How will PIONEERAF change our practice Roxana Mehran







- Slides: 28

How will PIONEER-AF change our practice? Roxana Mehran MD, FACC, FSCAI, FAHA, FESC Professor of Medicine (Cardiology), and Population Health Science and Policy The Icahn School of Medicine at Mount Sinai CRT 2017 20 th Anniversary Washington, DC

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Consulting Fees/Honoraria Company • The Medicines Co. , BMS, Astra Zeneca, Lilly/Daiichi Sankyo • Abbott Vascular, Boston Scientific, CSL Behring, Janssen (J+J), Claret • Advisory board for Janssen (J+J), Medscape, Osprey

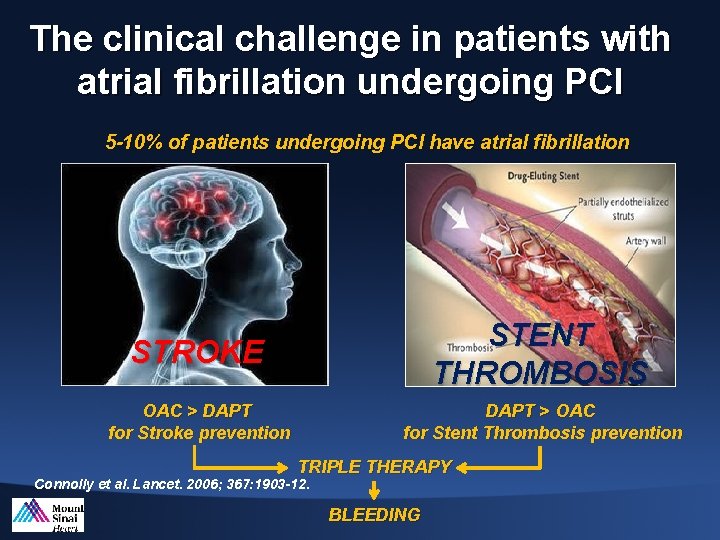
The clinical challenge in patients with atrial fibrillation undergoing PCI 5 -10% of patients undergoing PCI have atrial fibrillation STROKE STENT THROMBOSIS OAC > DAPT for Stroke prevention DAPT > OAC for Stent Thrombosis prevention TRIPLE THERAPY Connolly et al. Lancet. 2006; 367: 1903 -12. BLEEDING

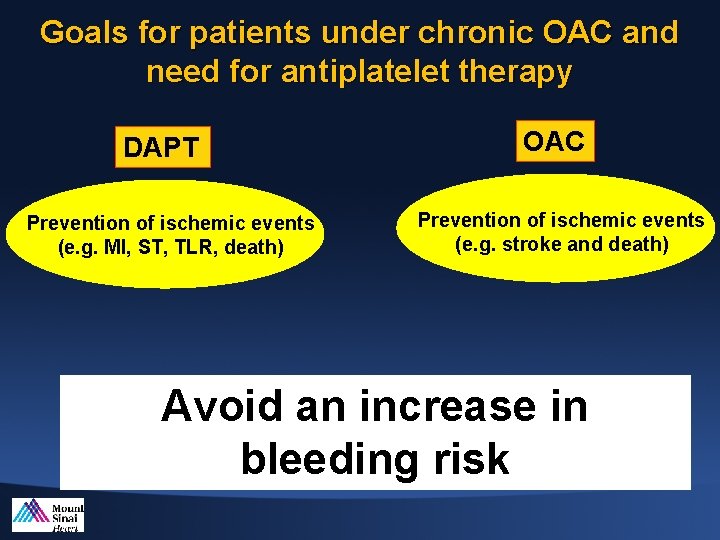
Goals for patients under chronic OAC and need for antiplatelet therapy DAPT Prevention of ischemic events (e. g. MI, ST, TLR, death) OAC Prevention of ischemic events (e. g. stroke and death) Avoid an increase in bleeding risk

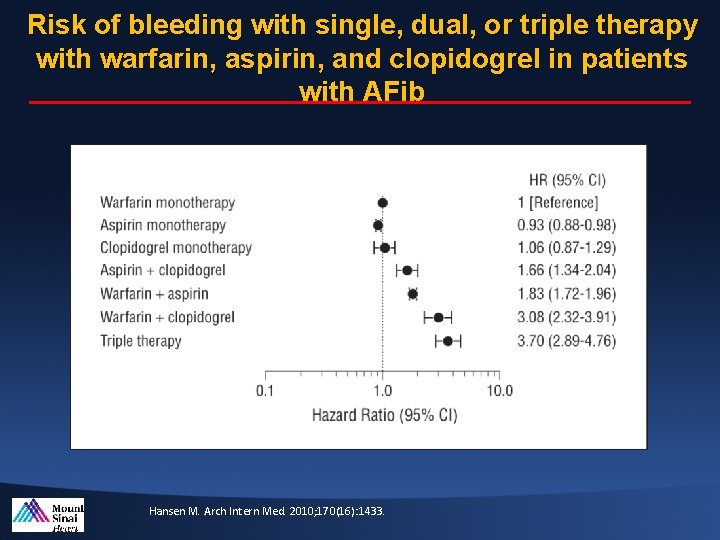
Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with AFib Hansen M. Arch Intern Med. 2010; 170(16): 1433.

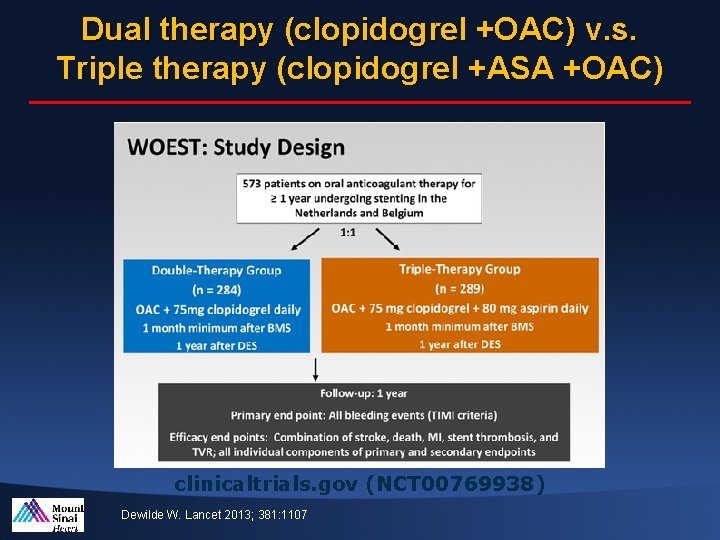
Dual therapy (clopidogrel +OAC) v. s. Triple therapy (clopidogrel +ASA +OAC) clinicaltrials. gov (NCT 00769938) Dewilde W. Lancet 2013; 381: 1107

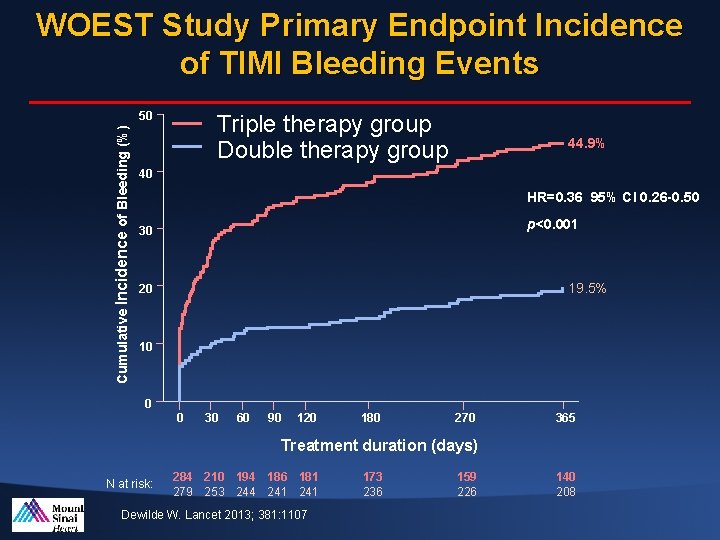
WOEST Study Primary Endpoint Incidence of TIMI Bleeding Events Cumulative Incidence of Bleeding (%) 50 Triple therapy group Double therapy group 44. 9% 40 HR=0. 36 95% CI 0. 26 -0. 50 p<0. 001 30 19. 5% 20 10 0 0 30 60 90 120 180 270 365 Treatment duration (days) N at risk: 284 210 194 186 181 279 253 244 241 Dewilde W. Lancet 2013; 381: 1107 173 236 159 226 140 208

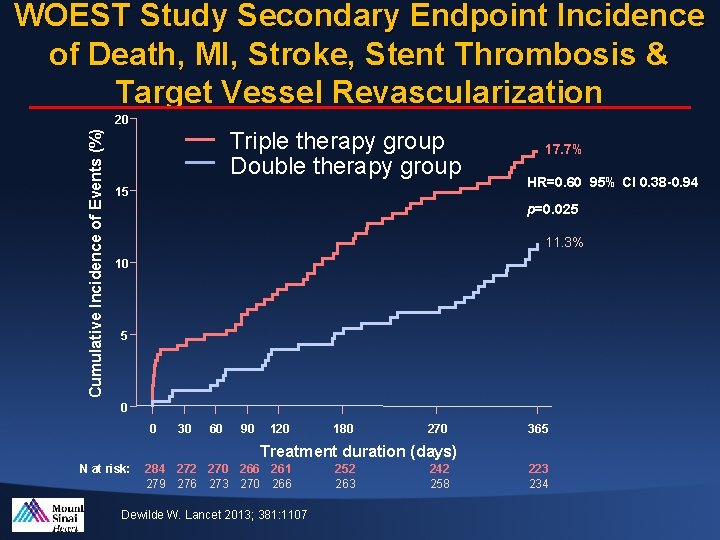
WOEST Study Secondary Endpoint Incidence of Death, MI, Stroke, Stent Thrombosis & Target Vessel Revascularization Cumulative Incidence of Events (%) 20 Triple therapy group Double therapy group 15 17. 7% HR=0. 60 95% CI 0. 38 -0. 94 p=0. 025 11. 3% 10 5 0 0 30 60 90 120 180 270 365 Treatment duration (days) N at risk: 284 272 270 266 261 279 276 273 270 266 Dewilde W. Lancet 2013; 381: 1107 252 263 242 258 223 234

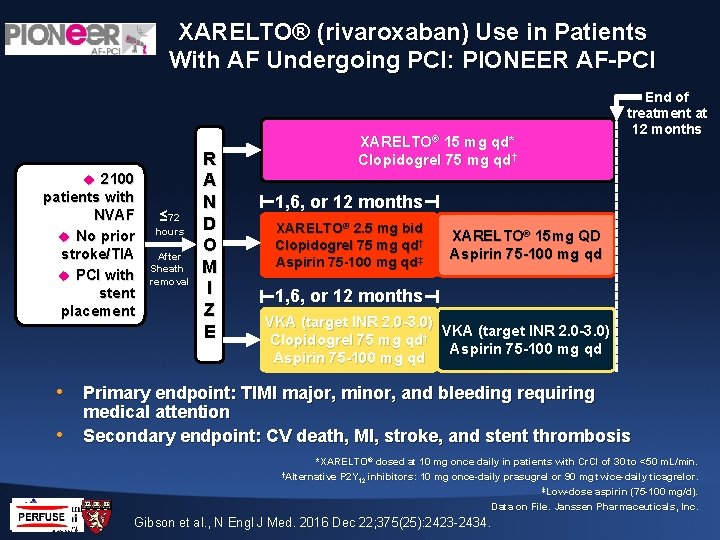
XARELTO® (rivaroxaban) Use in Patients With AF Undergoing PCI: PIONEER AF-PCI 2100 patients with NVAF No prior stroke/TIA PCI with stent placement ≤ 72 hours After Sheath removal R A N D O M I Z E End of treatment at 12 months XARELTO® 15 mg qd* Clopidogrel 75 mg qd† 1, 6, or 12 months XARELTO® 2. 5 mg bid Clopidogrel 75 mg qd† Aspirin 75 -100 mg qd‡ XARELTO® 15 mg QD Aspirin 75 -100 mg qd 1, 6, or 12 months VKA (target INR 2. 0 -3. 0) Clopidogrel 75 mg qd† Aspirin 75 -100 mg qd • Primary endpoint: TIMI major, minor, and bleeding requiring • medical attention Secondary endpoint: CV death, MI, stroke, and stent thrombosis *XARELTO® dosed at 10 mg once daily in patients with Cr. Cl of 30 to <50 m. L/min. †Alternative P 2 Y inhibitors: 10 mg once-daily prasugrel or 90 mg twice-daily ticagrelor. 12 ‡Low-dose aspirin (75 -100 mg/d). Data on File. Janssen Pharmaceuticals, Inc. Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434.

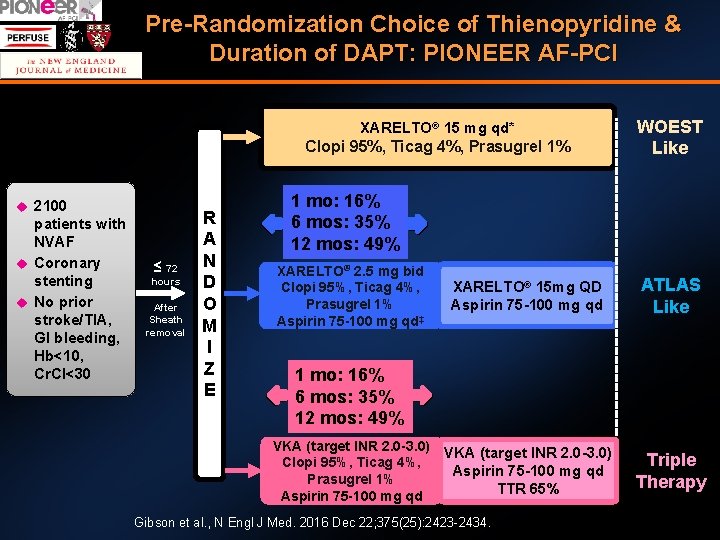
Pre-Randomization Choice of Thienopyridine & Duration of DAPT: PIONEER AF-PCI XARELTO® 15 mg qd* Clopi 95%, Ticag 4%, Prasugrel 1% 2100 patients with NVAF Coronary stenting No prior stroke/TIA, GI bleeding, Hb<10, Cr. Cl<30 ≤ 72 hours After Sheath removal R A N D O M I Z E WOEST Like 1 mo: 16% 6 mos: 35% 12 mos: 49% XARELTO® 2. 5 mg bid Clopi 95%, Ticag 4%, Prasugrel 1% Aspirin 75 -100 mg qd‡ XARELTO® 15 mg QD Aspirin 75 -100 mg qd ATLAS Like VKA (target INR 2. 0 -3. 0) Aspirin 75 -100 mg qd TTR 65% Triple Therapy 1 mo: 16% 6 mos: 35% 12 mos: 49% VKA (target INR 2. 0 -3. 0) Clopi 95%, Ticag 4%, Prasugrel 1% Aspirin 75 -100 mg qd Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434.

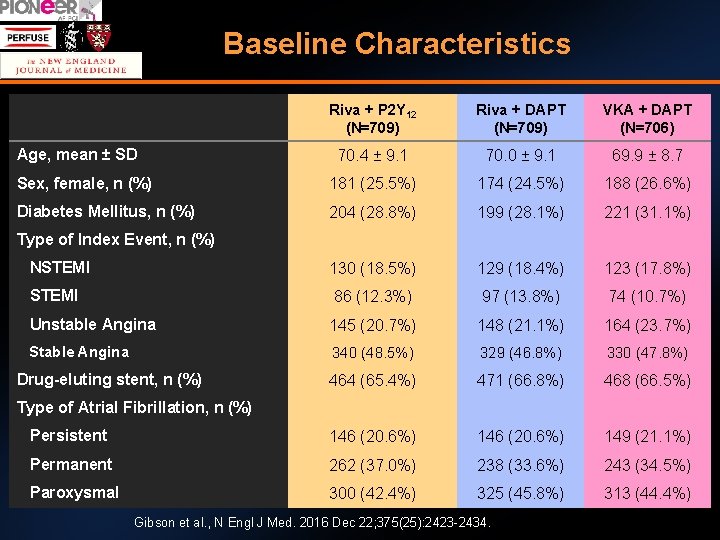
Baseline Characteristics Riva + P 2 Y 12 (N=709) Riva + DAPT (N=709) VKA + DAPT (N=706) 70. 4 ± 9. 1 70. 0 ± 9. 1 69. 9 ± 8. 7 Sex, female, n (%) 181 (25. 5%) 174 (24. 5%) 188 (26. 6%) Diabetes Mellitus, n (%) 204 (28. 8%) 199 (28. 1%) 221 (31. 1%) NSTEMI 130 (18. 5%) 129 (18. 4%) 123 (17. 8%) STEMI 86 (12. 3%) 97 (13. 8%) 74 (10. 7%) Unstable Angina 145 (20. 7%) 148 (21. 1%) 164 (23. 7%) Stable Angina 340 (48. 5%) 329 (46. 8%) 330 (47. 8%) 464 (65. 4%) 471 (66. 8%) 468 (66. 5%) Persistent 146 (20. 6%) 149 (21. 1%) Permanent 262 (37. 0%) 238 (33. 6%) 243 (34. 5%) Paroxysmal 300 (42. 4%) 325 (45. 8%) 313 (44. 4%) Age, mean ± SD Type of Index Event, n (%) Drug-eluting stent, n (%) Type of Atrial Fibrillation, n (%) Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434.

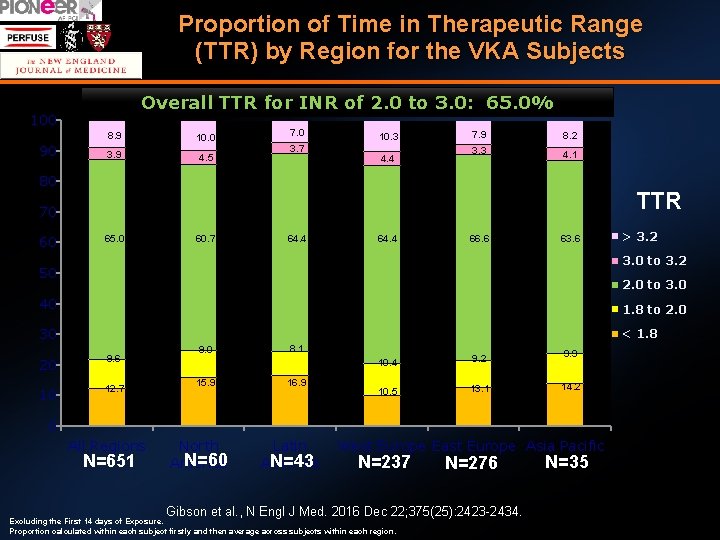
Proportion of Time in Therapeutic Range (TTR) by Region for the VKA Subjects Overall TTR for INR of 2. 0 to 3. 0: 65. 0% 100 90 8. 9 10. 0 3. 9 4. 5 7. 0 3. 7 10. 3 4. 4 7. 9 8. 2 3. 3 4. 1 80 TTR 70 60 65. 0 60. 7 64. 4 66. 6 63. 6 > 3. 2 3. 0 to 3. 2 50 2. 0 to 3. 0 40 1. 8 to 2. 0 30 < 1. 8 20 9. 6 10 12. 7 9. 0 15. 9 8. 1 16. 9 10. 4 9. 2 9. 9 10. 5 13. 1 14. 2 0 All Regions N=651 North N=60 America Latin N=43 America West Europe East Europe Asia Pacific N=237 N=276 Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434. Excluding the First 14 days of Exposure. Proportion calculated within each subject firstly and then average across subjects within each region. N=35

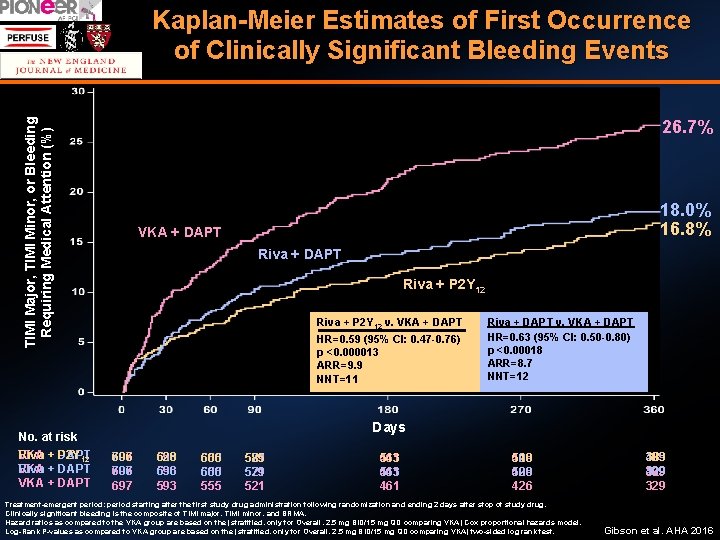
Kaplan-Meier Estimates of First Occurrence of Clinically Significant Bleeding Events TIMI Major, TIMI Minor, or Bleeding Requiring Medical Attention (%) 26. 7% p<0. 00018 p<0. 000013 18. 0% 16. 8% VKA +DAPT VKA + +DAPT Riva + P 2 Y 12 HR = 0. 63 (95% CI 0. 50 -0. 80) = (95% 8. 7 HR +ARR =DAPT 0. 59 0. 47 -0. 76) Riva + P 2 Y 12 v. VKA Riva +CI DAPT v. VKA + DAPT NNT = 12 HR=0. 63 (95% CI: 0. 50 -0. 80) HR=0. 59 (95% CI: 0. 47 -0. 76) ARR = 9. 9 p <0. 00018 p <0. 000013 NNT = 11 ARR=8. 7 ARR=9. 9 NNT=12 NNT=11 No. at risk VKA + DAPT Riva P 2 Y 12 VKA + DAPT Riva VKA + DAPT Days 697 706 697 593 636 628 593 636 593 555 600 606 555 600 555 521 579 585 521 579 521 461 543 461 426 509 510 426 509 426 Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. Clinically significant bleeding is the composite of TIMI major, TIMI minor, and BRMA. Hazard ratios as compared to the VKA group are based on the (stratified, only for Overall, 2. 5 mg BID/15 mg QD comparing VKA) Cox proportional hazards model. Log-Rank P-values as compared to VKA group are based on the (stratified, only for Overall, 2. 5 mg BID/15 mg QD comparing VKA) two-sided log rank test. 329 409 383 329 409 329 Gibson et al. AHA 2016

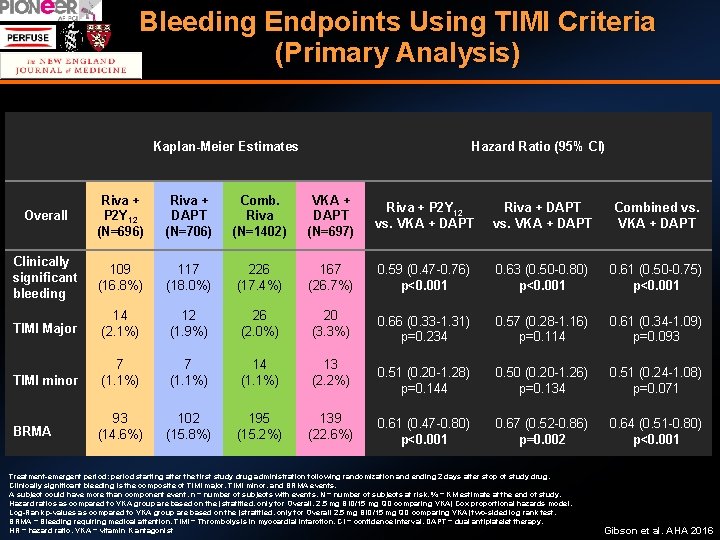
Bleeding Endpoints Using TIMI Criteria (Primary Analysis) Kaplan-Meier Estimates Hazard Ratio (95% CI) Overall Riva + P 2 Y 12 (N=696) Riva + DAPT (N=706) Comb. Riva (N=1402) VKA + DAPT (N=697) Riva + P 2 Y 12 vs. VKA + DAPT Riva + DAPT vs. VKA + DAPT Combined vs. VKA + DAPT Clinically significant bleeding 109 (16. 8%) 117 (18. 0%) 226 (17. 4%) 167 (26. 7%) 0. 59 (0. 47 -0. 76) p<0. 001 0. 63 (0. 50 -0. 80) p<0. 001 0. 61 (0. 50 -0. 75) p<0. 001 TIMI Major 14 (2. 1%) 12 (1. 9%) 26 (2. 0%) 20 (3. 3%) 0. 66 (0. 33 -1. 31) p=0. 234 0. 57 (0. 28 -1. 16) p=0. 114 0. 61 (0. 34 -1. 09) p=0. 093 TIMI minor 7 (1. 1%) 14 (1. 1%) 13 (2. 2%) 0. 51 (0. 20 -1. 28) p=0. 144 0. 50 (0. 20 -1. 26) p=0. 134 0. 51 (0. 24 -1. 08) p=0. 071 BRMA 93 (14. 6%) 102 (15. 8%) 195 (15. 2%) 139 (22. 6%) 0. 61 (0. 47 -0. 80) p<0. 001 0. 67 (0. 52 -0. 86) p=0. 002 0. 64 (0. 51 -0. 80) p<0. 001 Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. Clinically significant bleeding is the composite of TIMI major, TIMI minor, and BRMA events. A subject could have more than component event. n = number of subjects with events, N = number of subjects at risk, % = KM estimate at the end of study. Hazard ratios as compared to VKA group are based on the (stratified, only for Overall, 2. 5 mg BID/15 mg QD comparing VKA) Cox proportional hazards model. Log-Rank p-values as compared to VKA group are based on the (stratified, only for Overall 2. 5 mg BID/15 mg QD comparing VKA) two-sided log rank test. BRMA = Bleeding requiring medical attention, TIMI = Thrombolysis in myocardial infarction, CI = confidence interval, DAPT = dual antiplatelet therapy, HR = hazard ratio, VKA = vitamin K antagonist Gibson et al. AHA 2016

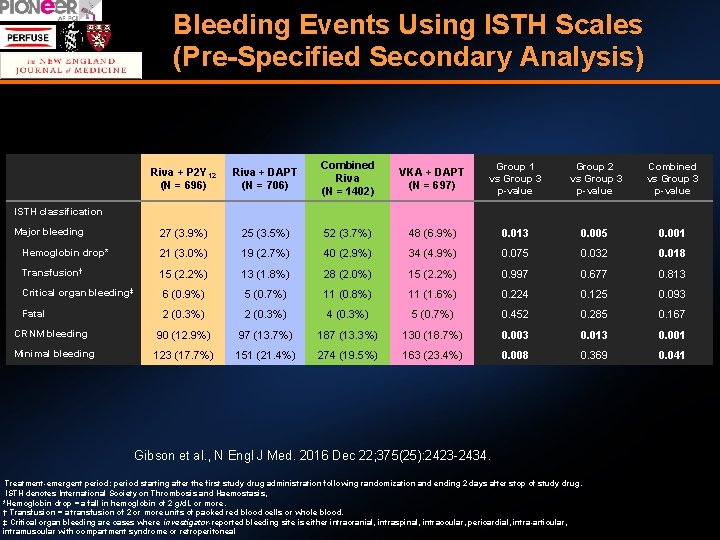
Bleeding Events Using ISTH Scales (Pre-Specified Secondary Analysis) Riva + P 2 Y 12 (N = 696) Riva + DAPT (N = 706) Combined Riva (N = 1402) VKA + DAPT (N = 697) Group 1 vs Group 3 p-value Group 2 vs Group 3 p-value Combined vs Group 3 p-value 27 (3. 9%) 25 (3. 5%) 52 (3. 7%) 48 (6. 9%) 0. 013 0. 005 0. 001 Hemoglobin drop* 21 (3. 0%) 19 (2. 7%) 40 (2. 9%) 34 (4. 9%) 0. 075 0. 032 0. 018 Transfusion† 15 (2. 2%) 13 (1. 8%) 28 (2. 0%) 15 (2. 2%) 0. 997 0. 677 0. 813 Critical organ bleeding‡ 6 (0. 9%) 5 (0. 7%) 11 (0. 8%) 11 (1. 6%) 0. 224 0. 125 0. 093 Fatal 2 (0. 3%) 4 (0. 3%) 5 (0. 7%) 0. 452 0. 285 0. 167 90 (12. 9%) 97 (13. 7%) 187 (13. 3%) 130 (18. 7%) 0. 003 0. 013 0. 001 123 (17. 7%) 151 (21. 4%) 274 (19. 5%) 163 (23. 4%) 0. 008 0. 369 0. 041 ISTH classification Major bleeding CRNM bleeding Minimal bleeding Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434. Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. ISTH denotes International Society on Thrombosis and Haemostasis, *Hemoglobin drop = a fall in hemoglobin of 2 g/d. L or more. † Transfusion = a transfusion of 2 or more units of packed red blood cells or whole blood. ‡ Critical organ bleeding are cases where investigator-reported bleeding site is either intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome or retroperitoneal

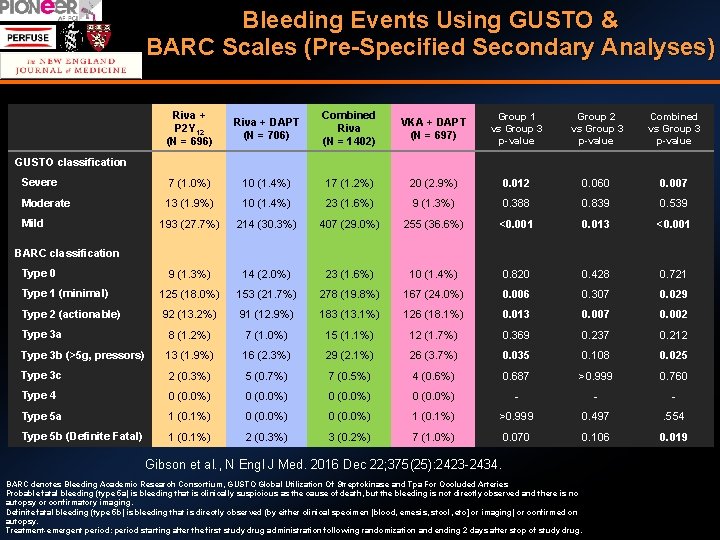
Bleeding Events Using GUSTO & BARC Scales (Pre-Specified Secondary Analyses) Riva + P 2 Y 12 (N = 696) Riva + DAPT (N = 706) Combined Riva (N = 1402) VKA + DAPT (N = 697) Group 1 vs Group 3 p-value Group 2 vs Group 3 p-value Combined vs Group 3 p-value Severe 7 (1. 0%) 10 (1. 4%) 17 (1. 2%) 20 (2. 9%) 0. 012 0. 060 0. 007 Moderate 13 (1. 9%) 10 (1. 4%) 23 (1. 6%) 9 (1. 3%) 0. 388 0. 839 0. 539 193 (27. 7%) 214 (30. 3%) 407 (29. 0%) 255 (36. 6%) <0. 001 0. 013 <0. 001 9 (1. 3%) 14 (2. 0%) 23 (1. 6%) 10 (1. 4%) 0. 820 0. 428 0. 721 Type 1 (minimal) 125 (18. 0%) 153 (21. 7%) 278 (19. 8%) 167 (24. 0%) 0. 006 0. 307 0. 029 Type 2 (actionable) 92 (13. 2%) 91 (12. 9%) 183 (13. 1%) 126 (18. 1%) 0. 013 0. 007 0. 002 Type 3 a 8 (1. 2%) 7 (1. 0%) 15 (1. 1%) 12 (1. 7%) 0. 369 0. 237 0. 212 Type 3 b (>5 g, pressors) 13 (1. 9%) 16 (2. 3%) 29 (2. 1%) 26 (3. 7%) 0. 035 0. 108 0. 025 Type 3 c 2 (0. 3%) 5 (0. 7%) 7 (0. 5%) 4 (0. 6%) 0. 687 >0. 999 0. 760 Type 4 0 (0. 0%) - - - Type 5 a 1 (0. 1%) 0 (0. 0%) 1 (0. 1%) >0. 999 0. 497 . 554 Type 5 b (Definite Fatal) 1 (0. 1%) 2 (0. 3%) 3 (0. 2%) 7 (1. 0%) 0. 070 0. 106 0. 019 GUSTO classification Mild BARC classification Type 0 Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434. BARC denotes Bleeding Academic Research Consortium, GUSTO Global Utilization Of Streptokinase and Tpa For Occluded Arteries Probable fatal bleeding (type 5 a) is bleeding that is clinically suspicious as the cause of death, but the bleeding is not directly observed and there is no autopsy or confirmatory imaging. Definite fatal bleeding (type 5 b) is bleeding that is directly observed (by either clinical specimen [blood, emesis, stool, etc] or imaging) or confirmed on autopsy. Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug.

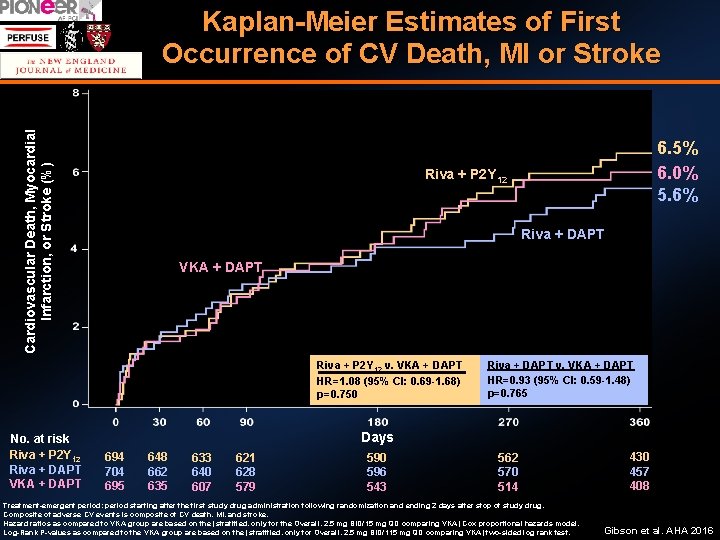
Cardiovascular Death, Myocardial Infarction, or Stroke (%) Kaplan-Meier Estimates of First Occurrence of CV Death, MI or Stroke Riva + P 2 Y 12 Riva + DAPT VKA + DAPT Riva + P 2 Y 12 v. VKA + DAPT HR=1. 08 (95% CI: 0. 69 -1. 68) p=0. 750 No. at risk Riva + P 2 Y 12 Riva + DAPT VKA + DAPT 6. 5% 6. 0% 5. 6% Riva + DAPT v. VKA + DAPT HR=0. 93 (95% CI: 0. 59 -1. 48) p=0. 765 Days 694 704 695 648 662 635 633 640 607 621 628 579 590 596 543 562 570 514 Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. Composite of adverse CV events is composite of CV death, MI, and stroke. Hazard ratios as compared to VKA group are based on the (stratified, only for the Overall, 2. 5 mg BID/15 mg QD comparing VKA) Cox proportional hazards model. Log-Rank P-values as compared to the VKA group are based on the (stratified, only for Overall, 2. 5 mg BID/115 mg QD comparing VKA) two-sided log rank test. 430 457 408 Gibson et al. AHA 2016

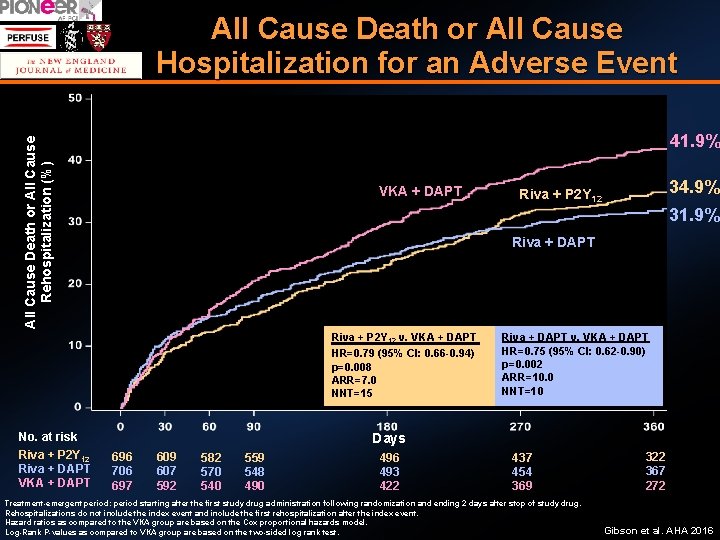
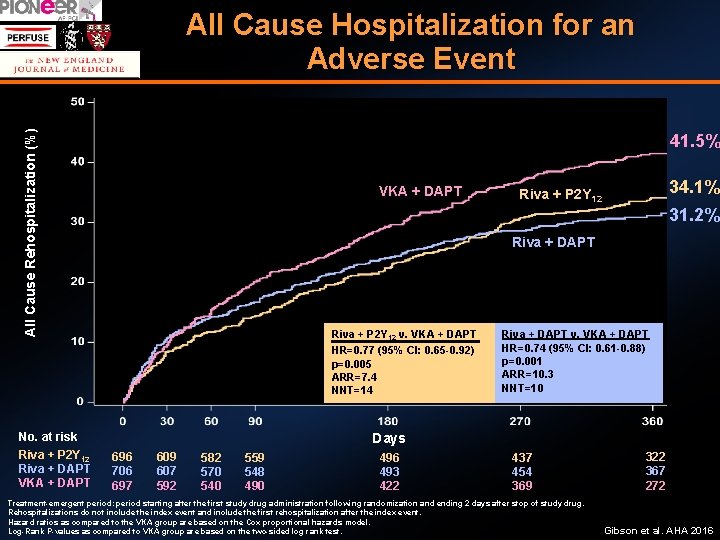
All Cause Death or All Cause Hospitalization for an Adverse Event Gibson et al. AHA 2016

All Cause Death or All Cause Hospitalization for an Adverse Event All Cause Death or All Cause Rehospitalization (%) 41. 9% VKA + DAPT 31. 9% Riva + DAPT Riva + P 2 Y 12 v. VKA + DAPT HR=0. 79 (95% CI: 0. 66 -0. 94) p=0. 008 ARR=7. 0 NNT=15 No. at risk Riva + P 2 Y 12 Riva + DAPT VKA + DAPT 34. 9% Riva + P 2 Y 12 Riva + DAPT v. VKA + DAPT HR=0. 75 (95% CI: 0. 62 -0. 90) p=0. 002 ARR=10. 0 NNT=10 Days 696 706 697 609 607 592 582 570 540 559 548 490 496 493 422 437 454 369 Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. Rehospitalizations do not include the index event and include the first rehospitalization after the index event. Hazard ratios as compared to the VKA group are based on the Cox proportional hazards model. Log-Rank P-values as compared to VKA group are based on the two-sided log rank test. 322 367 272 Gibson et al. AHA 2016

All Cause Rehospitalization (%) All Cause Hospitalization for an Adverse Event 41. 5% VKA + DAPT 31. 2% Riva + DAPT Riva + P 2 Y 12 v. VKA + DAPT HR=0. 77 (95% CI: 0. 65 -0. 92) p=0. 005 ARR=7. 4 NNT=14 No. at risk Riva + P 2 Y 12 Riva + DAPT VKA + DAPT 34. 1% Riva + P 2 Y 12 Riva + DAPT v. VKA + DAPT HR=0. 74 (95% CI: 0. 61 -0. 88) p=0. 001 ARR=10. 3 NNT=10 Days 696 706 697 609 607 592 582 570 540 559 548 490 496 493 422 437 454 369 Treatment-emergent period: period starting after the first study drug administration following randomization and ending 2 days after stop of study drug. Rehospitalizations do not include the index event and include the first rehospitalization after the index event. Hazard ratios as compared to the VKA group are based on the Cox proportional hazards model. Log-Rank P-values as compared to VKA group are based on the two-sided log rank test. 322 367 272 Gibson et al. AHA 2016

Summary A strategy of either rivaroxaban 15 mg daily plus a P 2 Y 12 or rivaroxaban 2. 5 mg BID + DAPT was associated with a reduction in clinically significant bleeding compared with conventional triple therapy of VKA + DAPT (HR = 0. 59 (0. 470. 76), p < 0. 001, NNT 11, and HR = 0. 63 (0. 50 -0. 80), p <0. 001, NNT 12 respectively ). CV death / MI / stroke were comparable among the groups (Riva 15 mg+ P 2 Y 12 = 6. 5%, Riva 2. 5 mg+ DAPT = 5. 6%, VKA + DAPT = 6. 0%) with broad confidence intervals Rates of all cause death or hospitalization were reduced in the Rivaroxaban arms (Riva 15 mg + P 2 Y 12 = NNT 15, Riva 2. 5 + DAPT, NNT =10) Gibson et al. , N Engl J Med. 2016 Dec 22; 375(25): 2423 -2434.

The Pioneer-AF-PCI trial

Gibson et al. AHA 2016

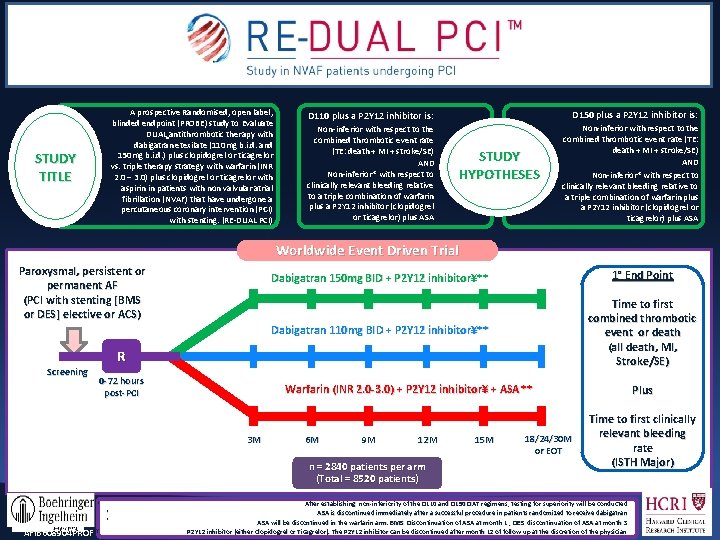
A prospective Randomised, open label, blinded endpoint (PROBE) study to Evaluate DUAL antithrombotic therapy with dabigatran etexilate (110 mg b. i. d. and 150 mg b. i. d. ) plus clopidogrel or ticagrelor vs. triple therapy strategy with warfarin (INR 2. 0 – 3. 0) plus clopidogrel or ticagrelor with aspirin in patients with non valvular atrial fibrillation (NVAF) that have undergone a percutaneous coronary intervention (PCI)) with stenting. (RE-DUAL PCI) STUDY TITLE D 110 plus a P 2 Y 12 inhibitor is: D 150 plus a P 2 Y 12 inhibitor is: Non-inferior with respect to the combined thrombotic event rate (TE: death + MI + stroke/SE) AND Non-inferior* with respect to clinically relevant bleeding relative to a triple combination of warfarin plus a P 2 Y 12 inhibitor (clopidogrel or ticagrelor) plus ASA STUDY HYPOTHESES Worldwide Event Driven Trial Paroxysmal, persistent or permanent AF (PCI with stenting [BMS or DES] elective or ACS) Dabigatran 150 mg BID + P 2 Y 12 inhibitor¥** 1° End Point Dabigatran 110 mg BID + P 2 Y 12 inhibitor¥** Time to first combined thrombotic event or death (all death, MI, Stroke/SE) R Screening 0 -72 hours post-PCI Warfarin (INR 2. 0 -3. 0) + P 2 Y 12 inhibitor¥ + ASA ** 3 M 6 M 9 M 12 M n = 2840 patients per arm (Total = 8520 patients) * * * AFIB 608904 PROF ¥ 15 M 18/24/30 M or EOT Plus Time to first clinically relevant bleeding rate (ISTH Major) After establishing non-inferiority of the D 110 and D 150 DAT regimens, testing for superiority will be conducted ASA is discontinued immediately after a successful procedure in patients randomized to receive dabigatran ASA will be discontinued in the warfarin arm. BMS: Discontinuation of ASA at month 1 ; DES: discontinuation of ASA at month 3 P 2 Y 12 inhibitor (either Clopidogrel or Ticagrelor). The P 2 Y 12 inhibitor can be discontinued after month 12 of follow up at the discretion of the physician

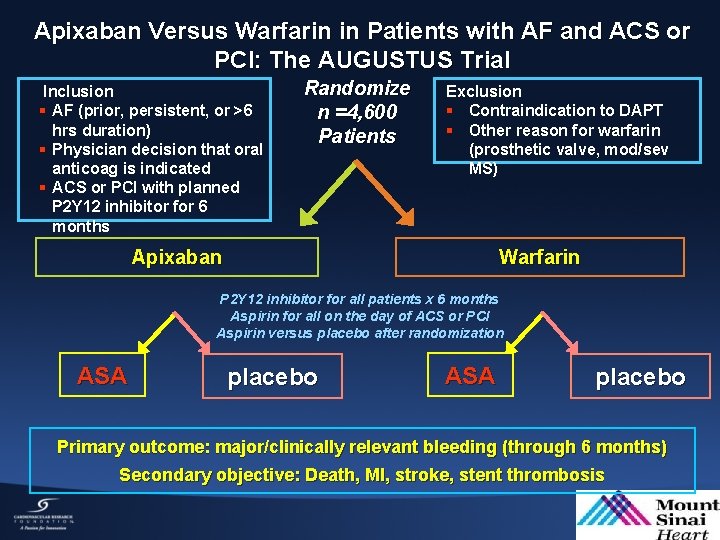
Apixaban Versus Warfarin in Patients with AF and ACS or PCI: The AUGUSTUS Trial Inclusion § AF (prior, persistent, or >6 hrs duration) § Physician decision that oral anticoag is indicated § ACS or PCI with planned P 2 Y 12 inhibitor for 6 months Randomize n =4, 600 Patients Exclusion § Contraindication to DAPT § Other reason for warfarin (prosthetic valve, mod/sev MS) Warfarin Apixaban P 2 Y 12 inhibitor for all patients x 6 months Aspirin for all on the day of ACS or PCI Aspirin versus placebo after randomization ASA placebo Primary outcome: major/clinically relevant bleeding (through 6 months) Secondary objective: Death, MI, stroke, stent thrombosis

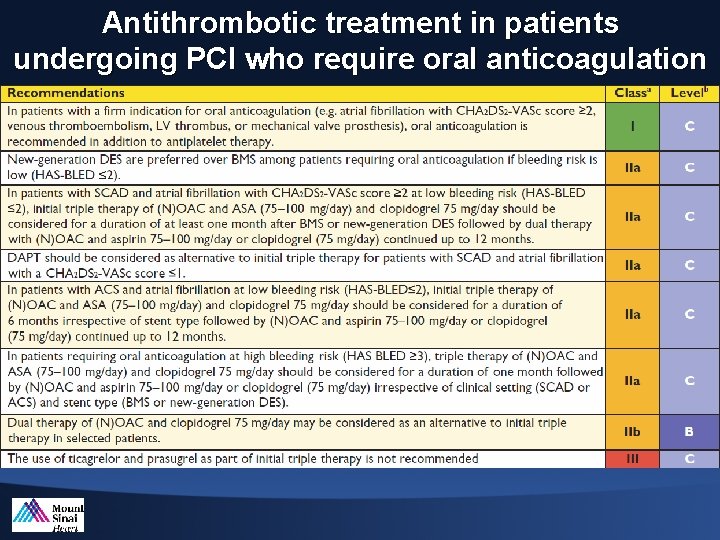
Antithrombotic treatment in patients undergoing PCI who require oral anticoagulation

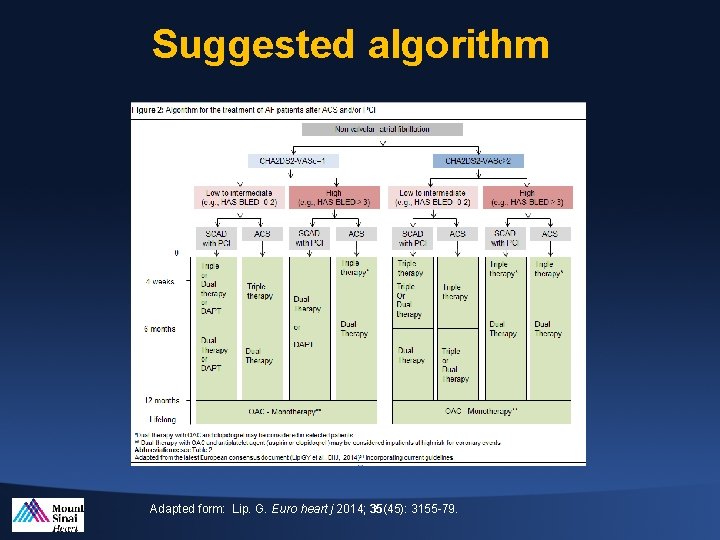
Suggested algorithm Adapted form: Lip. G. Euro heart j 2014; 35(45): 3155 -79.

How will Pioneer-AF change my practice? 1. Can now incorporate NOAC (Rivaroxaban) to the regimen of pts with AF undergoing PCI choose the Xeralto 15 mg/d +Clop in most patients. 2. In high risk PCI (multiple stents, LM), when DAPT is needed, use triple therapy (Riva 2. 5 mg BID, ASA, Clop-not approved in US) for one month and change to Clop and Xeralto as above