Diaparities in Enrollment into Clinical Trials Roxana Mehran
















- Slides: 42

Diaparities in Enrollment into Clinical Trials Roxana Mehran, MD Professor of Medicine, Health Evidence and Policy The Icahn School of Medicine at Mount Sinai

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. These relationships may lead to bias in my presentation. Affiliation/Financial Relationship Company • Grant/Research Support • The Medicines Co. , BMS, • Advisory Board • Janssen (J+J)- Executive (Institutional) • Consulting Fees/Honoraria Astra Zeneca, Lilly/Daiichi Sankyo; Orbus Neich Committee- PIONEER AF • CSL Behring, Janssen (J+J), Osprey Medical

“The lack of female representation in both preclinical studies and clinical trials has put women at greater risk for adverse ” events from medical interventions “The goal now is to ensure that every patient receives the correct treatment and dose at the right time, with minimum adverse side effects. Women may have been neglected by medical research for a couple of decades but the march of technology is now bound to take them forward as individuals” Washington Post, 11 February 2015

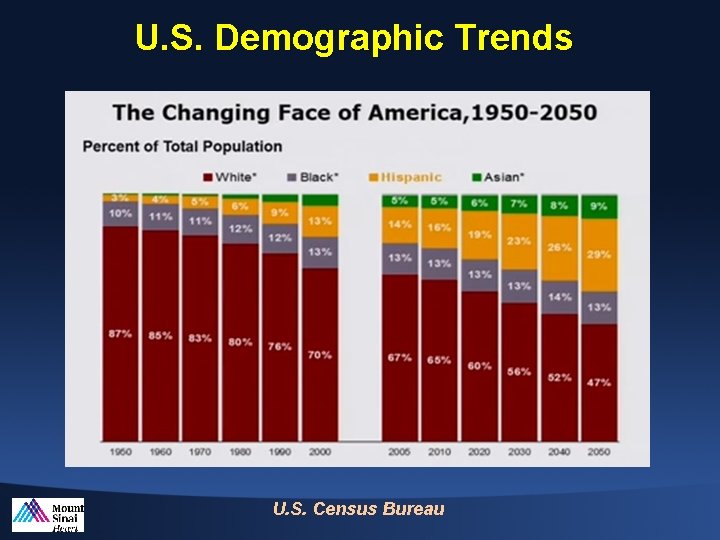
U. S. Demographic Trends U. S. Census Bureau

Changing Face of America

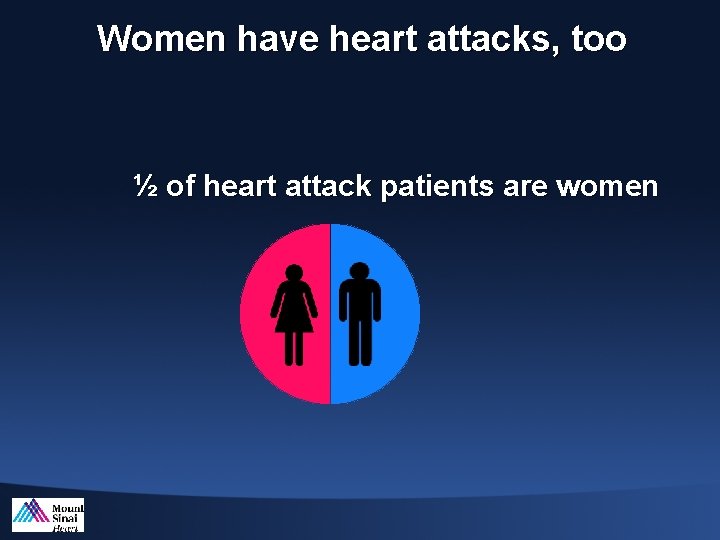
Women have heart attacks, too ½ of heart attack patients are women

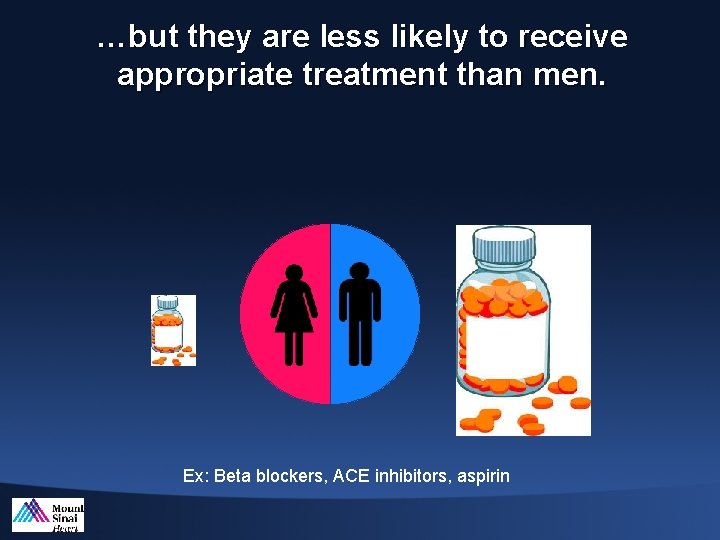
…but they are less likely to receive appropriate treatment than men. Ex: Beta blockers, ACE inhibitors, aspirin

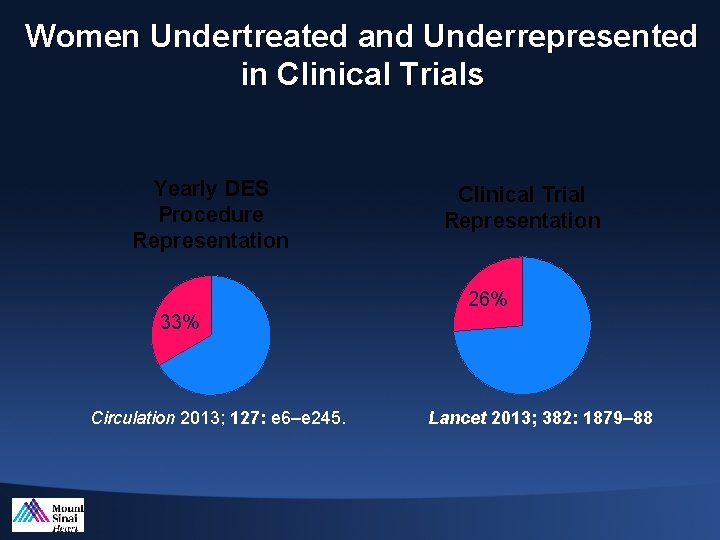
Women Undertreated and Underrepresented in Clinical Trials Yearly DES Procedure Representation 33% Circulation 2013; 127: e 6–e 245. Clinical Trial Representation 26% Lancet 2013; 382: 1879– 88

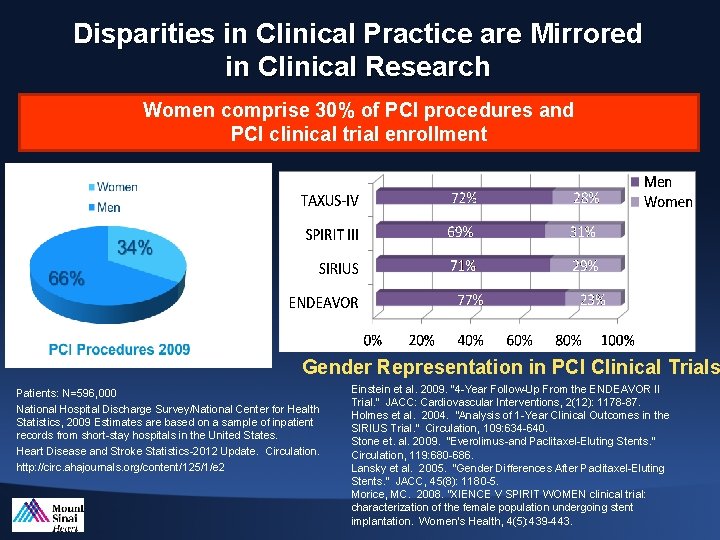
Disparities in Clinical Practice are Mirrored in Clinical Research Women comprise 30% of PCI procedures and PCI clinical trial enrollment Gender Representation in PCI Clinical Trials Patients: N=596, 000 National Hospital Discharge Survey/National Center for Health Statistics, 2009 Estimates are based on a sample of inpatient records from short-stay hospitals in the United States. Heart Disease and Stroke Statistics-2012 Update. Circulation. http: //circ. ahajournals. org/content/125/1/e 2 Einstein et al. 2009. “ 4 -Year Follow-Up From the ENDEAVOR II Trial. ” JACC: Cardiovascular Interventions, 2(12): 1178 -87. Holmes et al. 2004. “Analysis of 1 -Year Clinical Outcomes in the SIRIUS Trial. ” Circulation, 109: 634 -640. Stone et. al. 2009. “Everolimus-and Paclitaxel-Eluting Stents. ” Circulation, 119: 680 -686. Lansky et al. 2005. “Gender Differences After Paclitaxel-Eluting Stents. ” JACC, 45(8): 1180 -5. Morice, MC. 2008. “XIENCE V SPIRIT WOMEN clinical trial: characterization of the female population undergoing stent implantation. Women’s Health, 4(5): 439 -443.

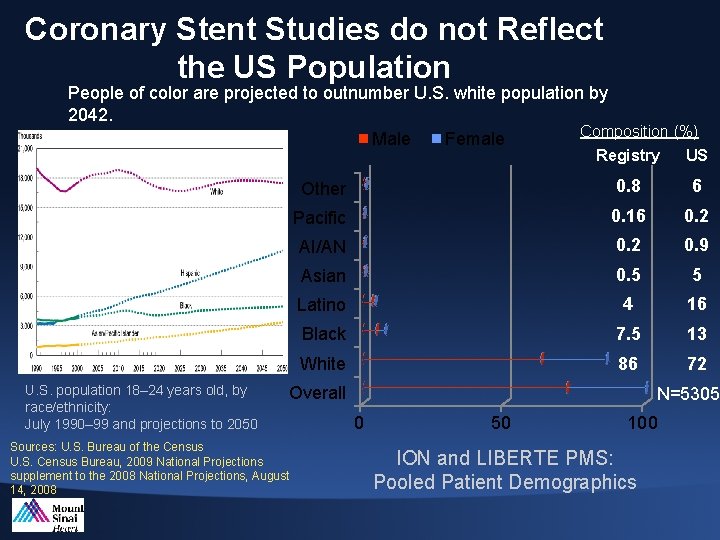
Coronary Stent Studies do not Reflect the US Population People of color are projected to outnumber U. S. white population by 2042. Composition (%) Male Female U. S. population 18– 24 years old, by race/ethnicity: July 1990– 99 and projections to 2050 Registry US Other 0. 8 6 Pacific 0. 16 0. 2 AI/AN 0. 2 0. 9 Asian 0. 5 5 Latino 4 16 Black 7. 5 13 White 86 72 Overall Sources: U. S. Bureau of the Census U. S. Census Bureau, 2009 National Projections supplement to the 2008 National Projections, August 14, 2008 N=5305 0 50 100 ION and LIBERTE PMS: Pooled Patient Demographics

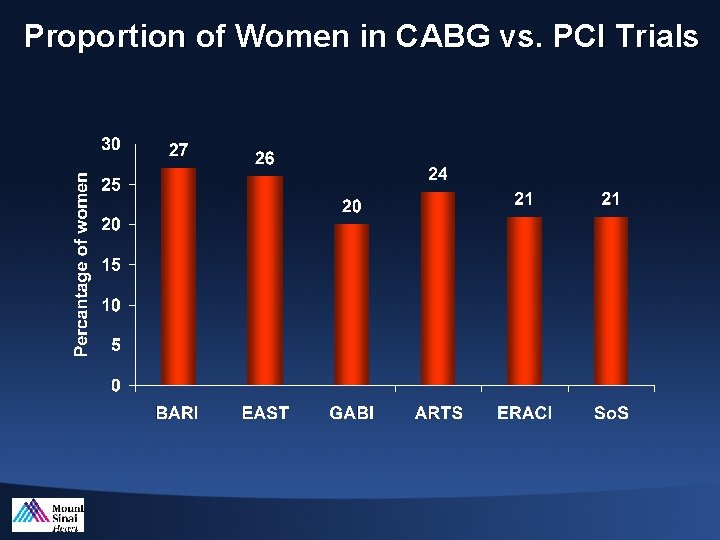
Proportion of Women in CABG vs. PCI Trials

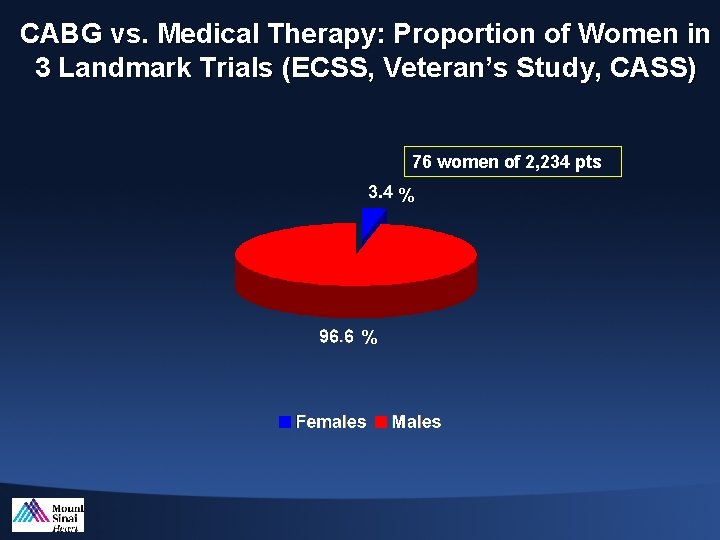
CABG vs. Medical Therapy: Proportion of Women in 3 Landmark Trials (ECSS, Veteran’s Study, CASS) 76 women of 2, 234 pts % %

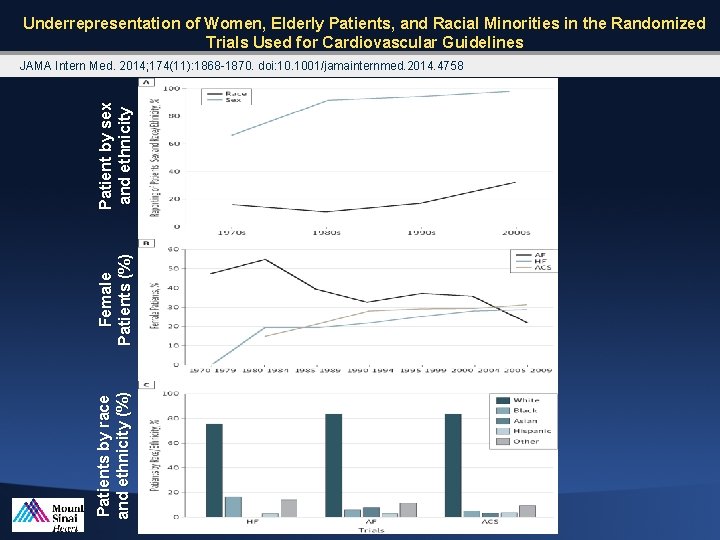
Underrepresentation of Women, Elderly Patients, and Racial Minorities in the Randomized Trials Used for Cardiovascular Guidelines Patients by race and ethnicity (%) Female Patients (%) Patient by sex and ethnicity JAMA Intern Med. 2014; 174(11): 1868 -1870. doi: 10. 1001/jamainternmed. 2014. 4758

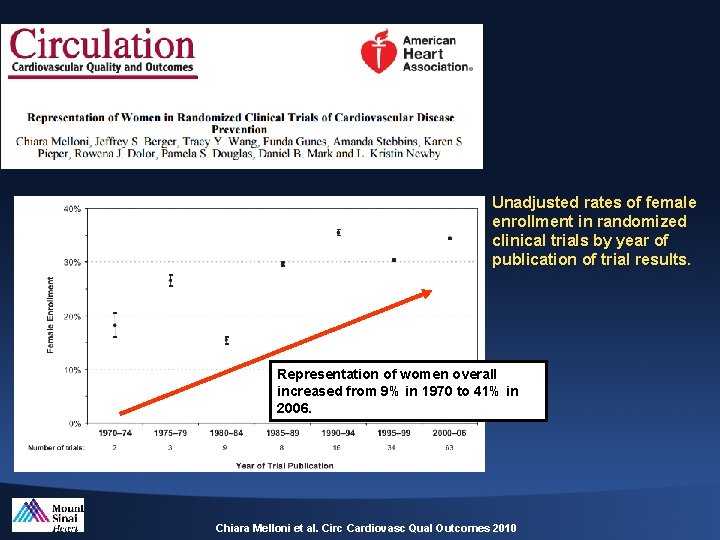
Unadjusted rates of female enrollment in randomized clinical trials by year of publication of trial results. Representation of women overall increased from 9% in 1970 to 41% in 2006. Chiara Melloni et al. Circ Cardiovasc Qual Outcomes 2010

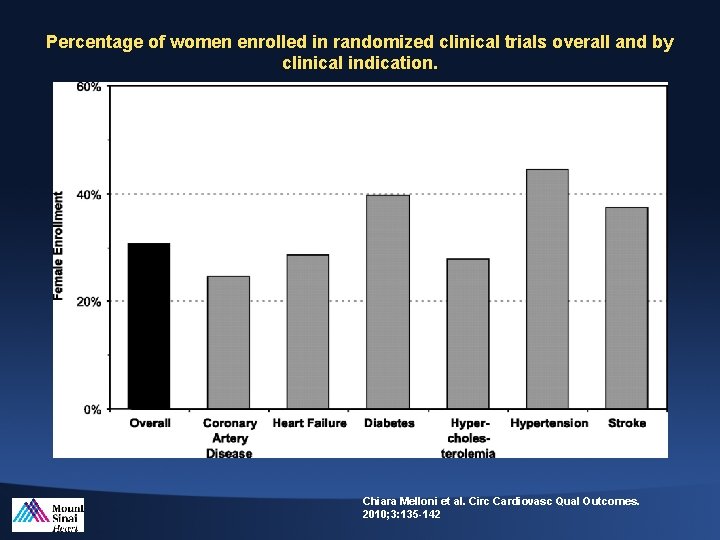
Percentage of women enrolled in randomized clinical trials overall and by clinical indication. Chiara Melloni et al. Circ Cardiovasc Qual Outcomes. 2010; 3: 135 -142

Gender-based Inconsistencies • Despite improvements in therapies overall in the past several years: ¡ the sex gap persists ¡ sex-associated risk factors contribute to excess risk in women including: • lower BMI • lower creatinine clearance • smaller vessel size • sex-specific platelet biology Further studies are required to identify any additional sex-associated risk factors and adjust therapy accordingly in women

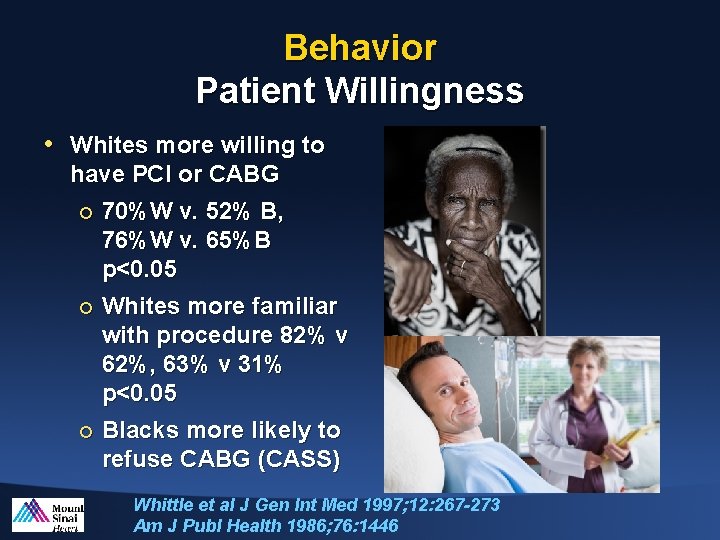
Behavior Patient Willingness • Whites more willing to have PCI or CABG ¡ 70%W v. 52% B, 76%W v. 65%B p<0. 05 ¡ Whites more familiar with procedure 82% v 62%, 63% v 31% p<0. 05 ¡ Blacks more likely to refuse CABG (CASS) Whittle et al J Gen Int Med 1997; 12: 267 -273 Am J Publ Health 1986; 76: 1446

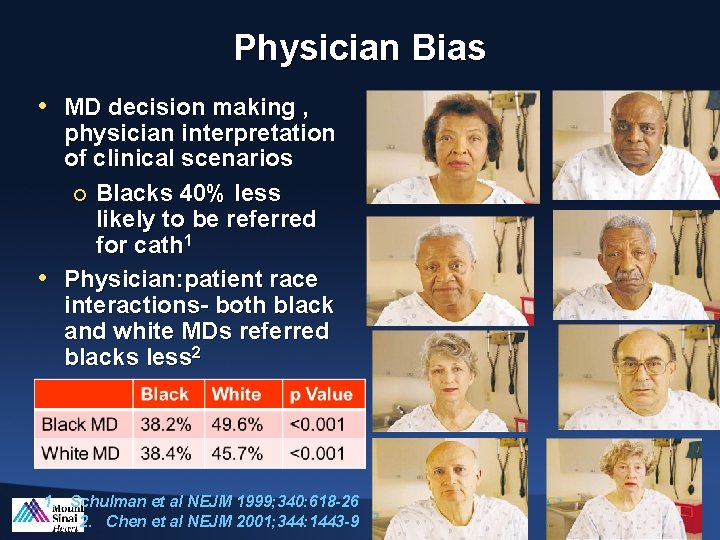
Physician Bias • MD decision making , physician interpretation of clinical scenarios ¡ Blacks 40% less likely to be referred for cath 1 • Physician: patient race interactions- both black and white MDs referred blacks less 2 1. Schulman et al NEJM 1999; 340: 618 -26 2. Chen et al NEJM 2001; 344: 1443 -9

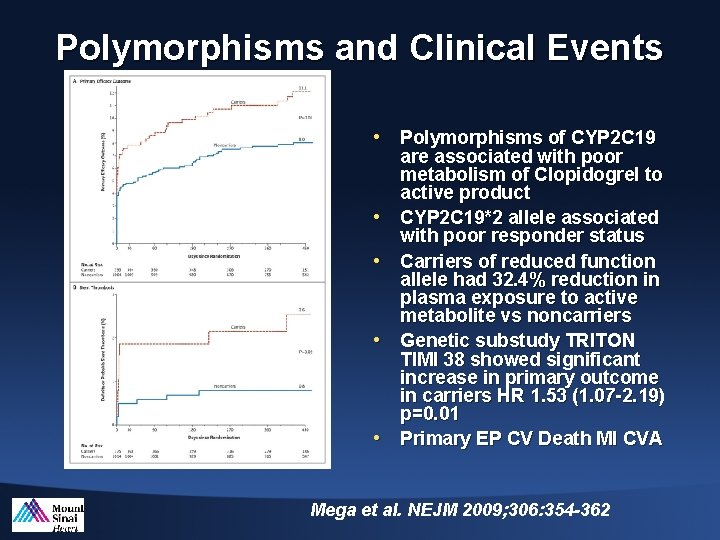
Polymorphisms and Clinical Events • Polymorphisms of CYP 2 C 19 • • are associated with poor metabolism of Clopidogrel to active product CYP 2 C 19*2 allele associated with poor responder status Carriers of reduced function allele had 32. 4% reduction in plasma exposure to active metabolite vs noncarriers Genetic substudy TRITON TIMI 38 showed significant increase in primary outcome in carriers HR 1. 53 (1. 07 -2. 19) p=0. 01 Primary EP CV Death MI CVA Mega et al. NEJM 2009; 306: 354 -362

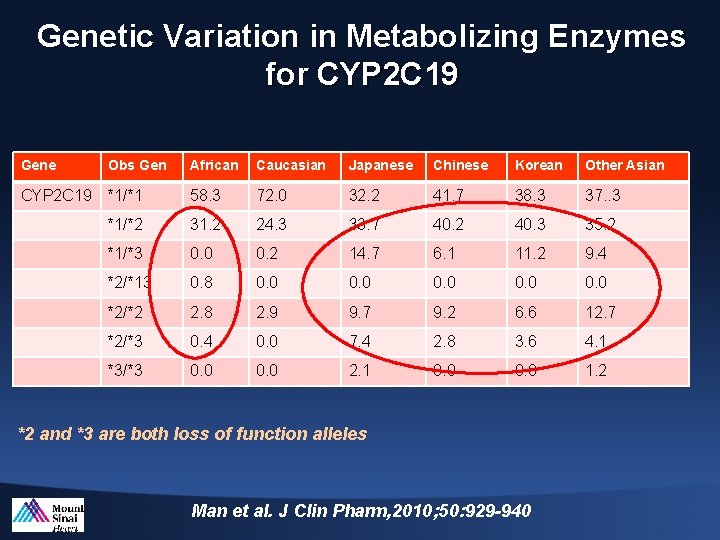
Genetic Variation in Metabolizing Enzymes for CYP 2 C 19 Gene Obs Gen African Caucasian Japanese Chinese Korean Other Asian CYP 2 C 19 *1/*1 58. 3 72. 0 32. 2 41. 7 38. 3 37. . 3 *1/*2 31. 2 24. 3 33. 7 40. 2 40. 3 35. 2 *1/*3 0. 0 0. 2 14. 7 6. 1 11. 2 9. 4 *2/*13 0. 8 0. 0 0. 0 *2/*2 2. 8 2. 9 9. 7 9. 2 6. 6 12. 7 *2/*3 0. 4 0. 0 7. 4 2. 8 3. 6 4. 1 *3/*3 0. 0 2. 1 0. 0 1. 2 *2 and *3 are both loss of function alleles Man et al. J Clin Pharm, 2010; 50: 929 -940

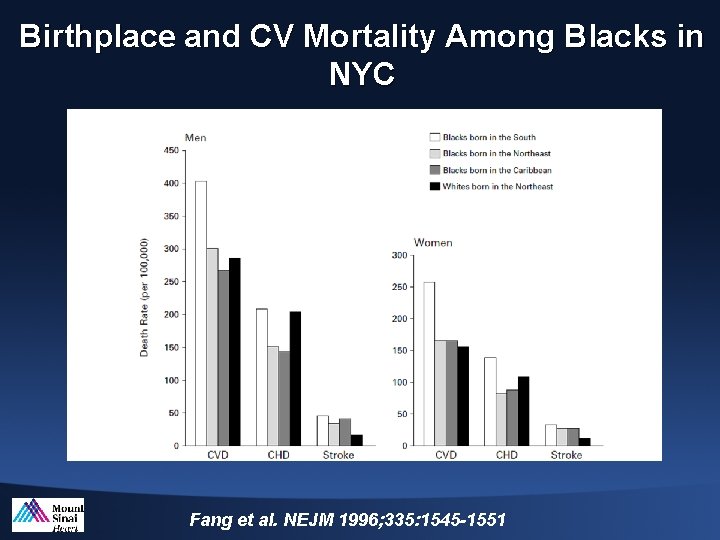
Birthplace and CV Mortality Among Blacks in NYC Fang et al. NEJM 1996; 335: 1545 -1551

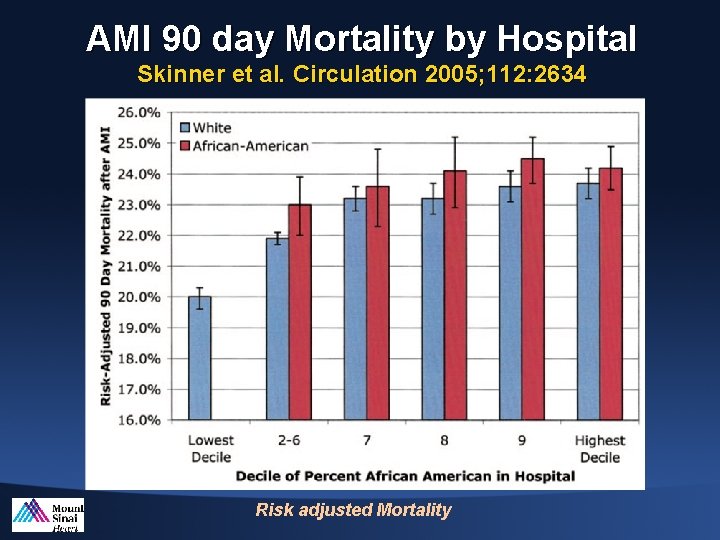
AMI 90 day Mortality by Hospital Skinner et al. Circulation 2005; 112: 2634 Risk adjusted Mortality

How to improve participation of women in clinical trials?

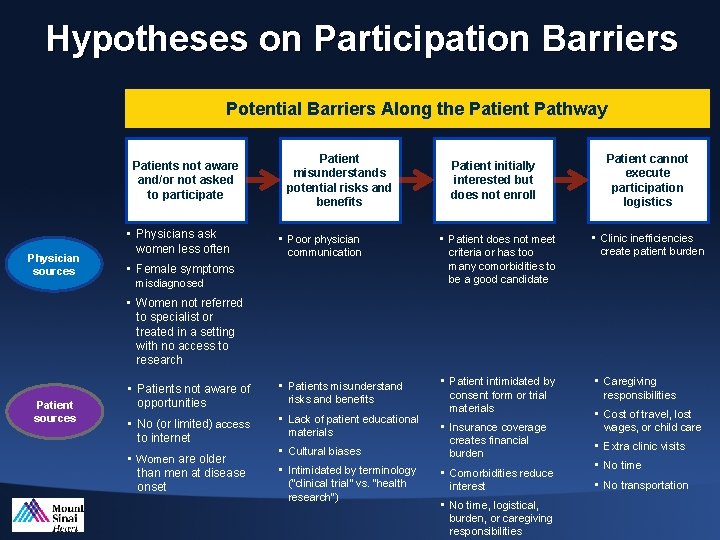
Addressing Participation Barriers

Hypotheses on Participation Barriers Potential Barriers Along the Patient Pathway Patients not aware and/or not asked to participate Physician sources • Physicians ask women less often Patient misunderstands potential risks and benefits Patient initially interested but does not enroll • Poor physician communication • Patient does not meet criteria or has too many comorbidities to be a good candidate • Patients not aware of opportunities • Patients misunderstand risks and benefits • No (or limited) access to internet • Lack of patient educational materials • Patient intimidated by consent form or trial materials • Female symptoms misdiagnosed Patient cannot execute participation logistics • Clinic inefficiencies create patient burden • Women not referred to specialist or treated in a setting with no access to research Patient sources • Women are older than men at disease onset • Cultural biases • Intimidated by terminology (“clinical trial” vs. “health research”) • Insurance coverage creates financial burden • Comorbidities reduce interest • No time, logistical, burden, or caregiving responsibilities • Caregiving responsibilities • Cost of travel, lost wages, or child care • Extra clinic visits • No time • No transportation

2012 Market Research Results Hypothesis #1: Patients are not aware of the opportunity to participate § A large number of patients are not approached to consider participation § Many patients who participated sought out the trial on their own § PCPs and primary cardiologists are not aware of trial opportunities Hypothesis #2: Patient misunderstands the potential risks and benefits § Fear of randomization and/or fear of riskiness of the trial § Women tend to consult with more friends/family members than men and may be more swayed by anyone who is nervous on their behalf Hypothesis #3: Patient initially interested but does not enroll § Many interested patients do not qualify due to concomitant medications, comorbities, age, or other related reasons § Some trial sites never followed-up with patients to complete enrollment § Fear of randomization § Women may need more information, discussion, or time to decide and may not be receiving enough of these to convince them to enroll (and it may be cheaper/faster to enroll men) § Logistical burden too high (travel distance, missed work, child care logistics, etc) § Insurance coverage is an issue in many cases Hypothesis #4: Patient cannot execute participation logistics § Once enrolled, loss to follow-up was not a significant issue

Opportunities for Change Increase awareness around participation opportunities 1 ¡ Patient-focused awareness around the benefits of participation ¡ Make it easier for patients to locate research opportunities (e. g. database) ¡ Tools to increase awareness of participation opportunities among PCPs and General Cardiologists ¡ Leverage social networks to encourage women to participate (through interaction with their peers who have participated, etc) 2 Examine trial design elements/protocols and propose changes to increase the number of women who qualify 3 Reduce the perceptions and misperceptions around participation risk ¡ Patient education materials that describe the research process as well as the benefits of participation ¡ Education for investigators and trial coordinators on how to more effectively approach and communicate with female patients Each of these opportunities requires participation from multiple stakeholders

Women Opt-In for Heart Research (WIN-Her) • Boston Scientific is currently launching an initiative focused on increasing female enrollment in clinical trials to speed trial enrollment and to improve the scientific robustness of the results ¡ WIN-Her initiative (Women Opt-In for Heart Research) ¡ Physician leads will be Dr. Jeanne Poole from the University of Washington and Dr. Marye Gleva from Washington University • WIN-Her will be piloted in two upcoming randomized IDE trials ¡ MADIT-SICD (SICD) ¡ ASAP-TOO (WATCHMAN)

How to fill the sexspecific gap in medical knowledge? Examples… Confidential

Issued on December 19 th, 2011

Gender Data Forum On Devices September 24, 2012 | Heart House, Washington, DC

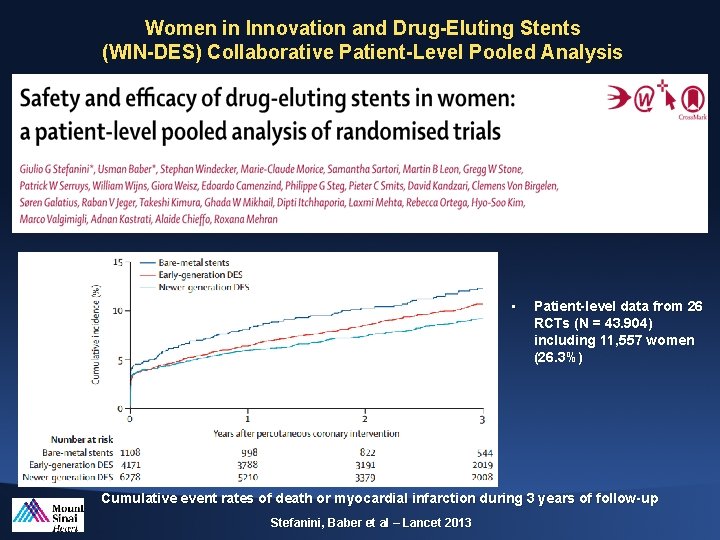
Women in Innovation and Drug-Eluting Stents (WIN-DES) Collaborative Patient-Level Pooled Analysis • Patient-level data from 26 RCTs (N = 43. 904) including 11, 557 women (26. 3%) Cumulative event rates of death or myocardial infarction during 3 years of follow-up Stefanini, Baber et al – Lancet 2013

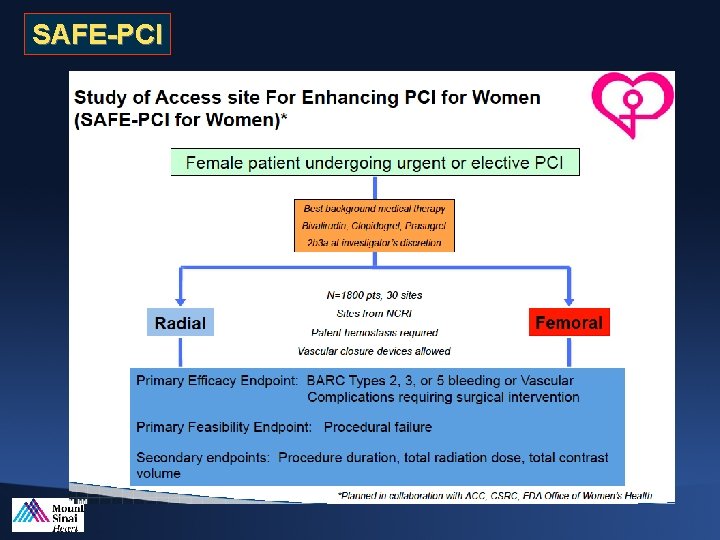
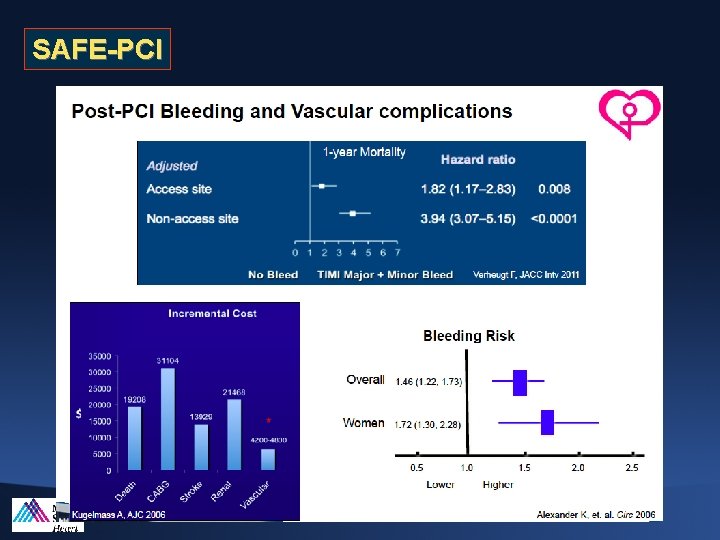
SAFE-PCI

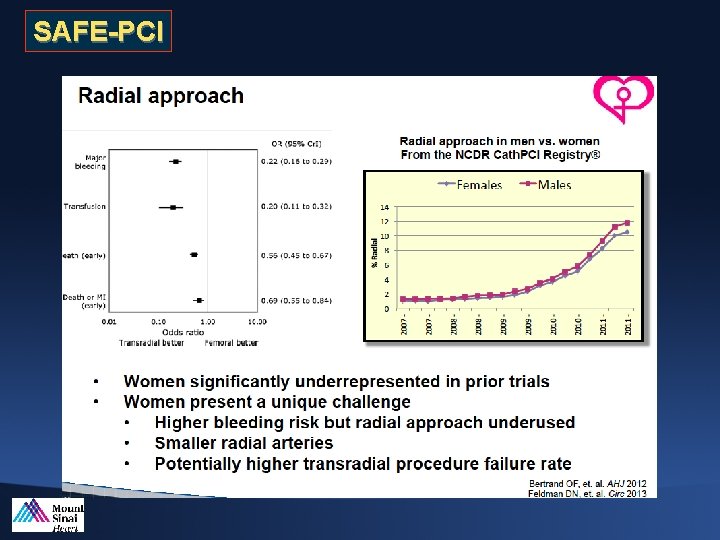
SAFE-PCI

SAFE-PCI

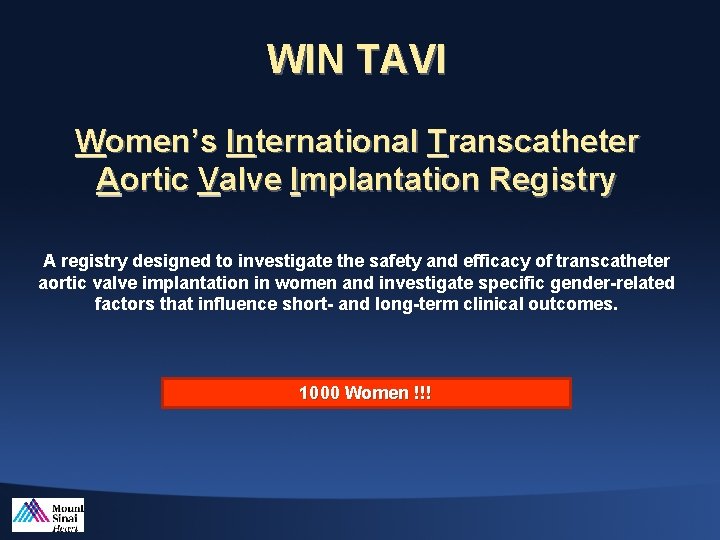
WIN TAVI Women’s International Transcatheter Aortic Valve Implantation Registry A registry designed to investigate the safety and efficacy of transcatheter aortic valve implantation in women and investigate specific gender-related factors that influence short- and long-term clinical outcomes. 1000 Women !!!

Total Enrollment & Enrollment by Site University of Munich San Raffaele Hospital Institut Hospitalier Jacques Cartier University of Pisa 123 102 90 79 Istituto Clinico Humanitas Clinique Pasteur University of Catania 71 70 61 University of Siena University of Rome Erasmus Medical Center Rangueil University Hospital University of Padova Mauriziano Hospital Centro Cardiologico Monzino IRCCS Imperial College Mount Sinai Hospital 57 55 34 33 27 24 23 19 16 Hospital Universitario Miguel Servet Elisabeth-Krankenhaus Radboud University Nijmegen Medical Center Queen Elizabeth Hospital 14 11 4 1 0 10 20 30 40 50 60 70 Enrollment Completed 80 90 100 110 120 130

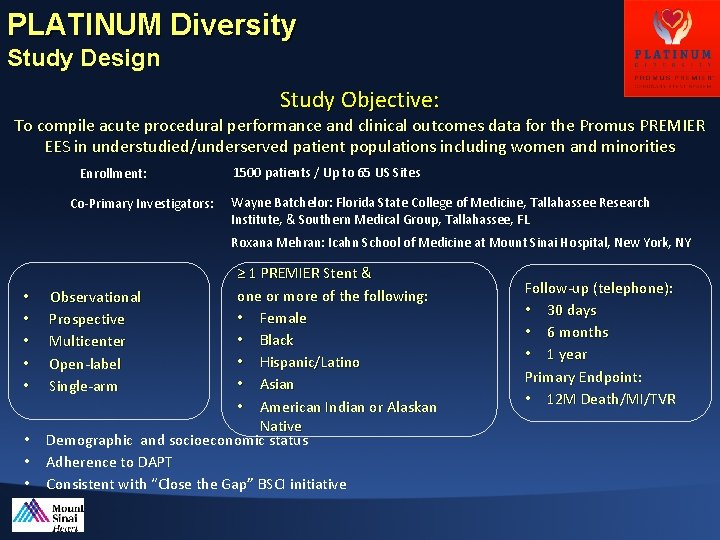
PLATINUM Diversity: Outcomes with the Promus PREMIERTM Stent in Women and Minorities

PLATINUM Diversity Study Design Study Objective: To compile acute procedural performance and clinical outcomes data for the Promus PREMIER EES in understudied/underserved patient populations including women and minorities Enrollment: Co-Primary Investigators: 1500 patients / Up to 65 US Sites Wayne Batchelor: Florida State College of Medicine, Tallahassee Research Institute, & Southern Medical Group, Tallahassee, FL Roxana Mehran: Icahn School of Medicine at Mount Sinai Hospital, New York, NY • • ≥ 1 PREMIER Stent & one or more of the following: Observational • Female Prospective • Black Multicenter • Hispanic/Latino Open-label • Asian Single-arm • American Indian or Alaskan Native Demographic and socioeconomic status Adherence to DAPT Consistent with “Close the Gap” BSCI initiative Follow-up (telephone): • 30 days • 6 months • 1 year Primary Endpoint: • 12 M Death/MI/TVR

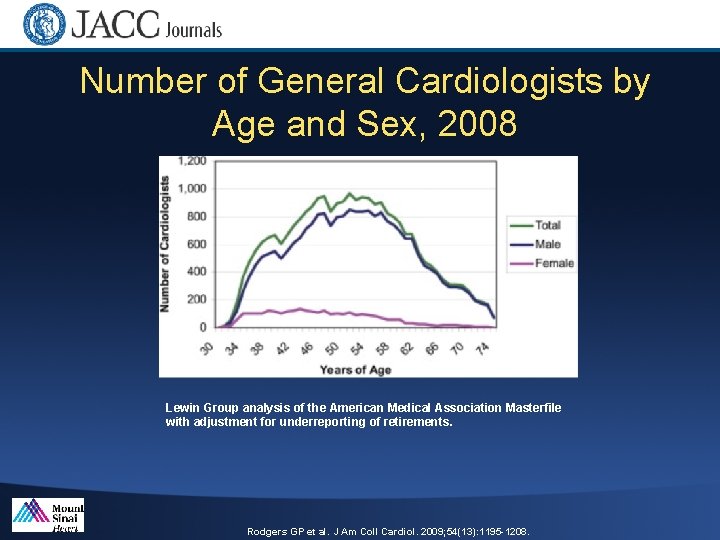
Number of General Cardiologists by Age and Sex, 2008 Lewin Group analysis of the American Medical Association Masterfile with adjustment for underreporting of retirements. Rodgers GP et al. J Am Coll Cardiol. 2009; 54(13): 1195 -1208.

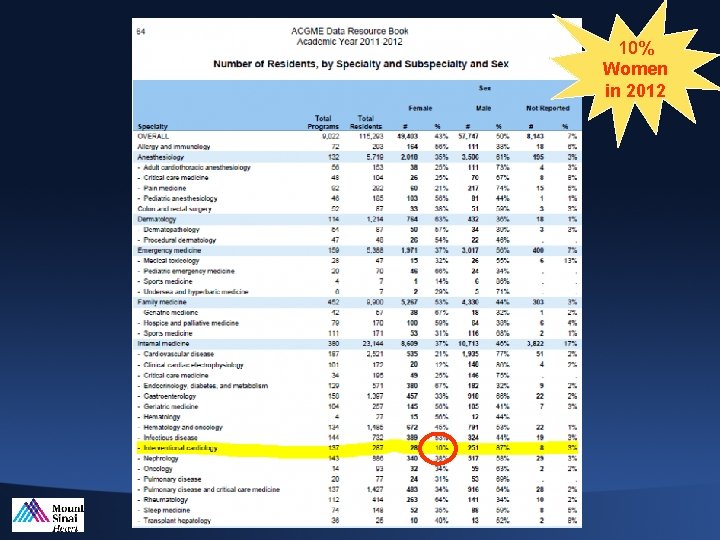
10% Women in 2012

Summary • >75% of clinical trials in Cardiology include less than 30% women. None have the power to show benefit or harm in this subset • The inconsistent results of registries and post-hoc analyses from randomized trials do not give any guidance which revascularization or pharmacological strategies should be chosen for women • Clinical trials should be designed with adequate power to detect hazards and benefits of treatments or strategies studied across minority groups
 Roxana mehran
Roxana mehran Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Lej hub customs dhl
Lej hub customs dhl Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Iwr clinical trial
Iwr clinical trial Audits and inspections of clinical trials
Audits and inspections of clinical trials Role of statistician in clinical trials
Role of statistician in clinical trials Readyset ohsu
Readyset ohsu Mpn clinical trials
Mpn clinical trials York trials unit
York trials unit Nida clinical trials network
Nida clinical trials network Clinical trials quality by design
Clinical trials quality by design Mrc clinical trials unit
Mrc clinical trials unit Prs clinical trial
Prs clinical trial Clinical trials prs
Clinical trials prs Site initiation visit powerpoint presentation
Site initiation visit powerpoint presentation Professor claire harrison
Professor claire harrison Stratified randomization
Stratified randomization Clinical trials.gov login
Clinical trials.gov login Clinical trials
Clinical trials Clinical trials api
Clinical trials api Mehran najafi
Mehran najafi Mehran ahmadlou
Mehran ahmadlou Mehran najafi
Mehran najafi Mehran convex mirror
Mehran convex mirror Mehran shakerinava
Mehran shakerinava Mehran convex mirror
Mehran convex mirror Mehran andalibi
Mehran andalibi Sfetea roxana
Sfetea roxana Roxana gavriloaia
Roxana gavriloaia Hta clasificare
Hta clasificare Roxana chapter 6
Roxana chapter 6 Carmen costache
Carmen costache Dr roxana viera
Dr roxana viera Profesora roxana
Profesora roxana Motifs in shawshank redemption
Motifs in shawshank redemption Roxana del aguila
Roxana del aguila Roxana bravo
Roxana bravo Projeto de reforço escolar para séries iniciais
Projeto de reforço escolar para séries iniciais Samanta computer
Samanta computer Roxana mayer johnson
Roxana mayer johnson Roxana knobel
Roxana knobel Dr roxana viera
Dr roxana viera































































