotropinoma Other mixed endocrine active adenomas • Malignant pituitary tumors: Functional and non-functional pituitary carcinoma • Metastases in the pituitary (breast, lung, stomach, kidney) • Pituitary cysts: Rathke's cleft cyst, Mucocoeles, Others • Empty sella syndrome

Etiology of Pituitary Adenomas • Developmental abnormalities: Craniopharyngioma (occasionally intrasellar location), Germinoma, Others • Primary Tumors of the central nervous system: Perisellar meningioma, Optic glioma • Vascular tumors: Hemangioblastoma • Malignant systemic diseases: Hodgkin's disease, Non-Hodgkin lymphoma, Leukemic infiltration, Histiocystosis X, Eosinophilic granuloma, Giant cell granuloma (tumor) • Granulomatous diseases: Neurosarcoidosis, Wegener's granulomatosis, Tuberculosis, Syphilis • Vascular aneurysms (intrasellar location)

Evaluation of an Incidental Pituitary Mass • Clinical Evaluation • Hormonal Evaluation • Radiologic Evaluation

Clinical Evaluation • Inappropriate pituitary hormone secretion • Visual field deficits • Cranial nerve palsies • Temporal lobe epilepsy • Hydrocephalus • Cerebrospinal fluid rhinorrhea

Hormonal Evaluation • May include of both basal hormone measurement and dynamic stimulation testing. • All pituitary masses should have screening basal hormone measurements, including: – Prolactin – TSH, FT 4 – ACTH, AM cortisol, midnight salivary cortisol – LH, FSH, estradiol or testosterone – GH, Insulin-like growth factor-1 (IGF-1)

Hormonal Evaluation (continued) Dynamic stimulation/suppression testing: – may be useful in selected cases to further evaluate pituitary reserve and/or for pituitary hyperfunction • • Dexamethasone suppression testing Oral glucose GH suppression test GHRH, L-dopa, arginine test CRH stimulation test Metyrapone test TRH stimulation test Gn. RH stimulation test Insulin-induced hypoglycemia test

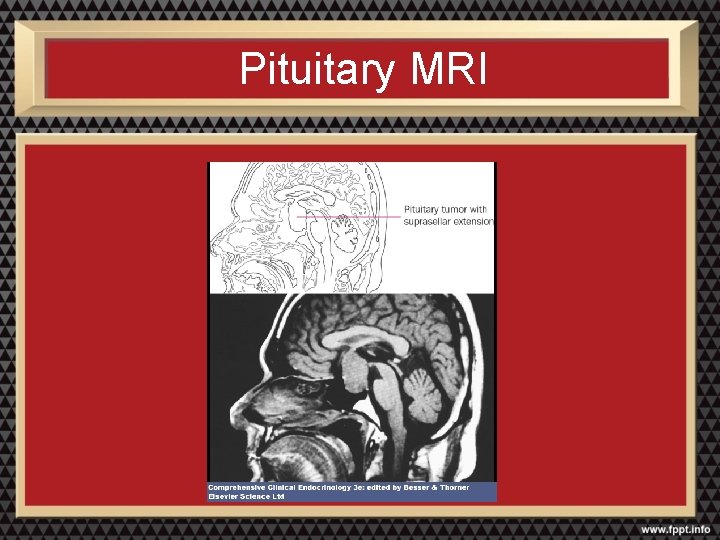
Radiologic Evaluation: MRI • Preferred imaging study for the pituitary • Better visualization of soft tissues and vascular structures than CT • No exposure to ionizing radiation

Radiologic Evaluation: CT • Better at visualizing bony structures and calcifications within soft tissues • Better at determining diagnosis of tumors with calcification, such as germinomas, craniopharyngiomas, and meningiomas • May be useful when MRI is contraindicated, such as in patients with pacemakers or metallic implants in the brain or eyes • Disadvantages include: – less optimal soft tissue imaging compared to MRI – exposure to radiation

Management of Pituitary Neoplasia • Observation • Pharmacotherapy • Surgery • Radiation Therapy

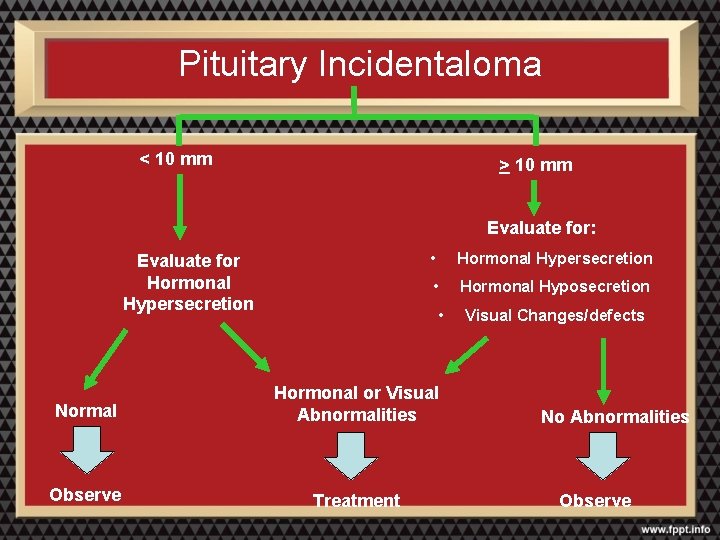
Pituitary Incidentaloma < 10 mm > 10 mm Evaluate for: Evaluate for Hormonal Hypersecretion • Hormonal Hyposecretion • Normal Hormonal or Visual Abnormalities Observe Treatment Visual Changes/defects No Abnormalities Observe

Observation and Follow-up • If less than 20 mm and no neurologic or hormonal abnormalities: – Monitor for adenoma size, visual changes, and hormonal hypersecretion in 6 and 12 months, then annually for a few years • Lesions less than 10 mm and proven to have no hormonal hypersecretion: – Lesions 2 to 4 mm: no further testing required – Lesions 5 to 9 mm: MRI can be done once or twice over the subsequent two years; if the lesion is stable in this period, the frequency can be decreased

Pharmacotherapy Which pharmacologic option to choose depends on type of tumor: • Dopamine agonists: Bromocriptine, Cabergoline- most useful for prolactinomas, less useful for GH secreting adenomas • Somatostatin analog: (Octreotide, Octreotide LAR, Lanreotide)- most useful for acromegaly • Pegvisomant (GH receptor blocker): useful in acromegaly refractory to somatostatin analogues • Other: Ketoconazole, Metyrapone, Mitotane- for Cushings disease- use limited by side effects, expense and lack of efficacy

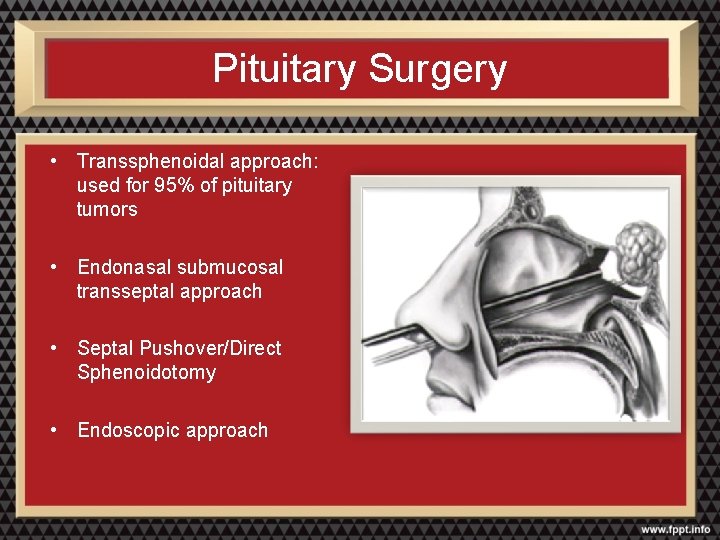
Pituitary Surgery • Transsphenoidal approach: used for 95% of pituitary tumors • Endonasal submucosal transseptal approach • Septal Pushover/Direct Sphenoidotomy • Endoscopic approach

Indications for Surgery • First-line treatment of some symptomatic pituitary adenomas. • Useful when medical therapy fails • Indicated in pituitary apoplexy with compressive symptoms • Surgery provides prompt relief from excess hormone secretion and mass effect.

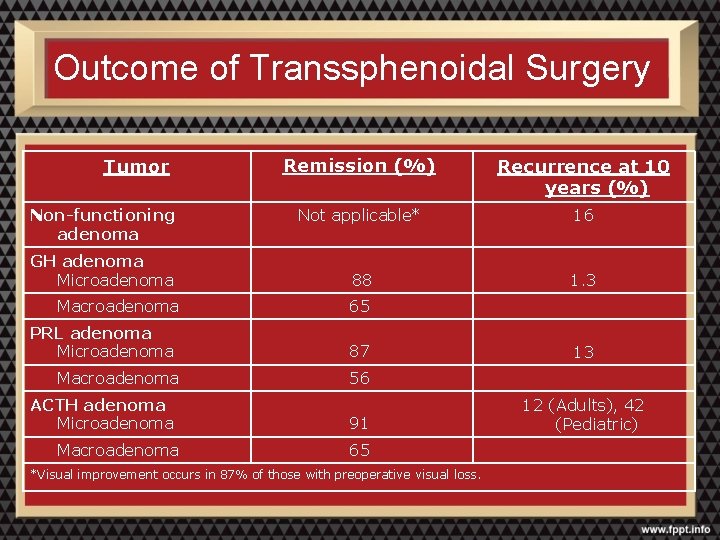
Outcome of Transsphenoidal Surgery Tumor Non-functioning adenoma GH adenoma Microadenoma Macroadenoma PRL adenoma Microadenoma Macroadenoma ACTH adenoma Microadenoma Macroadenoma Remission (%) Recurrence at 10 years (%) Not applicable* 16 88 1. 3 65 87 13 56 91 12 (Adults), 42 (Pediatric) 65 *Visual improvement occurs in 87% of those with preoperative visual loss.

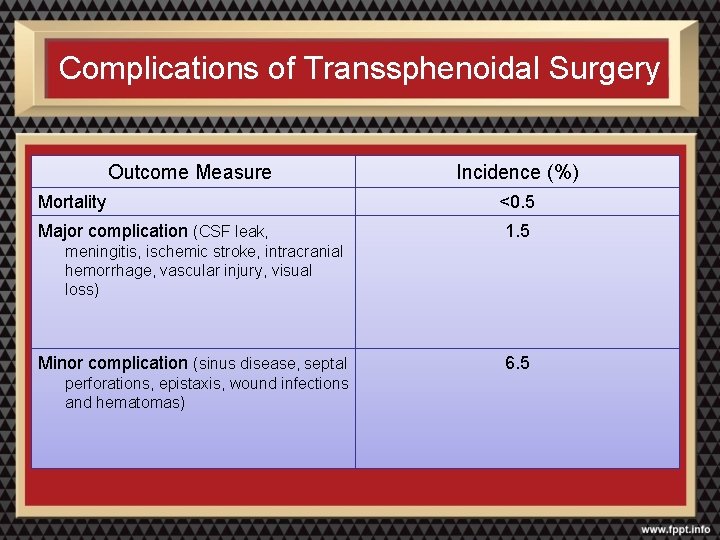
Complications of Transsphenoidal Surgery Outcome Measure Incidence (%) Mortality <0. 5 Major complication (CSF leak, 1. 5 meningitis, ischemic stroke, intracranial hemorrhage, vascular injury, visual loss) Minor complication (sinus disease, septal perforations, epistaxis, wound infections and hematomas) 6. 5

Radiation Therapy • Reserved for patients with larger tumors and/or persistent hormonal hyperfunction despite surgical intervention – Conventional radiotherapy – Gamma knife radiosurgery

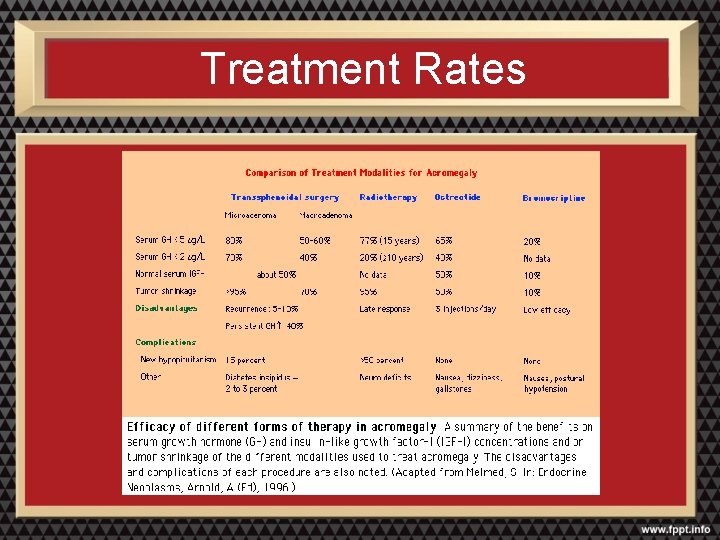
Conventional Radiotherapy • Response is slow, may take 5 to 10 years for full effect • Successful in up to 80% of acromegalics and 55 -60% of Cushing’s disease • High rate of hypopituitarism: up to 60% • Other complications: optic nerve damage, seizures, radionecrosis of brain tissue

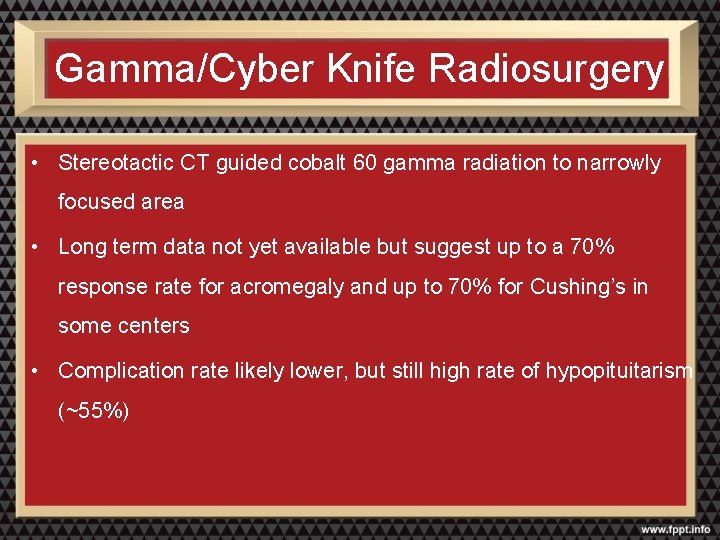
Gamma/Cyber Knife Radiosurgery • Stereotactic CT guided cobalt 60 gamma radiation to narrowly focused area • Long term data not yet available but suggest up to a 70% response rate for acromegaly and up to 70% for Cushing’s in some centers • Complication rate likely lower, but still high rate of hypopituitarism (~55%)

Abnormal Pituitary Function Associated with Pituitary Tumors

Disorders of Pituitary Function • Hypopituitarism – Central hypoadrenalism, hypogonadism, hypothyroidism or GH deficiency – Panhypopituitarism • Hypersecretion of Pituitary Hormones – Hyperprolactinemia – Acromegaly – Cushing’s Disease

Hyperprolactinemia (Prolactinomas)

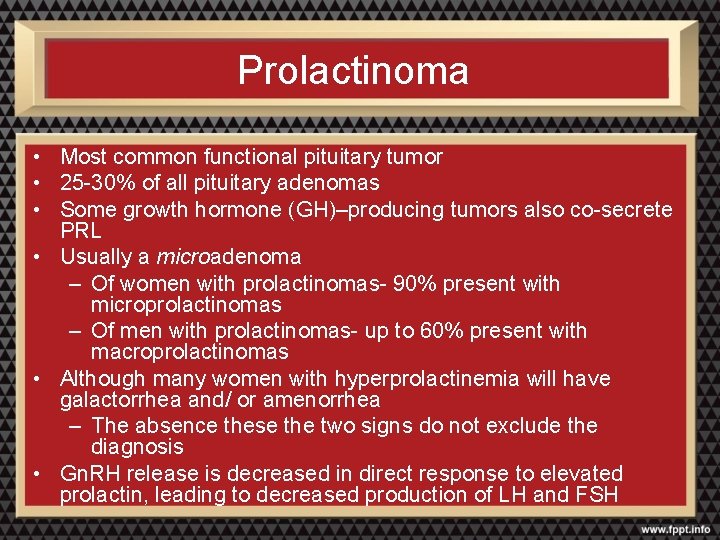
Prolactinoma • Most common functional pituitary tumor • 25 -30% of all pituitary adenomas • Some growth hormone (GH)–producing tumors also co-secrete PRL • Usually a microadenoma – Of women with prolactinomas- 90% present with microprolactinomas – Of men with prolactinomas- up to 60% present with macroprolactinomas • Although many women with hyperprolactinemia will have galactorrhea and/ or amenorrhea – The absence these the two signs do not exclude the diagnosis • Gn. RH release is decreased in direct response to elevated prolactin, leading to decreased production of LH and FSH

Signs and symptoms of prolactinomas • Galactorrhea • Oligomenorrhea or amenorrhea • Reduced fertility • Loss of libido • Erectile dysfunction • Headache • Visual field deficits


REMEMBER Not all hyperprolactinemia is due to a prolactinoma

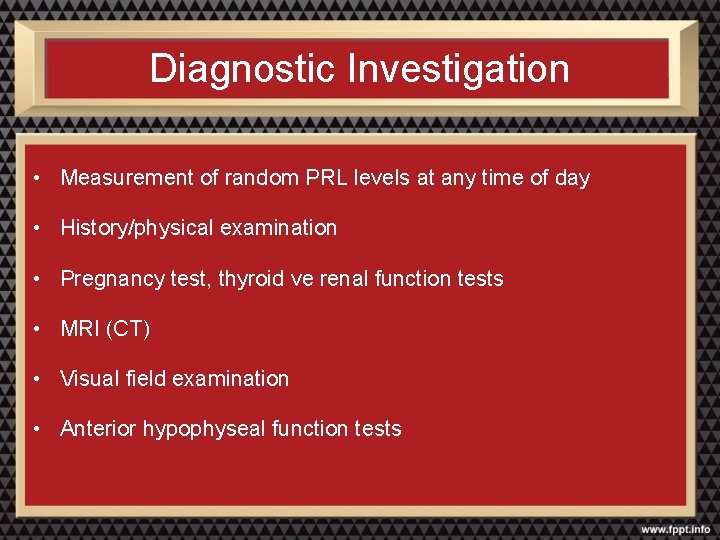
Diagnostic Investigation • Measurement of random PRL levels at any time of day • History/physical examination • Pregnancy test, thyroid ve renal function tests • MRI (CT) • Visual field examination • Anterior hypophyseal function tests

Misleading Prolactin Measurements • “Hook effect” • Macroprolactinemia

“Hook Effect” If there is a very high circulating concentration of prolactin, the excess number of prolactin molecules may consume or saturate added first antibody so that there none of the molecules of prolactin can attach to the fixed or second antibody.

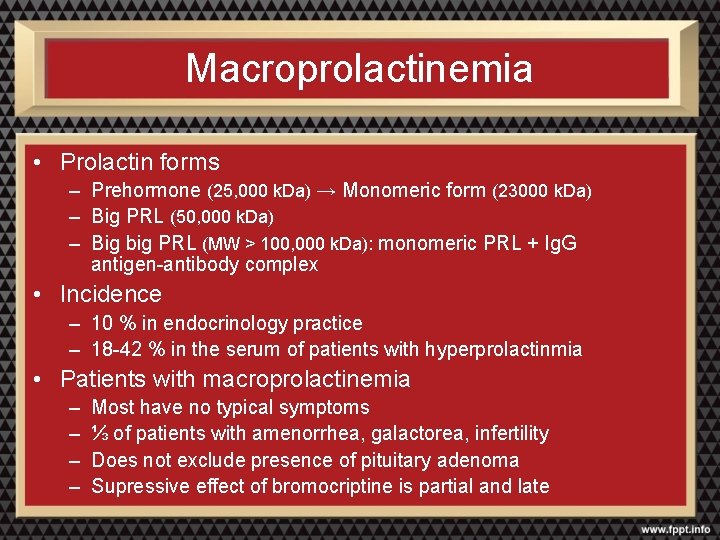
Macroprolactinemia • Prolactin forms – Prehormone (25, 000 k. Da) → Monomeric form (23000 k. Da) – Big PRL (50, 000 k. Da) – Big big PRL (MW > 100, 000 k. Da): monomeric PRL + Ig. G antigen-antibody complex • Incidence – 10 % in endocrinology practice – 18 -42 % in the serum of patients with hyperprolactinmia • Patients with macroprolactinemia – – Most have no typical symptoms ⅓ of patients with amenorrhea, galactorea, infertility Does not exclude presence of pituitary adenoma Supressive effect of bromocriptine is partial and late

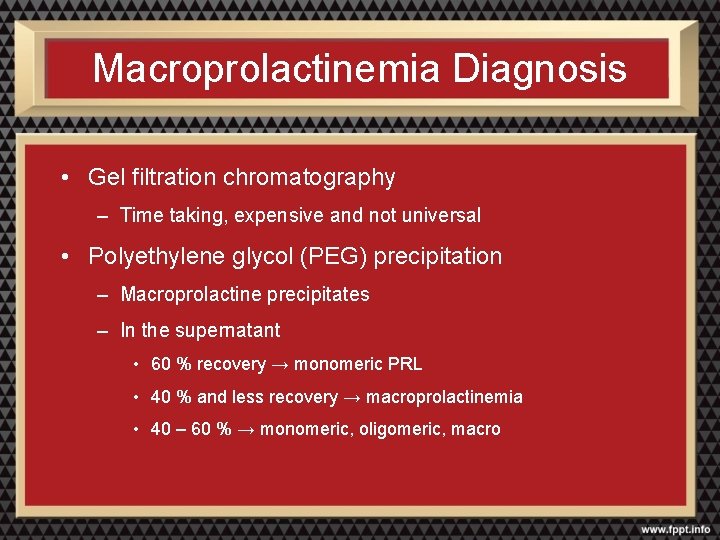
Macroprolactinemia Diagnosis • Gel filtration chromatography – Time taking, expensive and not universal • Polyethylene glycol (PEG) precipitation – Macroprolactine precipitates – In the supernatant • 60 % recovery → monomeric PRL • 40 % and less recovery → macroprolactinemia • 40 – 60 % → monomeric, oligomeric, macro

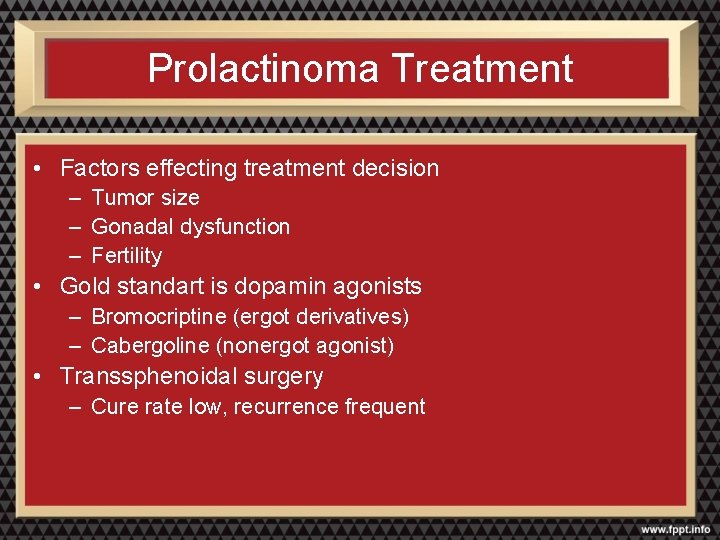
Prolactinoma Treatment • Factors effecting treatment decision – Tumor size – Gonadal dysfunction – Fertility • Gold standart is dopamin agonists – Bromocriptine (ergot derivatives) – Cabergoline (nonergot agonist) • Transsphenoidal surgery – Cure rate low, recurrence frequent

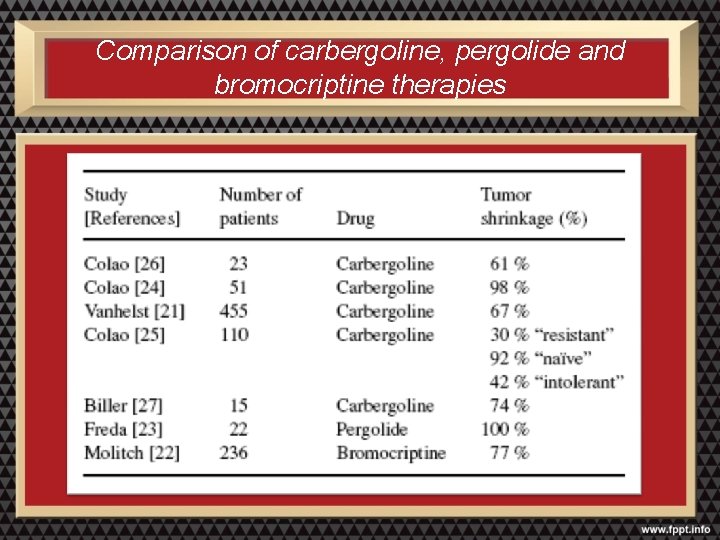
Comparison of carbergoline, pergolide and bromocriptine therapies

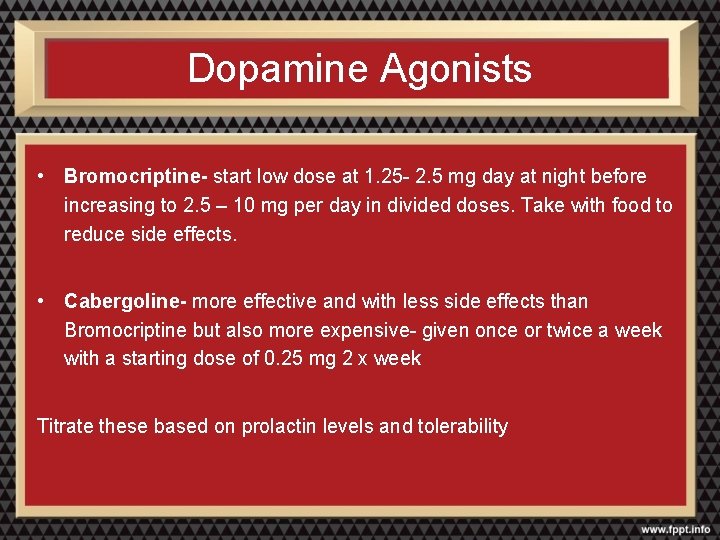
Dopamine Agonists • Bromocriptine- start low dose at 1. 25 - 2. 5 mg day at night before increasing to 2. 5 – 10 mg per day in divided doses. Take with food to reduce side effects. • Cabergoline- more effective and with less side effects than Bromocriptine but also more expensive- given once or twice a week with a starting dose of 0. 25 mg 2 x week Titrate these based on prolactin levels and tolerability

Acromegaly

Acromegaly • Caused by GH excess from the pituitary • Incidence 3 -4/million/year • MEN-1, Mc. Cune Albright Syndrome, Familial, Carney Complex • Diagnosis 6 -10 years after the onset of disease

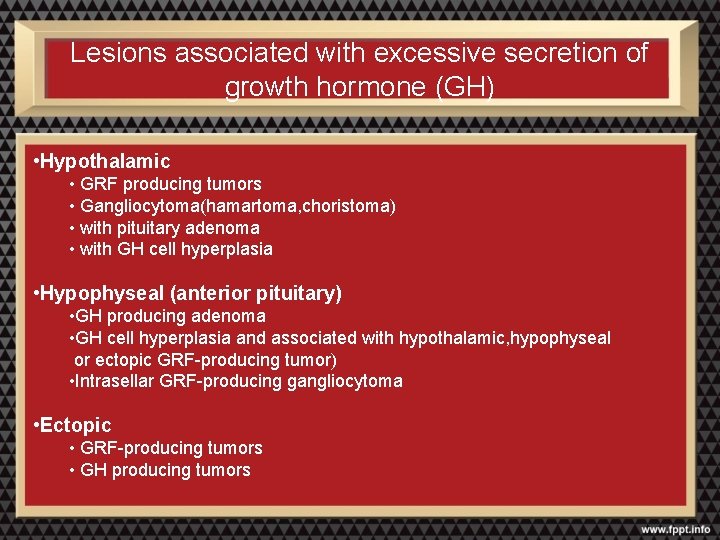
Lesions associated with excessive secretion of growth hormone (GH) • Hypothalamic • GRF producing tumors • Gangliocytoma(hamartoma, choristoma) • with pituitary adenoma • with GH cell hyperplasia • Hypophyseal (anterior pituitary) • GH producing adenoma • GH cell hyperplasia and associated with hypothalamic, hypophyseal or ectopic GRF-producing tumor) • Intrasellar GRF-producing gangliocytoma • Ectopic • GRF-producing tumors • GH producing tumors

Manifestations of Acromegaly • GH excess • Disturbances of other hormones • Parasellar manifestations

GH Excess • Acral growth • Osteoporosis • Prognathism • Soft tissue growth • Weight gain • Hypertrichosis • Hypermetabolism • Pigmentation • Hyperhidrosis • Fibroma mollescum • IGT / DM • Visceromegaly • Arthritic complaints • Goiter

Dısturbances of Other Hormones • Galactorrhea • Hyperadrenocorticism • Hyperthyroidism • Increased libido • Decreased libido, male

Parasellar Manifestations • Enlarged sella • Headache • Visual impairment • Uncinate fits • Rhinorrhea • Pituitary apoplexy • Papilledema

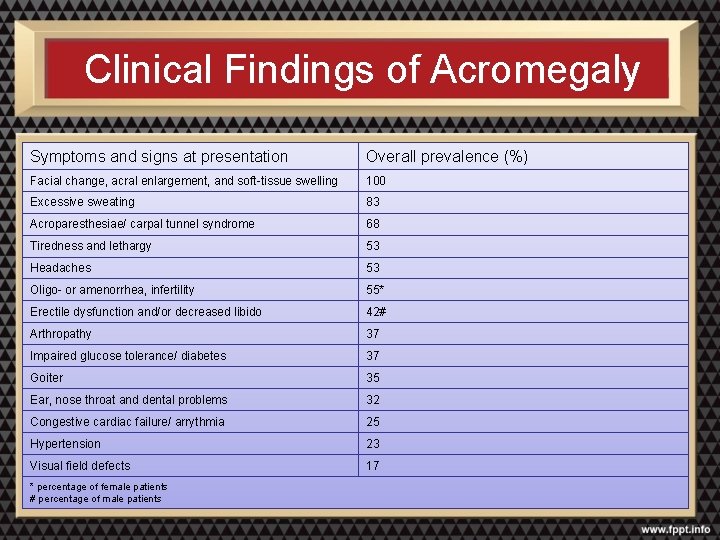
Clinical Findings of Acromegaly Symptoms and signs at presentation Overall prevalence (%) Facial change, acral enlargement, and soft-tissue swelling 100 Excessive sweating 83 Acroparesthesiae/ carpal tunnel syndrome 68 Tiredness and lethargy 53 Headaches 53 Oligo- or amenorrhea, infertility 55* Erectile dysfunction and/or decreased libido 42# Arthropathy 37 Impaired glucose tolerance/ diabetes 37 Goiter 35 Ear, nose throat and dental problems 32 Congestive cardiac failure/ arrythmia 25 Hypertension 23 Visual field defects 17 * percentage of female patients # percentage of male patients


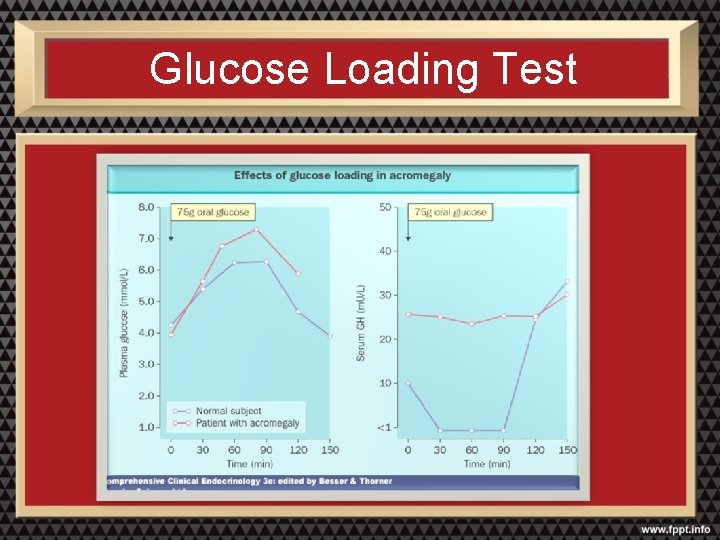
Tests For Acromegaly • Increased resting growth hormone • Elevated IGF-1(somatomedin -C) • GH not below 3 mg/ml after oral glucose load • Skull, hand foot x-rays • CT or MRI scan

Glucose Loading Test

Pituitary MRI

Treatment Options • Surgery • Transsphenoidal adenoma excision • Medical • Somatostatin analogues (Octreotide, lanreotide) • Dopamin agonists ( Bromocriptine, pergolide, cabergoline) • GH reseptor agonists (pegvisomant) • Radioterapy • Lineer accelarator, Gamma-knife, proton beam

Treatment Rates

Acromegaly: Causes of Death • Cardiovascular- 38 to 62 % • Respiratory- 0 to 25 % • Malignancy- 9 to 25 %

Cushing’s Disease

Cushing’s Syndrome vs. Cushing’s Disease • Cushing’s syndrome is a syndrome due to excess cortisol from pituitary, adrenal or other sources (exogenous glucocorticoids, ectopic ACTH, etc. ) • Cushing’s disease is hypercortisolism due to excess pituitary secretion of ACTH (about 70% of cases of endogenous Cushing’s syndrome)

Thyrotropin-Secreting Adenoma

Thyrotropin-secreting Adenoma • <1% of all hyperthyroidism cases • 25% of adenomas secrete other hormones • Goiter, visual defects, menstual irregularities, galactorrhea Lab: • Normal or High TSH • High total and free T 4 and T 3 MRI

Rare • Incidence – 7/1224 pituitary adenoma (%0, 65) – 7/800 adenoma – 2/1000 adenoma • >300 case report up to now

Clinical Presentation • Clinical presentation with goiter or hyperthyroidism mostly • Most of the patients are operated or treated with radioiodine • Recurrence with neurological symptoms lead to diagnosis

Clinical Presentation • Most of the are giant adenomas • Hypopituitarism frequent • GH and PRL excess can complicate

Treatment • Surgery – First line treatment option – Cure rate with transcranial surgery 29 % – Surgery + Radiotherapy cure rate 42 % – TSH normalization rate 75 -100 % – Increased α-subunit demonstrate residual tumor or rekurrens

Treatment • Drug treatment • Bromocriptine – 31 % of 51 pts significant decrease in TSH • Glucocorticoids – Use in providing euthyroidism before surgery – Dexamethasone 4 x 2 -4 mg • Antithyroid drugs – Use in providing euthyroidism before surgery – Propylthiouracil or KI – Avoid long term use

Treatment • Octreotide – Most successful option – Single dose 50 -100 mcg sc injection decrease TSH levels in 20/21 patients – Rapid decrease in tumor size after 3 weeks – TSH normalization rate of 78 % in long term use

Gonadotroph Adenoma

Gonadotroph Adenoma • Usually considered non-functional – Secrete inefficiently, variably • Presents with neurologic symptoms • Difficult to diagnose – Rule out other adenomas – Prepubertal girls=breast development, vaginal bleeding – Premenopausal=amenorrhea, oligomenorrhea

Gonadotroph Adenoma • Clinically nonfunctional adenomas originate – 85 % gonadotroph cells – %10 thyrotroph cells – %5 other cells • Incidence 10 %

Clinical Presentation

Diagnosis • Men and Women < 45 y old: – Increased FSH, LH or α-subunit diagnostic • Women (>45 yrs): – – High FSH with low LH High serum free alpha subunit High estradiol Thickened endometrium and polycystic ovaries

Treatment • Transsphenoidal surgery • Medical treatment • Radiotherapy

Treatment • Surgery – First line treatment – Not totally resectable • Radiotherapy – After inadequate surgery – Adenomas without neurological symptoms or visual disturbance

Treatment • Medical Treatment – Dopamin agonists(Bromocriptine) • Decreases ıntact gonadotropin ve α-subunit levels • No decrease in adenoma size – Somatostatin analogues(Octreotide) • Few case studies • Minimal decrease in tumor size and vision – Gn. RH analogues (Nal-Glu-Gn. RH) • Decrease in gonadotropin levels • No decrease in adenoma size

 Pituitary gland
Pituitary gland Hypothalamus hormones
Hypothalamus hormones Anterior pituitary
Anterior pituitary Eating disorder nursing diagnosis
Eating disorder nursing diagnosis Pituitary gland disorders
Pituitary gland disorders Sinan aydın mali müşavir
Sinan aydın mali müşavir Nizamettin aydin
Nizamettin aydin Nizamettin aydin
Nizamettin aydin Aydın kendirci
Aydın kendirci Thede loder
Thede loder Sevil aydın
Sevil aydın Aydın bir türk kadınıyım
Aydın bir türk kadınıyım Bushra aydin
Bushra aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Coordination
Coordination Nizamettin aydin
Nizamettin aydin Aydin marine
Aydin marine Aydın başar
Aydın başar This generation of computers used transistors
This generation of computers used transistors Nazmi aydın
Nazmi aydın Aydin bal
Aydin bal Aydın kekemelik merkezi
Aydın kekemelik merkezi Thalamus and hypothalamus function
Thalamus and hypothalamus function Difference between anterior and posterior pituitary
Difference between anterior and posterior pituitary Hypophyseal fossa and pituitary gland
Hypophyseal fossa and pituitary gland Diaphragma sellae
Diaphragma sellae Pituitary gland and pineal gland spiritual
Pituitary gland and pineal gland spiritual Hypophyseal fossa and pituitary gland
Hypophyseal fossa and pituitary gland Cl channel
Cl channel Janos lobe
Janos lobe Pituitary hormones and their targets
Pituitary hormones and their targets Pituitary and optic chiasm
Pituitary and optic chiasm Difference between anterior and posterior pituitary
Difference between anterior and posterior pituitary Preoptic area hypothalamus
Preoptic area hypothalamus Autonomic reflexes
Autonomic reflexes Geniculate nucleus of the thalamus
Geniculate nucleus of the thalamus Eugenia martinez vallejo
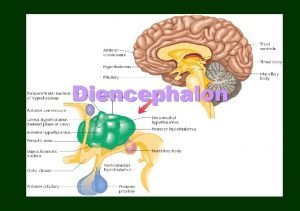
Eugenia martinez vallejo Hypothalamus
Hypothalamus Periventricular nucleus vs paraventricular
Periventricular nucleus vs paraventricular Nontropic hormones
Nontropic hormones Psychology chapter 9 motivation and emotion
Psychology chapter 9 motivation and emotion Tropic hormones hypothalamus
Tropic hormones hypothalamus Mamdouh mahfouz
Mamdouh mahfouz Hypothalamus hormones
Hypothalamus hormones N
N Hypothalamus hormones
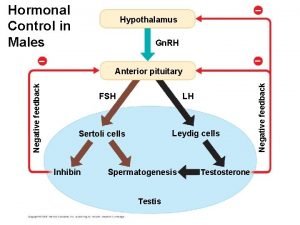
Hypothalamus hormones Lh and fsh in males
Lh and fsh in males Coronal section of hypothalamus
Coronal section of hypothalamus Presanted
Presanted Hypothalamus
Hypothalamus Hypothalamus epinephrine
Hypothalamus epinephrine Hypothalamus
Hypothalamus Hypothalamus
Hypothalamus Endocrine organ
Endocrine organ Hypothalamus
Hypothalamus Hypothalamus
Hypothalamus Epithalamus
Epithalamus Human reproductive system
Human reproductive system Zeng jinlian
Zeng jinlian Nervous sysytem
Nervous sysytem Hypothalamus
Hypothalamus Thermolyse
Thermolyse Melanocortin
Melanocortin Akhtar and hasan
Akhtar and hasan Akhtar & hasan actuaries
Akhtar & hasan actuaries Endocrine organ
Endocrine organ Pituitary gland venous drainage
Pituitary gland venous drainage Pituitary gland division
Pituitary gland division Hypothalamic pituitary portal system
Hypothalamic pituitary portal system Pituitary gland nerve supply
Pituitary gland nerve supply Endocrine weight loss
Endocrine weight loss Pituitary
Pituitary Hypophysis
Hypophysis Hypersecretion of prolactin
Hypersecretion of prolactin Health concerns
Health concerns