ADRENAL DISORDERS Hasan AYDIN MD Yeditepe University Medical























- Slides: 55

ADRENAL DISORDERS Hasan AYDIN, MD Yeditepe University Medical Faculty Department of Endocrinology and Metabolism

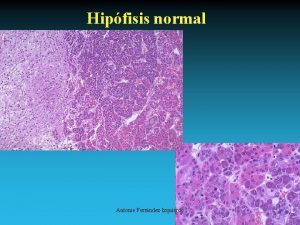
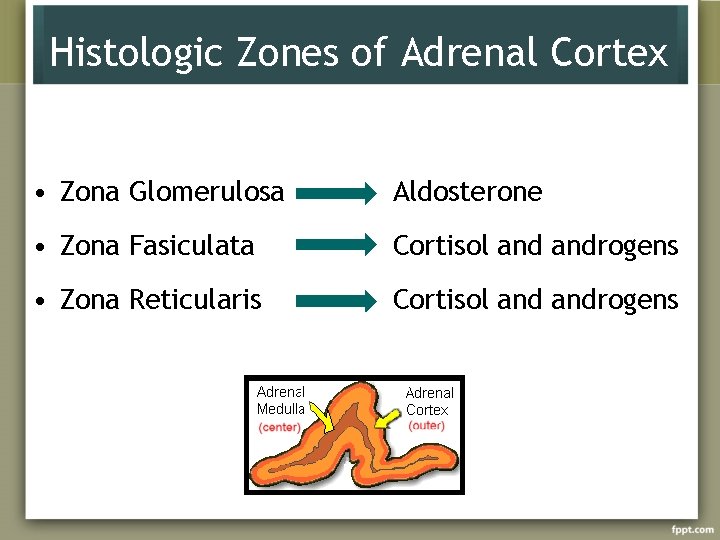
Histologic Zones of Adrenal Cortex • Zona Glomerulosa Aldosterone • Zona Fasiculata Cortisol androgens • Zona Reticularis Cortisol androgens

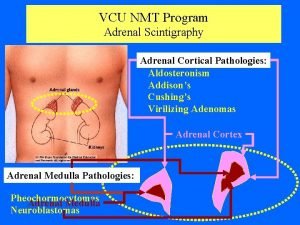
Diseases of Adrenal Gland • Diseses of Adrenal Cortex – Cushing syndrome – Adrenal failure – Congenital adrenal hyperplasia • Diseases of Adrenal Medulla – Pheochromocytoma – Hyperaldosteronism (Conn’s syndrome)

Cushing’s Syndrome

Cushing’s Sydrome vs. Disease • Chronic glucocorticoid excess, whatever it’s cause • Cushing’s diasease: a spesific type of Cushing’s sydrome due to excessive pituitary ACTH secretion from a pituitary tumor.

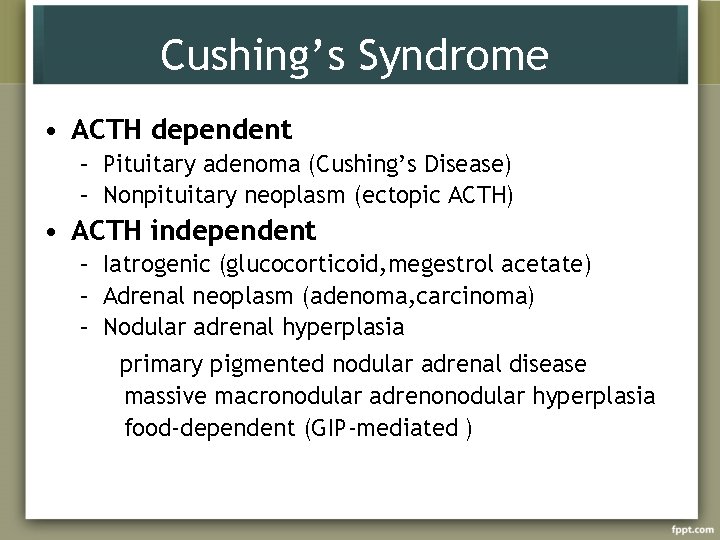
Cushing’s Syndrome • ACTH dependent – Pituitary adenoma (Cushing’s Disease) – Nonpituitary neoplasm (ectopic ACTH) • ACTH independent – Iatrogenic (glucocorticoid, megestrol acetate) – Adrenal neoplasm (adenoma, carcinoma) – Nodular adrenal hyperplasia primary pigmented nodular adrenal disease massive macronodular adrenonodular hyperplasia food-dependent (GIP-mediated )

Cushing’s Disease • 70 % of cases of Cushing’s sydrome • Female/male 8: 1 • Age at diagnosis : childhood – 70 years Etiology: Corticotroph-cell pituitary adenomas Corticotroph cell hyperplasia Tumors are typically smaller than 10 mm

Ectopic ACTH Hypersecretion • 15 -20 % of patients with ACTH dependent Cushing’s Sydrome • Tumors causing the ectopic ACTH syndrome – Small cell carcinoma of the lung (50% of cases) – Pancreatic islet tumors – Carcinoid tumors (lung, thymus, gut, pancreas, ovary) – Medullary carcinoma of the thyroid – Pheochromocytoma

Adrenal Cushing’s Syndrome – Adrenal neoplasm (adenoma, carcinoma) – Nodular adrenal hyperplasia – Primary pigmented nodular adrenal disease – Massive macronodular adrenonodular hyperplasia – Food-dependent (GIP-mediated )

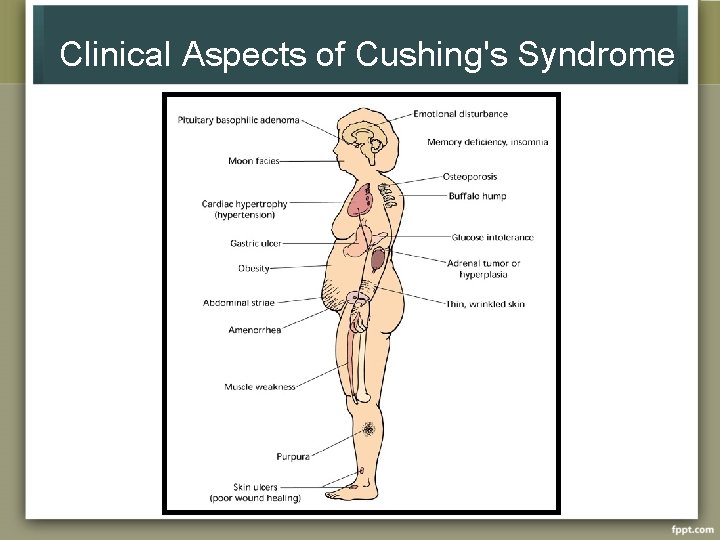
Clinical Aspects of Cushing's Syndrome

Diagnosis • Urine free cortisol in 24 hour urine samples – 80 -100 mg/24 h is normal • Used in screening

Diagnosis • Absence of diurnal rhythm is the hallmark of diagnosis Serum cortisol levels exceeding 7 mg/d. L at midnight indicates absence of diurnal rhythm

Diagnosis • Dexametasone supression test Low dose –for screening 1 mg dexametasone at bedtime (23 00 hour) Determine plasma cortisol early following morning Plasma cortisol < 1. 8 mcg/dl - normal Dexamethasone 4 x 0. 5 mg for two days 17 hydroxycorticosteroid excretion greater than 4 mg/24 h on the second day of dx administration= Cushing syndrome

Differential Diagnosis • Plasma ACTH: Differentiate ACTH dependent and non dependent forms Patients with ACTH –secreting neoplasms usually have plasma ACTH levels > 10 pg/ml frequently greater than 52 pg/ml. • Pituitary MRI ACTH-dependent patients adenoma on MRI likehood of cushing disease 98 -99% Incidentaloma 10%

Inferior Petrosal Sinus Sampling • Distinguishing pituitary from non pituitary ACTH dependent Cushing’s syndrome • Simultaneous inferior petrosal sinus and peripheral ACTH measurements before and after CRH stimulation • IPS/P > 2 pituitary ACTH secreting tumor • IPS/P < 1. 8 ectopic ACTH • Diagnostic accuracy 100% in the differential diagnosis of ACTH dependent Cushing’s syndrome

Treatment Remove or destroy the basic lesion Correct the hypersecretion of adrenal hormones • microsurgery • radiation therapy • pharmocologic inhibition of ACTH secretion ketoconasole, metyrapone, amimoglutethimide, mitotane

Adrenocortical Insufficiency (Addison’s Disease)

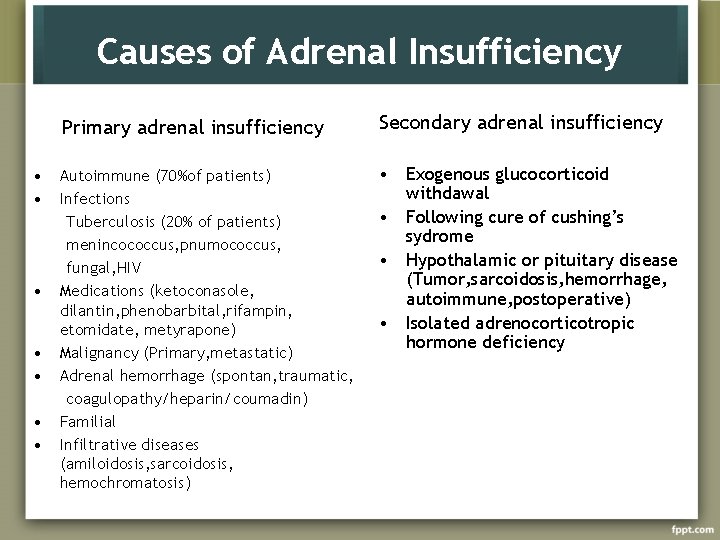
Causes of Adrenal Insufficiency • • Primary adrenal insufficiency Secondary adrenal insufficiency Autoimmune (70%of patients) Infections Tuberculosis (20% of patients) menincococcus, pnumococcus, fungal, HIV Medications (ketoconasole, dilantin, phenobarbital, rifampin, etomidate, metyrapone) Malignancy (Primary, metastatic) Adrenal hemorrhage (spontan, traumatic, coagulopathy/heparin/coumadin) Familial Infiltrative diseases (amiloidosis, sarcoidosis, hemochromatosis) • Exogenous glucocorticoid withdawal • Following cure of cushing’s sydrome • Hypothalamic or pituitary disease (Tumor, sarcoidosis, hemorrhage, autoimmune, postoperative) • Isolated adrenocorticotropic hormone deficiency

Presentation Highly variable Duration of disease Whether deficiency is primary or secondary

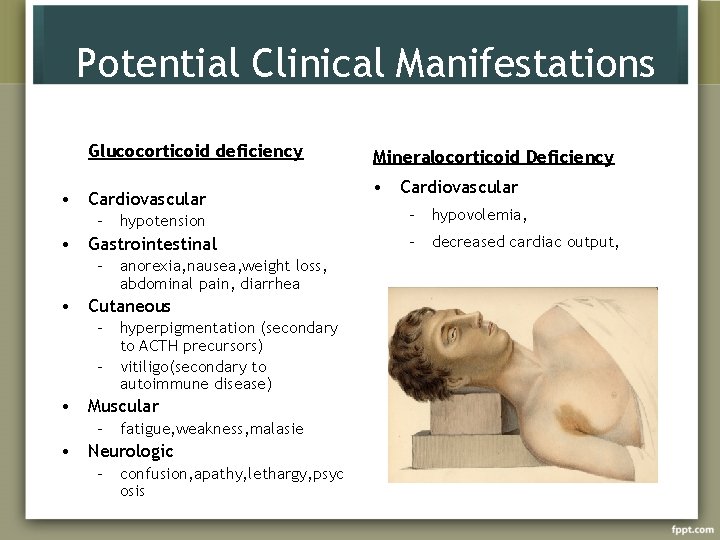
Potential Clinical Manifestations Glucocorticoid deficiency • Cardiovascular – hypotension • Gastrointestinal – anorexia, nausea, weight loss, abdominal pain, diarrhea • Cutaneous – hyperpigmentation (secondary to ACTH precursors) – vitiligo(secondary to autoimmune disease) • Muscular – fatigue, weakness, malasie • Neurologic – confusion, apathy, lethargy, psyc osis Mineralocorticoid Deficiency • Cardiovascular – hypovolemia, – decreased cardiac output,

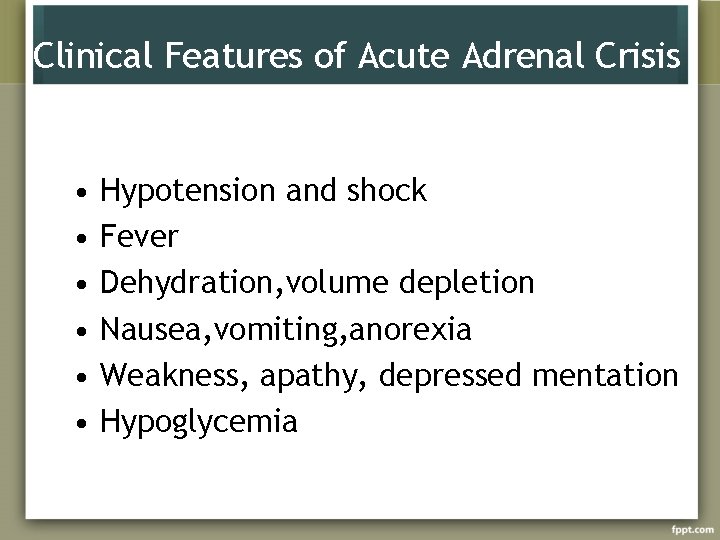
Acute Adrenal Crisis occurs in patients with Addison’s disease who are exposed to the stress of infection, trauma, surgery or dehydration

Clinical Features of Acute Adrenal Crisis • • • Hypotension and shock Fever Dehydration, volume depletion Nausea, vomiting, anorexia Weakness, apathy, depressed mentation Hypoglycemia

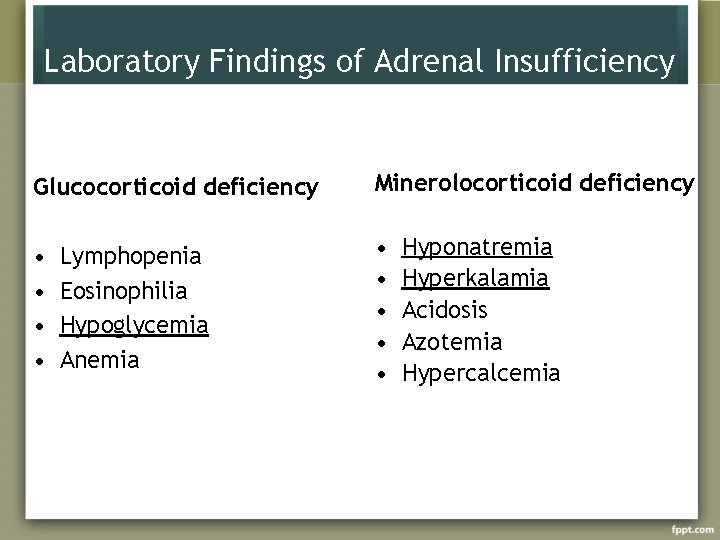
Laboratory Findings of Adrenal Insufficiency Glucocorticoid deficiency Minerolocorticoid deficiency • • • Lymphopenia Eosinophilia Hypoglycemia Anemia Hyponatremia Hyperkalamia Acidosis Azotemia Hypercalcemia

Diagnosis of Adrenocortical Insufficiency Since basal levels of adrenocortical steroids in either urine or plasma may be normal in partial adrenal insufficiency, tests of adrenal cortical reserve are necesseary to establish the diagnosis Cortisol > 20 mg/day at any time of day - diagnosis very unlikely Hemodynamic instability - cortisol < 20 mg/day - suspicious

ACTH Stimulation Test Performed at any time of day A baseline cortisol sample is obtained and 250 mg synthetic ACTH (cosyntropin) is then administered intravenously. Cortisol samples are drawn 30 and 60 min later. Plasma cortisol >18 mcg/dl excludes the diagnosis

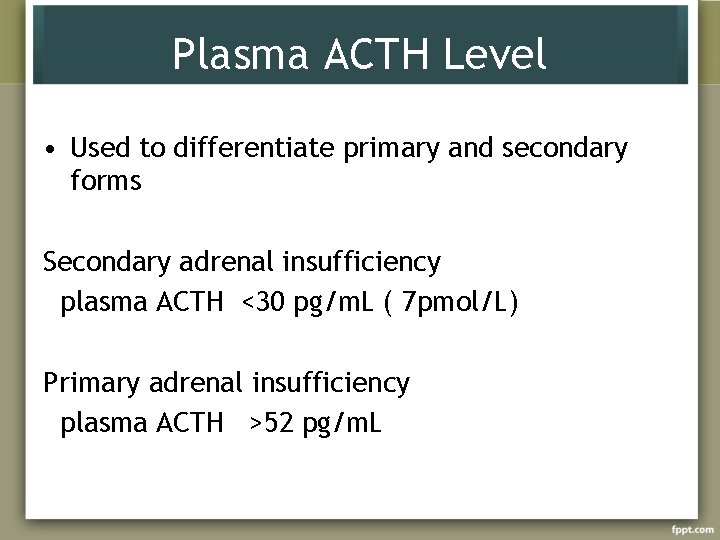
Plasma ACTH Level • Used to differentiate primary and secondary forms Secondary adrenal insufficiency plasma ACTH <30 pg/m. L ( 7 pmol/L) Primary adrenal insufficiency plasma ACTH >52 pg/m. L

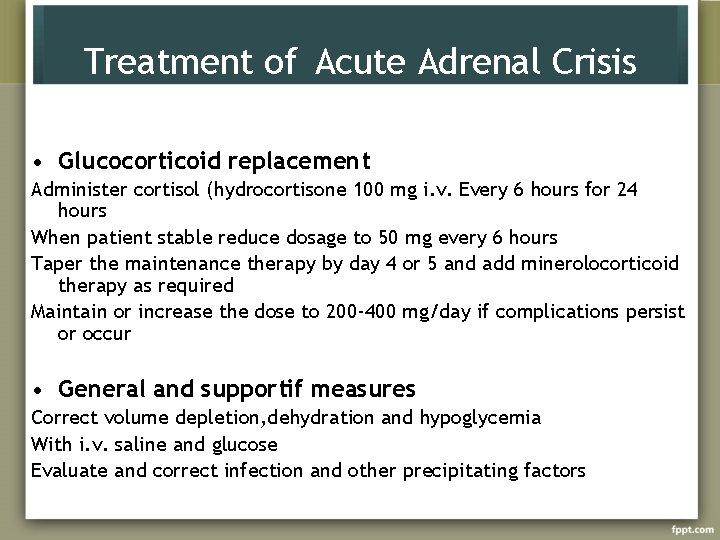
Treatment of Acute Adrenal Crisis • Glucocorticoid replacement Administer cortisol (hydrocortisone 100 mg i. v. Every 6 hours for 24 hours When patient stable reduce dosage to 50 mg every 6 hours Taper the maintenance therapy by day 4 or 5 and add minerolocorticoid therapy as required Maintain or increase the dose to 200 -400 mg/day if complications persist or occur • General and supportif measures Correct volume depletion, dehydration and hypoglycemia With i. v. saline and glucose Evaluate and correct infection and other precipitating factors

Maintenance Therapy • Cortisol 15 -20 mg in AM. and 10 mg at 4 -5 PM • Fludrocortisone, 0. 05 -0. 1 mg orally in AM • Clinical follow-up : maintenance of normal weight, blood pressure, and electrolytes • Patient education, identification card or bracelet • Increase cortisol dosage during stress

Congenital Adrenal Hyperplasia

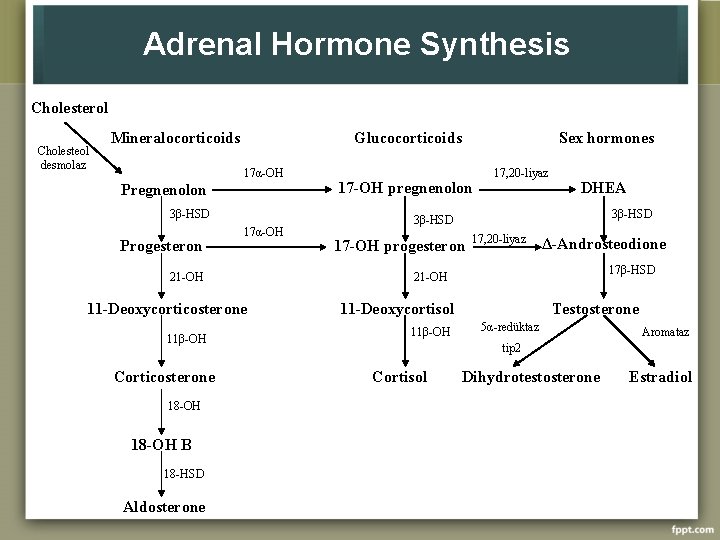
Adrenal Hormone Synthesis Cholesterol Cholesteol desmolaz Mineralocorticoids Glucocorticoids 17α-OH Pregnenolon 3β-HSD Progesteron 17α-OH 21 -OH 11 -Deoxycorticosterone 11β-OH Corticosterone 18 -OH B 18 -HSD Aldosterone Sex hormones 17 -OH pregnenolon 17, 20 -liyaz DHEA 3β-HSD 17 -OH progesteron 17, 20 -liyaz Δ-Androsteodione 17β-HSD 21 -OH 11 -Deoxycortisol 11β-OH Testosterone 5α-redüktaz Aromataz tip 2 Cortisol Dihydrotestosterone Estradiol

State 11 beta-hydroxylase 17 alpha-hydroxylase 3 beta-hydroxysteroid dehydrogenase Aldosterone synthase St. AR Incidence Clinical Picture ~1 in 100, 000 livebirth Virilization in women; salt wasting rare Virilization in men; no puberty in women. No salt wasting rare Virilization in men; mild virilization in women. Salt wasting possible rare Cortisol concentration normal; no virilisation. Salt wasting rare Virilization in men; no puberty in women. salt wasting

Treatment • Corticosteroid replacement • Mineralocorticoid replacement

Hyperaldosteronism (Conn’s Syndrome)

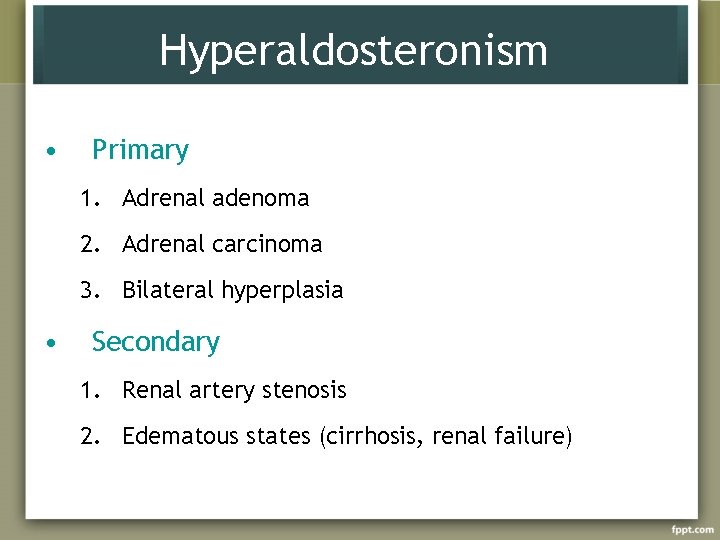
Hyperaldosteronism • Primary 1. Adrenal adenoma 2. Adrenal carcinoma 3. Bilateral hyperplasia • Secondary 1. Renal artery stenosis 2. Edematous states (cirrhosis, renal failure)

Hyperaldosteronism • ↑ BP + K+ ↓ or low normal K+ • ↑ BP in young person • ↑ BP difficult to control

Signs and Labs • • • Hypertension Hypokalemia Urine potassium wasting Increased aldosterone Low renin

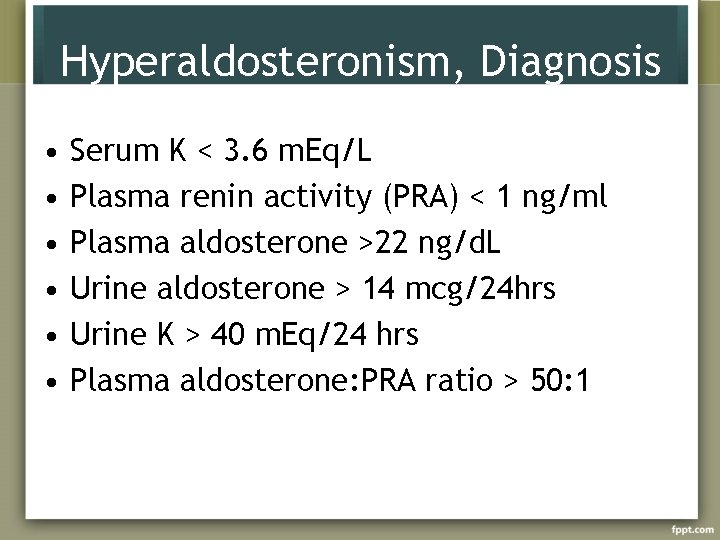
Hyperaldosteronism, Diagnosis • • • Serum K < 3. 6 m. Eq/L Plasma renin activity (PRA) < 1 ng/ml Plasma aldosterone >22 ng/d. L Urine aldosterone > 14 mcg/24 hrs Urine K > 40 m. Eq/24 hrs Plasma aldosterone: PRA ratio > 50: 1

Hyperaldosteronism, Diagnosis • CT scan • Adrenal vein sampling

Hyperaldosteronism, Treatment • Bilateral hyperplasia- medical, spironolactone, amiloride. • Unilateral adenoma- adrenalectomy.

Pheochromocytoma

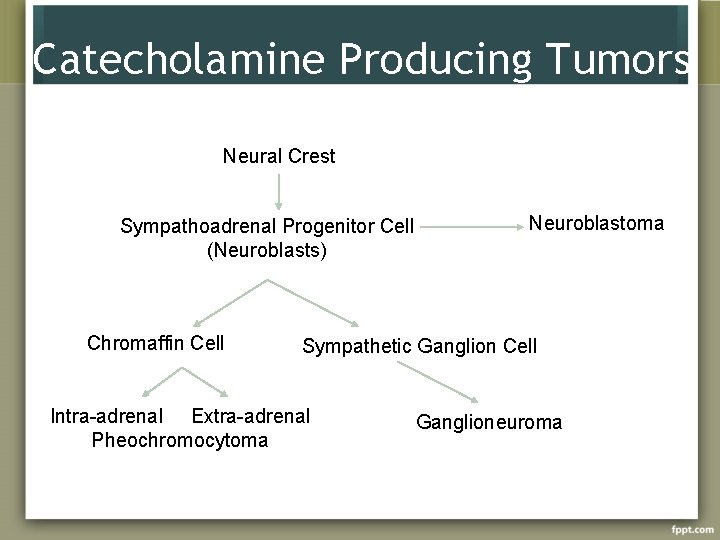
Catecholamine Producing Tumors Neural Crest Sympathoadrenal Progenitor Cell (Neuroblasts) Chromaffin Cell Neuroblastoma Sympathetic Ganglion Cell Intra-adrenal Extra-adrenal Pheochromocytoma Ganglioneuroma

Pheochromocytoma • 0. 01 -0. 1% of HTN population • Found in 0. 5% of those screened • M=F • 3 rd to 5 th decades of life • Rare, investigate only if clinically suspicion: • • • Signs or Symptoms Severe HTN, HTN crisis Refractory HTN (> 3 drugs) HTN present @ age < 20 or > 50 ? Adrenal lesion found on imaging (ex. Incidentaloma)

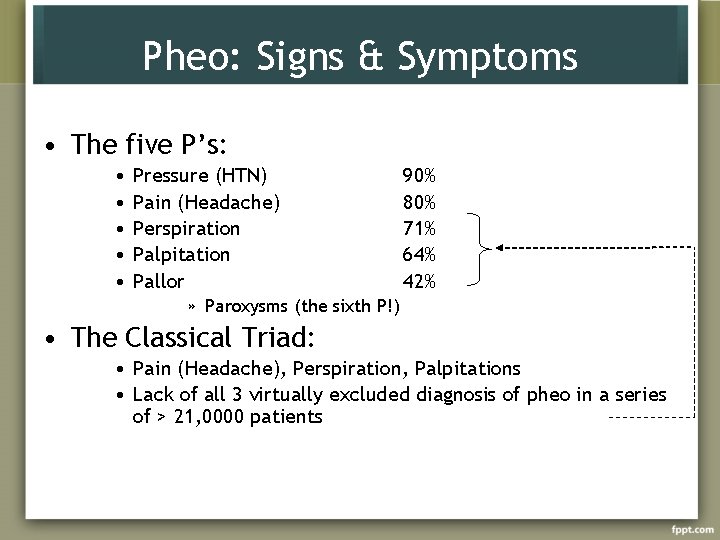
Pheo: Signs & Symptoms • The five P’s: • • • Pressure (HTN) Pain (Headache) Perspiration Palpitation Pallor 90% 80% 71% 64% 42% » Paroxysms (the sixth P!) • The Classical Triad: • Pain (Headache), Perspiration, Palpitations • Lack of all 3 virtually excluded diagnosis of pheo in a series of > 21, 0000 patients

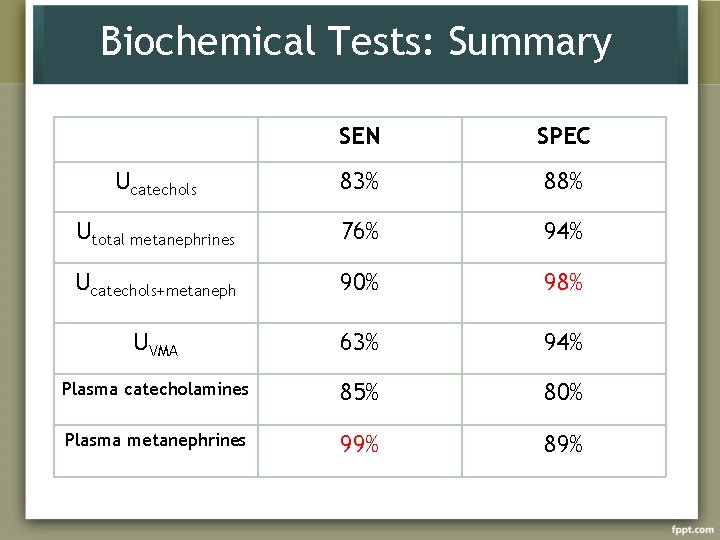
Biochemical Tests: Summary SEN SPEC Ucatechols 83% 88% Utotal metanephrines 76% 94% Ucatechols+metaneph 90% 98% UVMA 63% 94% Plasma catecholamines 85% 80% Plasma metanephrines 99% 89%

Localization: Imaging • CT abdomen • Adrenal pheo SEN 93 -100% • Extra-adrenal pheo SEN 90% • MRI • > SEN than CT for extra-adrenal pheo • MIBG Scan • SEN 77 -90% SPEC 95 -100%

Pheochromocytoma, Treatment • Treatment is surgery • Must medically optimize prior to surgery – Treat HTN – Expand intravascular volume – Control cardiac arrhythmias

Preop Preperation Regimens • Combined α + β blockade • Phenoxybenzamine • Selective α 1 -blocker (ex. Prazosin) • Propanolol • Metyrosine • Calcium Channel Blocker (CCB) • Nicardipine

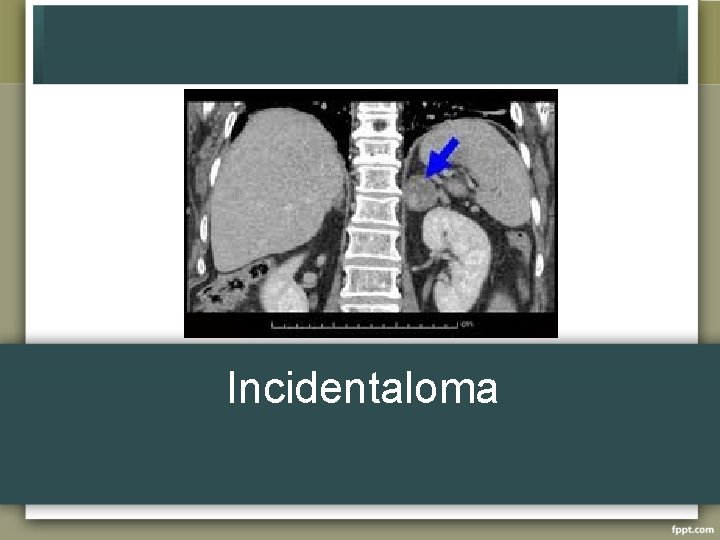
Incidentaloma

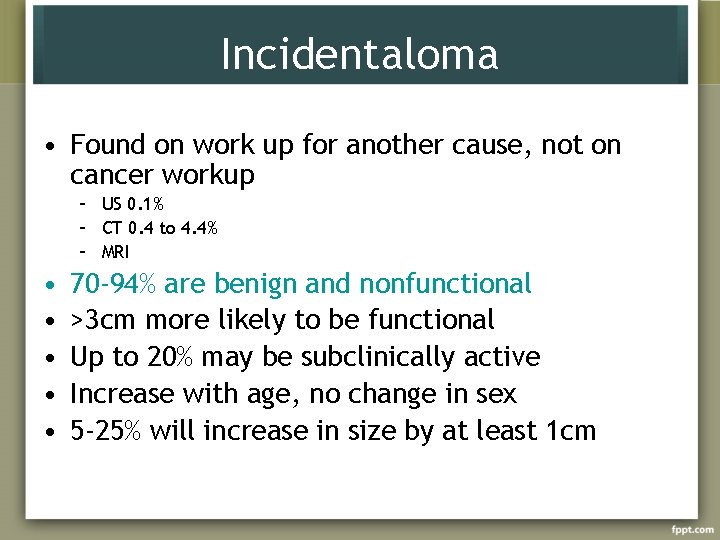
Incidentaloma • Found on work up for another cause, not on cancer workup – US 0. 1% – CT 0. 4 to 4. 4% – MRI • • • 70 -94% are benign and nonfunctional >3 cm more likely to be functional Up to 20% may be subclinically active Increase with age, no change in sex 5 -25% will increase in size by at least 1 cm


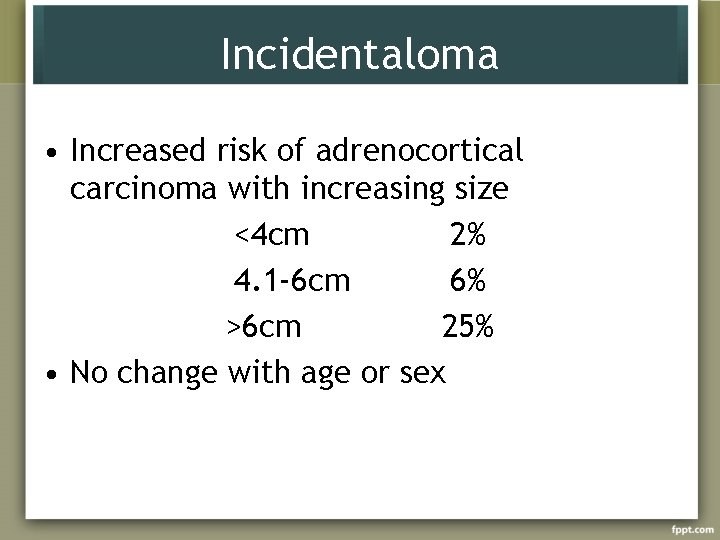
Incidentaloma • Increased risk of adrenocortical carcinoma with increasing size <4 cm 2% 4. 1 -6 cm 6% >6 cm 25% • No change with age or sex

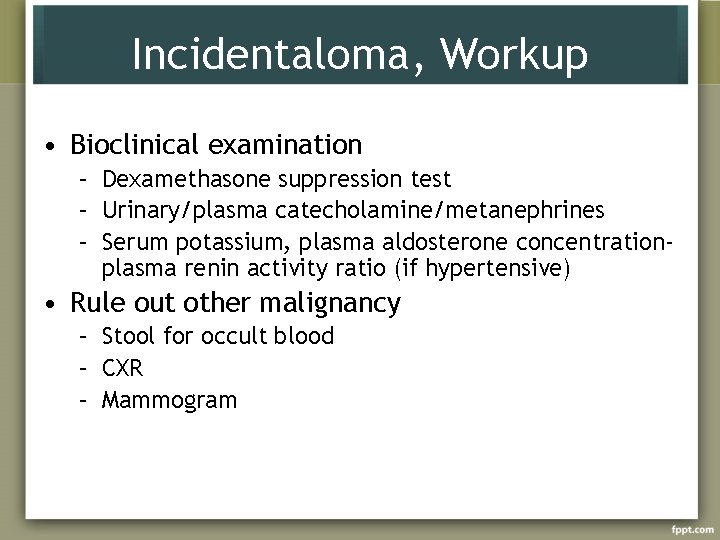
Incidentaloma, Workup • Bioclinical examination – Dexamethasone suppression test – Urinary/plasma catecholamine/metanephrines – Serum potassium, plasma aldosterone concentrationplasma renin activity ratio (if hypertensive) • Rule out other malignancy – Stool for occult blood – CXR – Mammogram

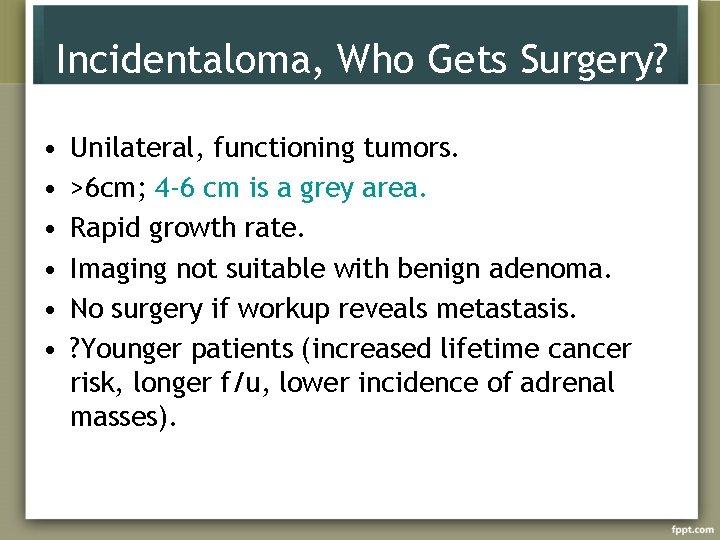
Incidentaloma, Who Gets Surgery? • • • Unilateral, functioning tumors. >6 cm; 4 -6 cm is a grey area. Rapid growth rate. Imaging not suitable with benign adenoma. No surgery if workup reveals metastasis. ? Younger patients (increased lifetime cancer risk, longer f/u, lower incidence of adrenal masses).

Incidentaloma, Watchful Waiting • <4 cm, nonfunctioning tumors. • CT in 3 and 12 months. If no increase in size, no data to support further imaging. • ? Periodic hormonal testing. If a tumor will start to hyperfunction, this will most likely happen in 3 -4 yrs.

 Yeditepe edu
Yeditepe edu Coadsys exam yeditepe
Coadsys exam yeditepe Yeditepe coadsys exam
Yeditepe coadsys exam Sinan aydın ymm
Sinan aydın ymm Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Nizamettin aydin
Nizamettin aydin Aydın kendirci
Aydın kendirci Unsent message to aydin
Unsent message to aydin Sevil aydın
Sevil aydın Aydın bir türk kadınıyım
Aydın bir türk kadınıyım Bushra aydin
Bushra aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Coordination
Coordination Package diagram
Package diagram Aydin marine
Aydin marine Aydın başar
Aydın başar Giga tera peta
Giga tera peta Nazmi aydın
Nazmi aydın Aydin bal
Aydin bal Aydın kekemelik merkezi
Aydın kekemelik merkezi Craniosacral region
Craniosacral region Adrenal bez histolojisi
Adrenal bez histolojisi Hipotálamo-hipófise-adrenal
Hipotálamo-hipófise-adrenal Dr dawn lim
Dr dawn lim Estadiamento de tanner
Estadiamento de tanner Konjenital lipoid adrenal hiperplazi
Konjenital lipoid adrenal hiperplazi Suprarenal gland relations
Suprarenal gland relations Raa pathway
Raa pathway What causes addisons disease
What causes addisons disease Adrenal kriz acilci
Adrenal kriz acilci Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Dr wilson adrenal rebuilder side effects
Dr wilson adrenal rebuilder side effects Summary of adrenal gland
Summary of adrenal gland Site:slidetodoc.com
Site:slidetodoc.com Cow adrenal gland
Cow adrenal gland 17 hydroxyprogesterone level
17 hydroxyprogesterone level Virilisation in females
Virilisation in females Adrenal cortex develops from
Adrenal cortex develops from Acth
Acth Adrenal medulla cortex
Adrenal medulla cortex Psödo cushing sendromu
Psödo cushing sendromu Adrenal cushing
Adrenal cushing Hymen
Hymen Adrenal sympathetic pathway
Adrenal sympathetic pathway Adrenal tumour
Adrenal tumour Congenital adrenal hyperplasia
Congenital adrenal hyperplasia Adrenal gland regions
Adrenal gland regions Thyroid follicle histology
Thyroid follicle histology Adrenal sympathetic pathway
Adrenal sympathetic pathway Cat heart
Cat heart Betoderm
Betoderm Adrenal glands
Adrenal glands Adrenal gland regions
Adrenal gland regions Conn's vs addison's
Conn's vs addison's