Detect Protect Perfect care of patients with Atrial








![References [1] NHS Improving Quality. Costs and benefits of Antithrombotic Therapy in Atrial Fibrillation References [1] NHS Improving Quality. Costs and benefits of Antithrombotic Therapy in Atrial Fibrillation](https://slidetodoc.com/presentation_image/124960b6bfc8b8e62e333c9944261ec1/image-51.jpg)
- Slides: 51

Detect, Protect, Perfect: care of patients with Atrial Fibrillation across Wessex A report for Wessex AHSN ‘Atrial Fibrillation: Detect, Perfect, Protect’ Programme Dr Anastasios Argyropoulos Centre for Implementation Science A. Argyropoulos@soton. ac. uk Wessex AHSN Atrial Fibrillation Senior Programme Manager: Vicki Rowse Wessex AHSN Atrial Fibrillation Clinical Lead: Sharron Gordon

Contents • Introduction page 2 • Detect page 3 • Protect page 12 • Perfect page 27 • Summary of opportunities page 33 • Clinical impact of planned interventions page 34 • Cost impact analysis page 37 1 • Appendix A: Data definitions, description-methodology page 40 • Appendix B: Cost impact analysis Year 1, Year 2 and Year 3 page 47 • References page 50

Introduction Atrial Fibrillation (AF) is the most common sustained cardiac arrhythmia affecting 1 -2% of the UK population. AF becomes more common with increasing age, affecting approximately 10% of the population over 75 years old and 18% of those over 85 years old. AF is associated with a 5 -fold increased risk of stroke compared to other stroke causes. Clinical outcomes in terms of increased disability, are considerably worse for AF-associated compared to stroke not associated with AF, and mortality from stroke is doubled in patients with AF. Overall 15% of strokes are caused by AF but AF is the predominant cause of stroke in the elderly which is clearly of concern with an ageing patient demographic. Bed days for patients with a primary or secondary diagnosis of AF are estimated to have cost the NHS £ 2. 8 billion in 2005 in direct care costs with wider costs in terms of lost productivity and social care amounting to an additional £ 4. 2 billion. [1]-[3] 2

DETECT Detection- Key Findings Increasing the detection of AF should result in increased anticoagulation rates and a reduction in stroke rates as a consequence. Detection rates are increasing across the region but 21, 043 potential AF patients remain to be found across Wessex. Evidence 1. Actual vs expected AF prevalence 2. Percentage of practices uploading GRASP-AF [6] data 3. AF prevalence 4. Patients potentially undiagnosed with AF in 2016 -2017 5. AF related strokes 3

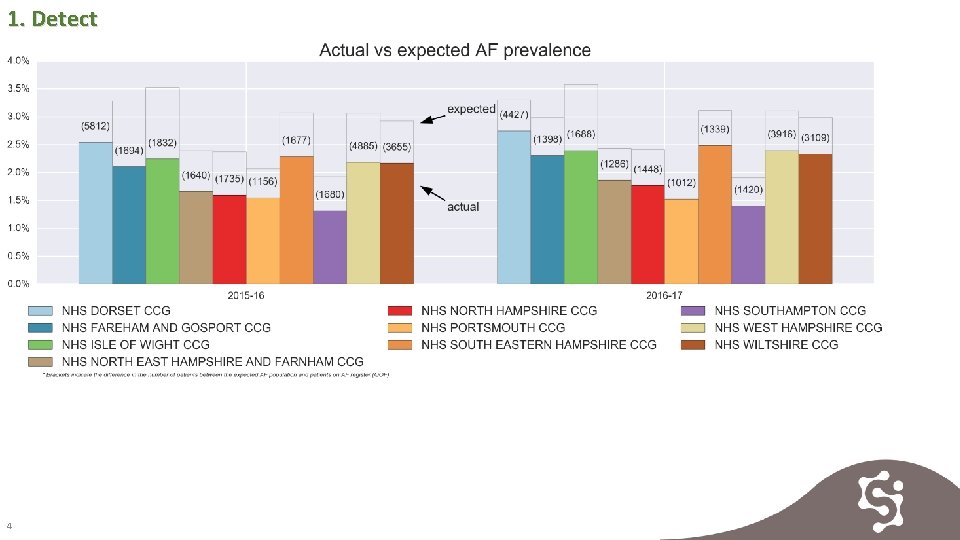
1. Detect 4

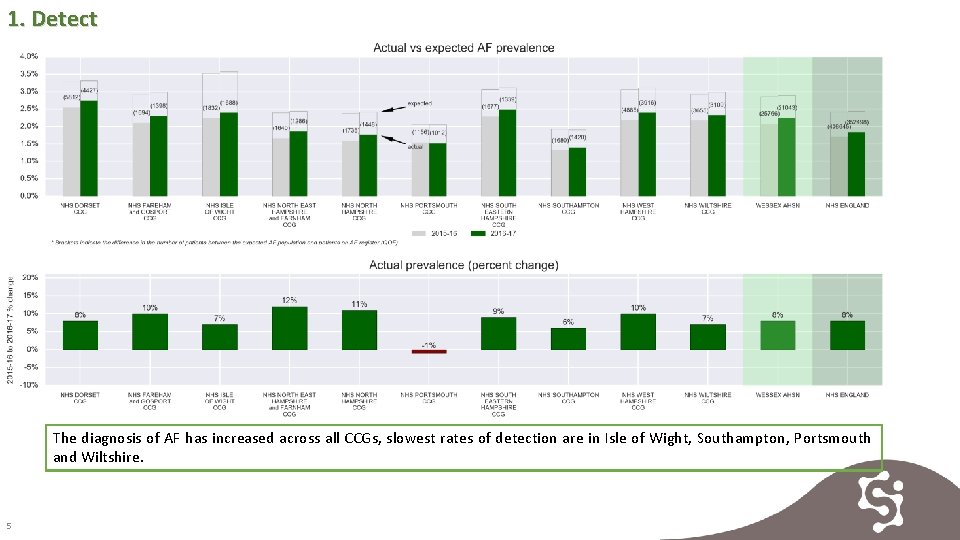
1. Detect The diagnosis of AF has increased across all CCGs, slowest rates of detection are in Isle of Wight, Southampton, Portsmouth and Wiltshire. 5

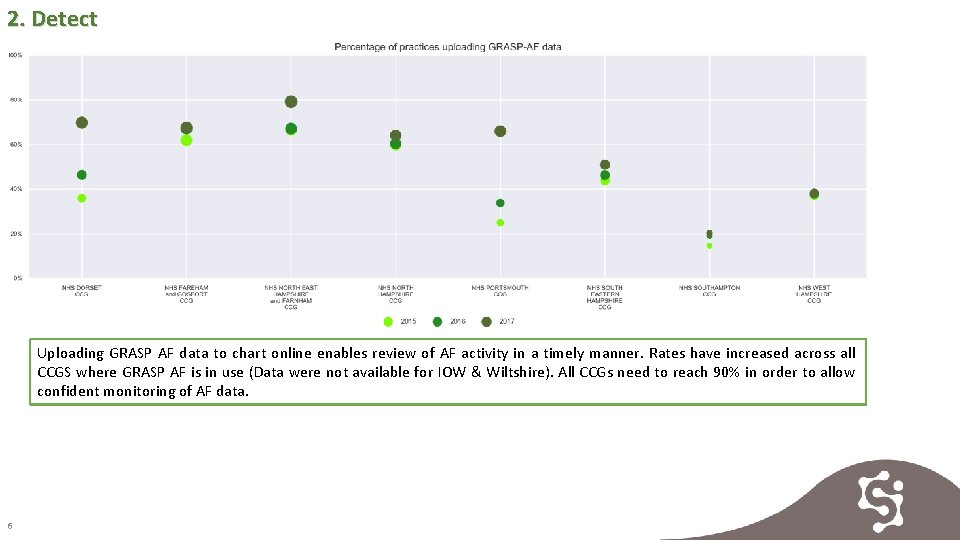
2. Detect Uploading GRASP AF data to chart online enables review of AF activity in a timely manner. Rates have increased across all CCGS where GRASP AF is in use (Data were not available for IOW & Wiltshire). All CCGs need to reach 90% in order to allow confident monitoring of AF data. 6

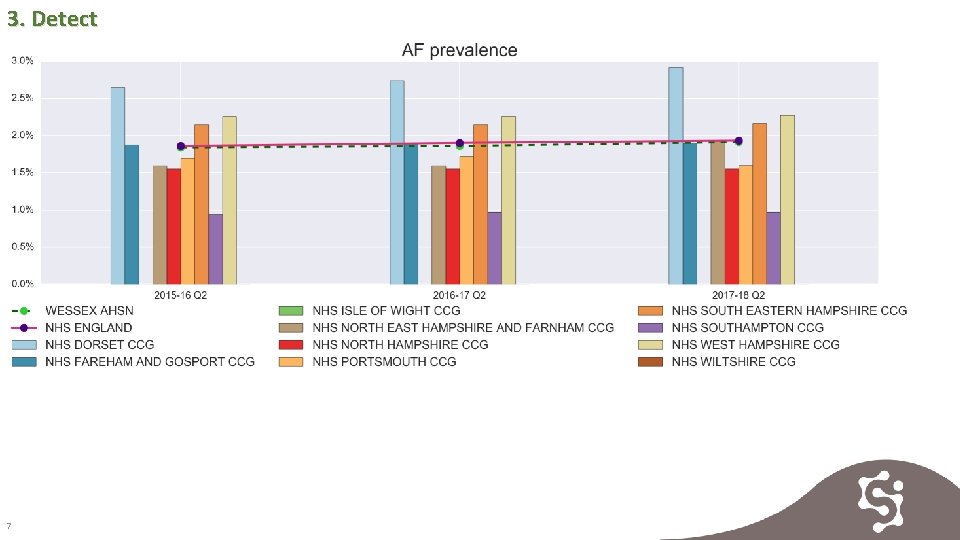
3. Detect 7

3. Detect 8

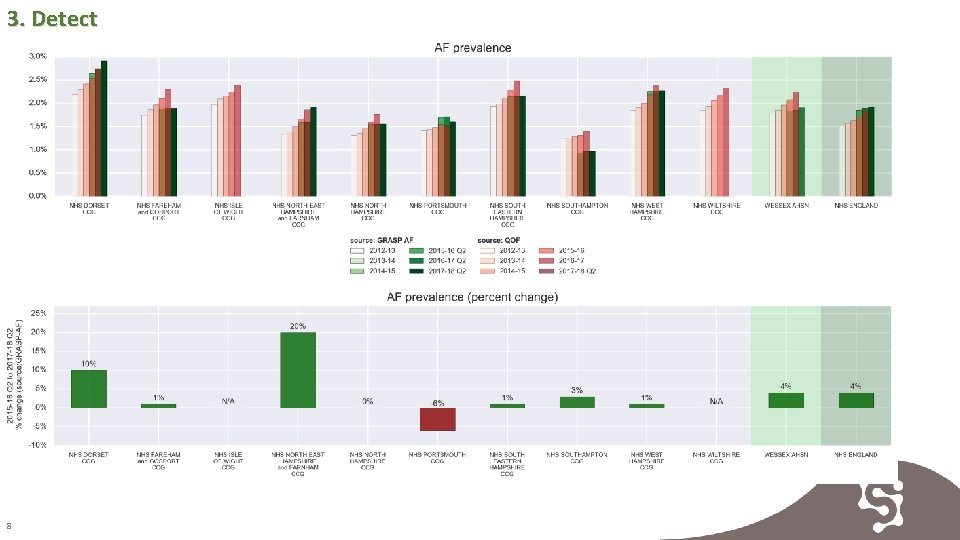
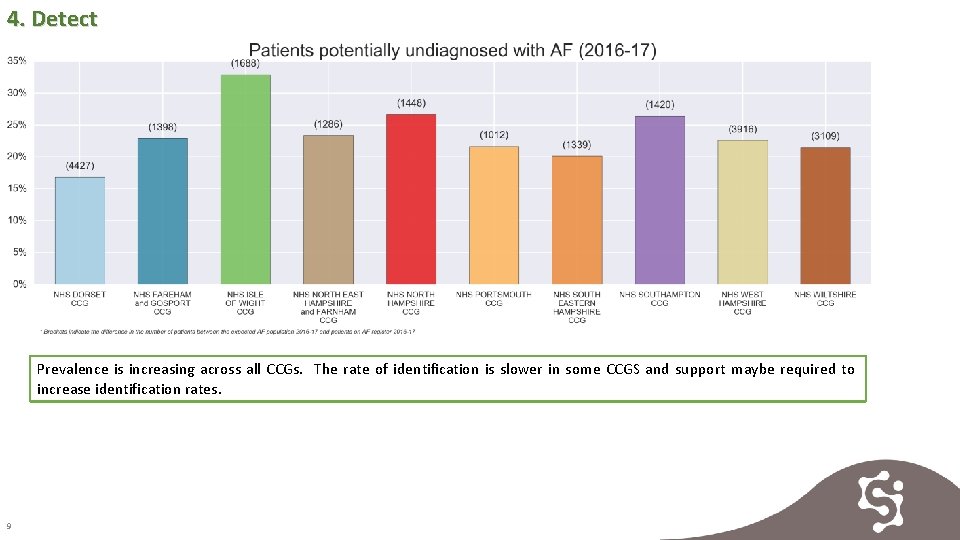
4. Detect Prevalence is increasing across all CCGs. The rate of identification is slower in some CCGS and support maybe required to increase identification rates. 9

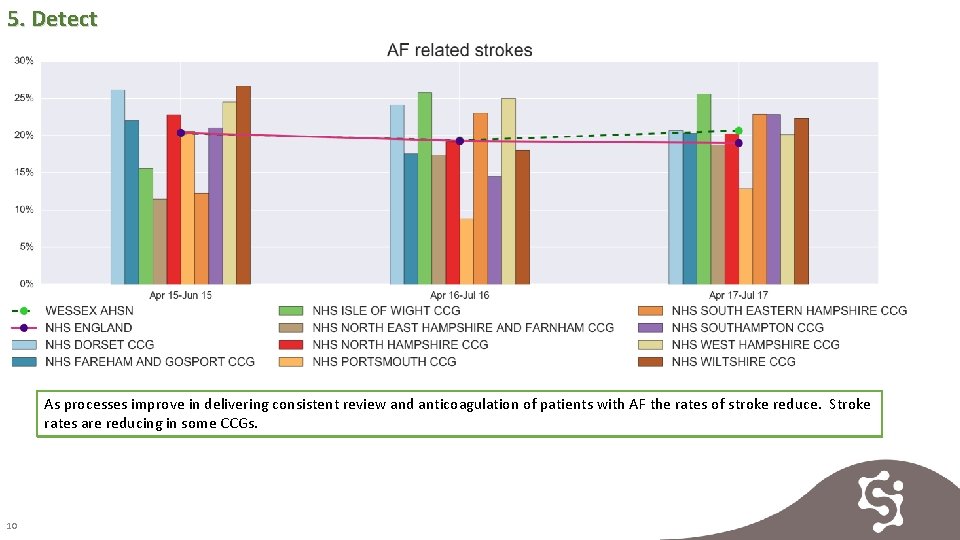
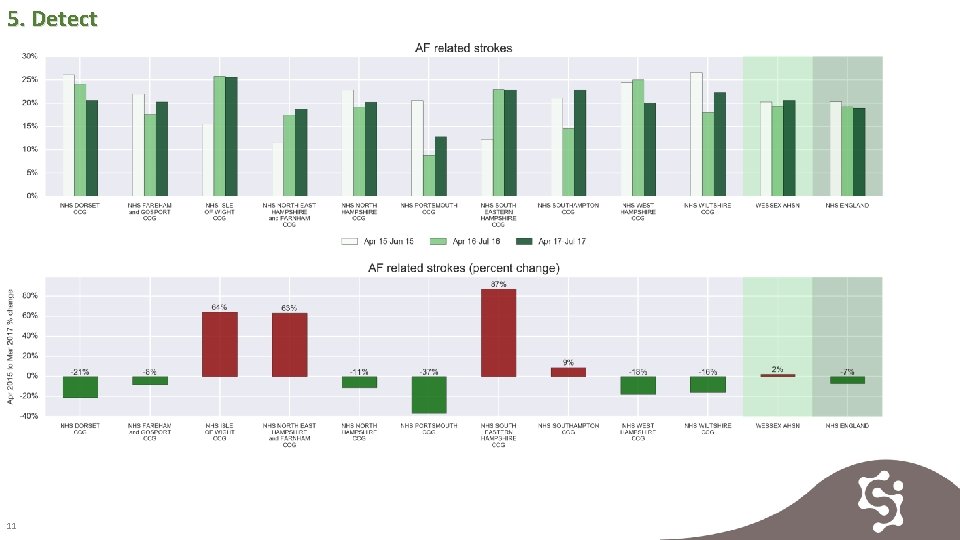
5. Detect As processes improve in delivering consistent review and anticoagulation of patients with AF the rates of stroke reduce. Stroke rates are reducing in some CCGs. 10

5. Detect 11

PROTECT Protection- Key Findings Anticoagulation reduces the risk of AF related stroke by 66% therefore increasing the rates of anticoagulation will result in reduced stroke rates. Anticoagulation rates are increasing across Wessex. Evidence 1. Patients diagnosed AF missing a risk assessment 2. Patients treated with anticoagulation 3. Patients treated with anticoagulation drug therapy with CHA 2 DS 2 -VASc≥ 2 4. Patients risk assessed and eligible for treatment not on anticoagulant 5. Patients contraindicated or declined anticoagulation 6. Patients treated with antiplatelets solely 7. AF related stroke patients not on anticoagulants 12

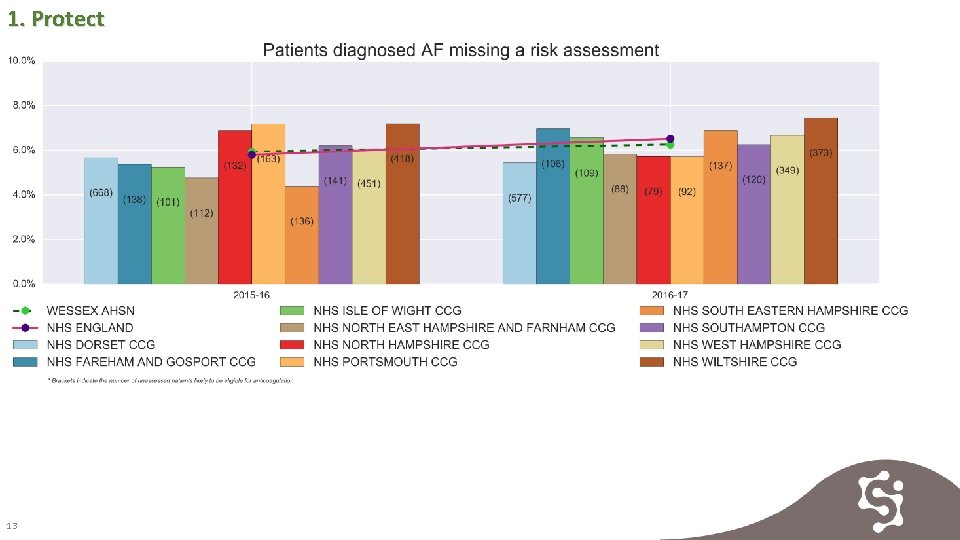
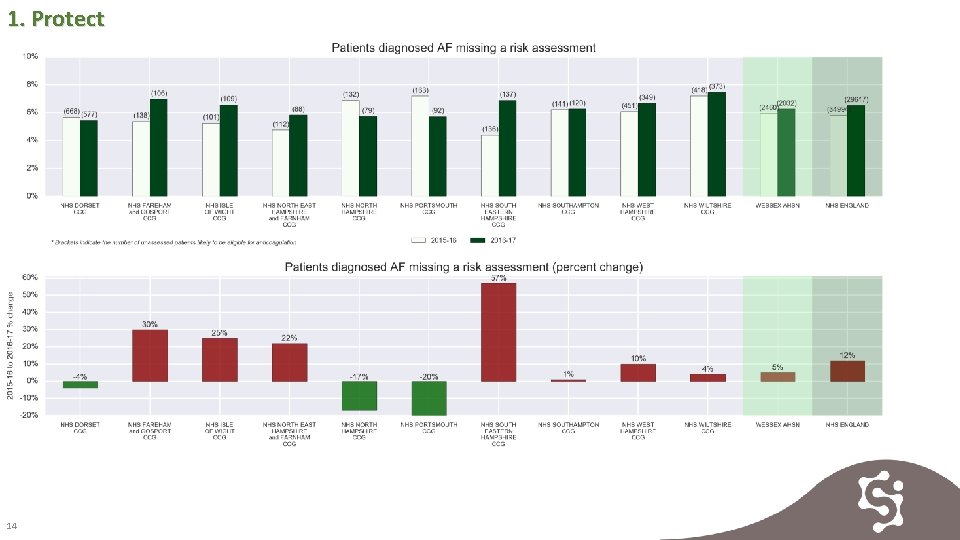
1. Protect 13

1. Protect 14

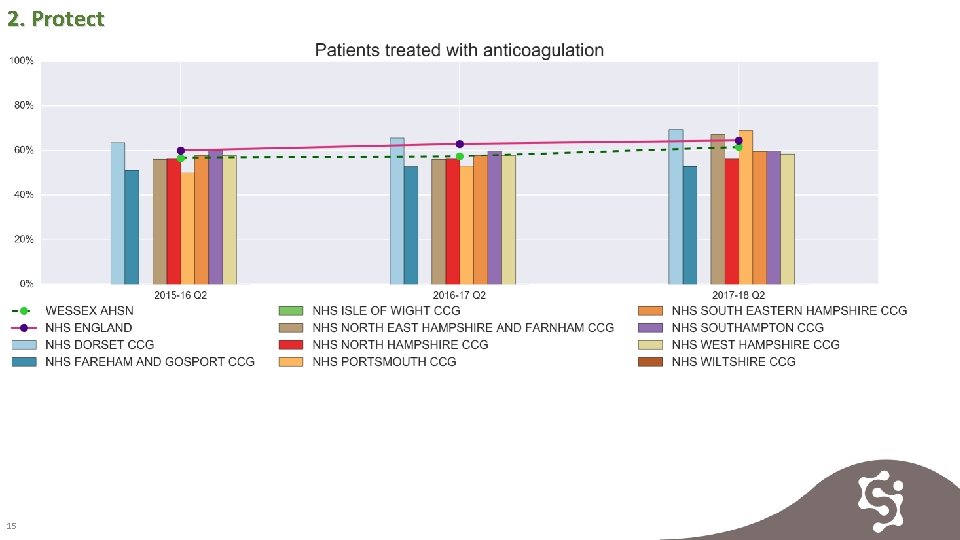
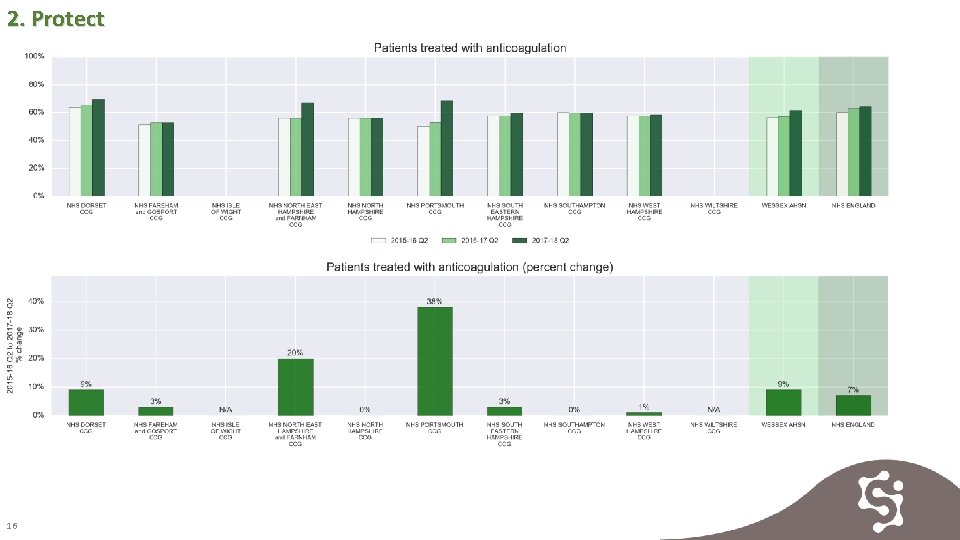
2. Protect 15

2. Protect 16

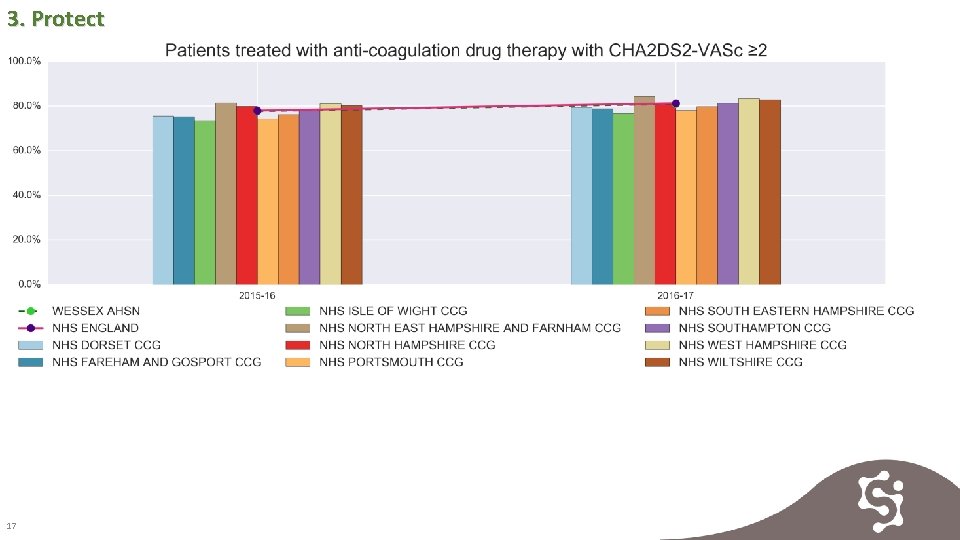
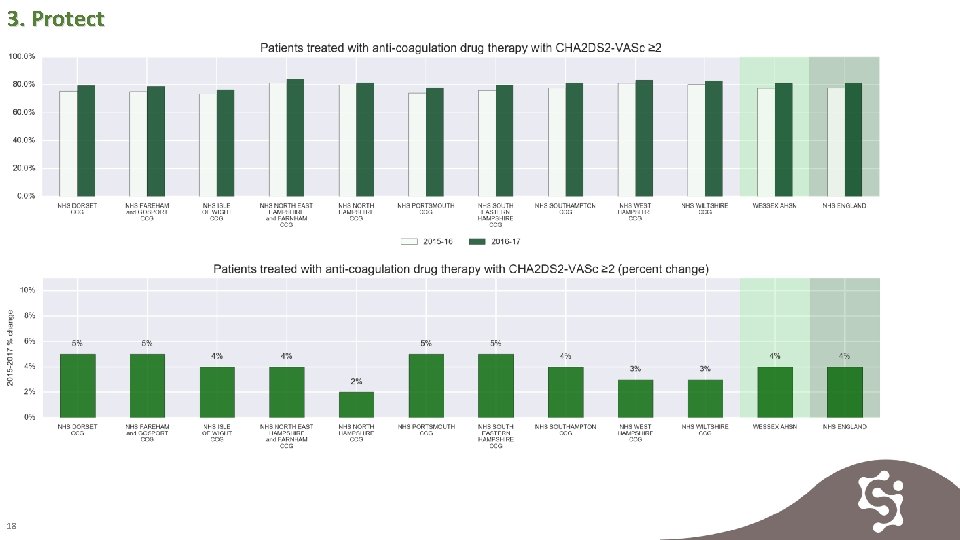
3. Protect 17

3. Protect 18

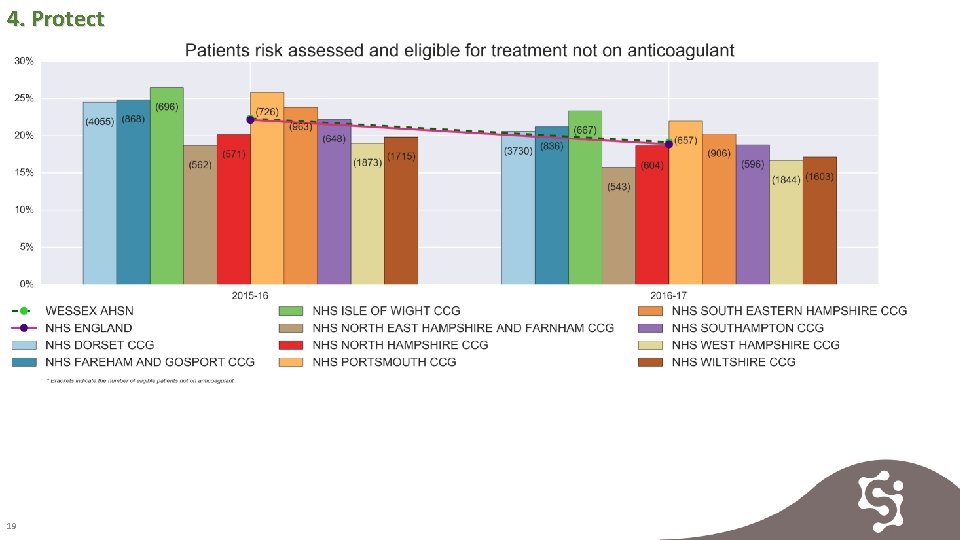
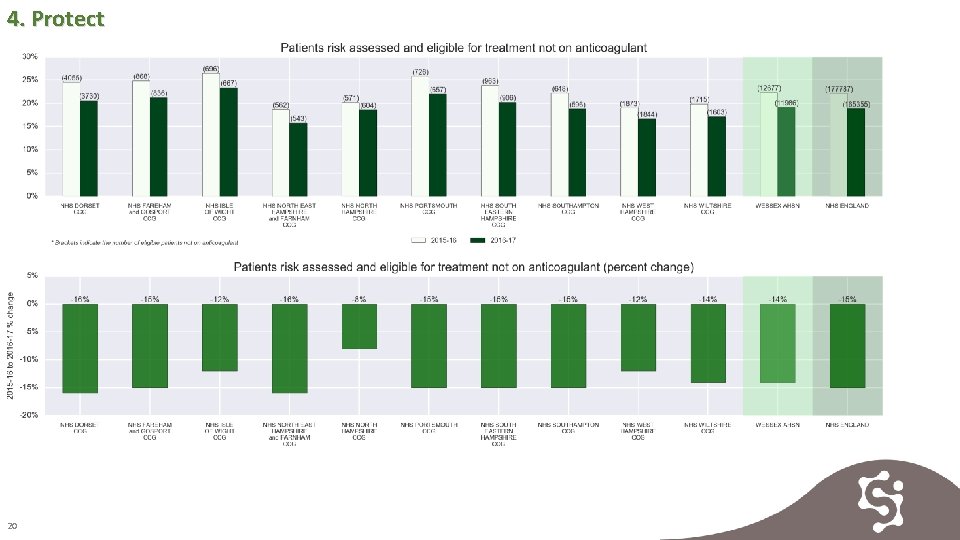
4. Protect 19

4. Protect 20

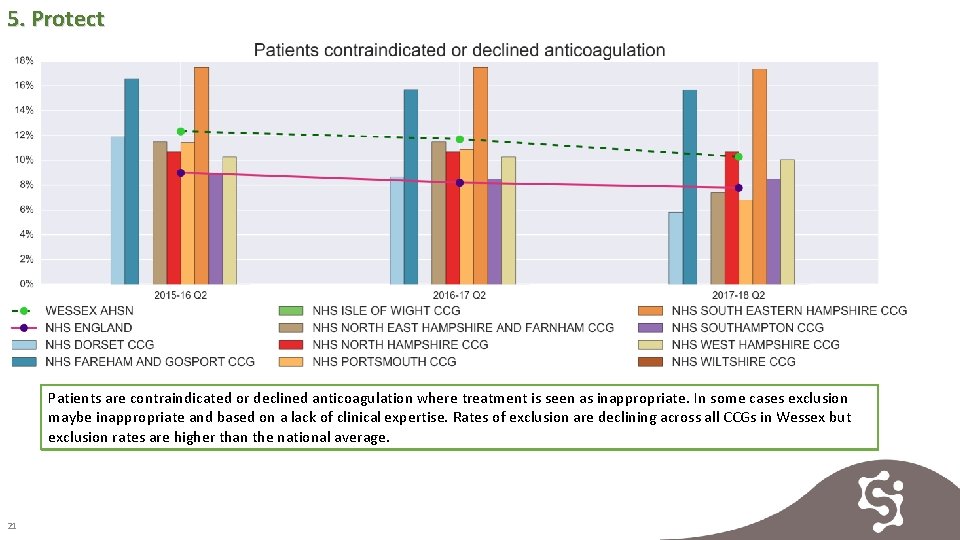
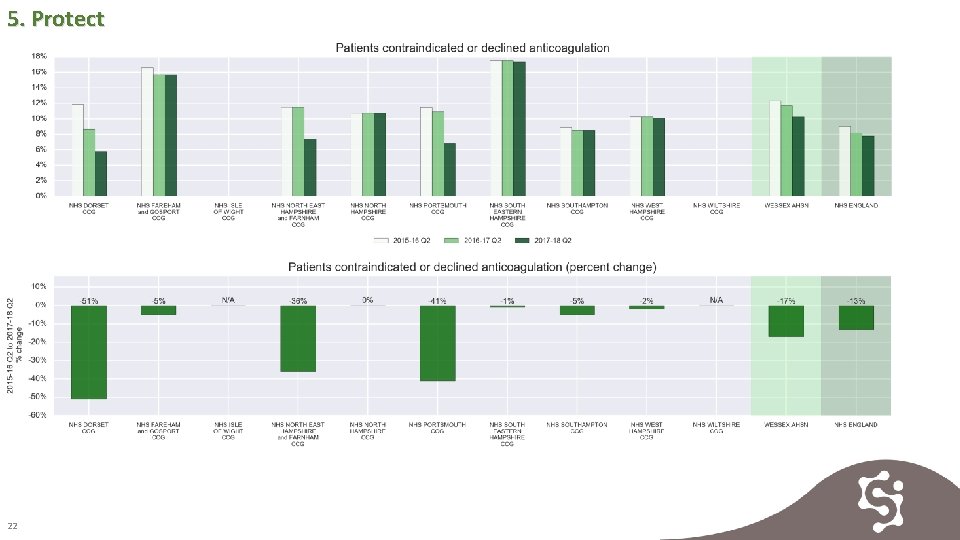
5. Protect Patients are contraindicated or declined anticoagulation where treatment is seen as inappropriate. In some cases exclusion maybe inappropriate and based on a lack of clinical expertise. Rates of exclusion are declining across all CCGs in Wessex but exclusion rates are higher than the national average. 21

5. Protect 22

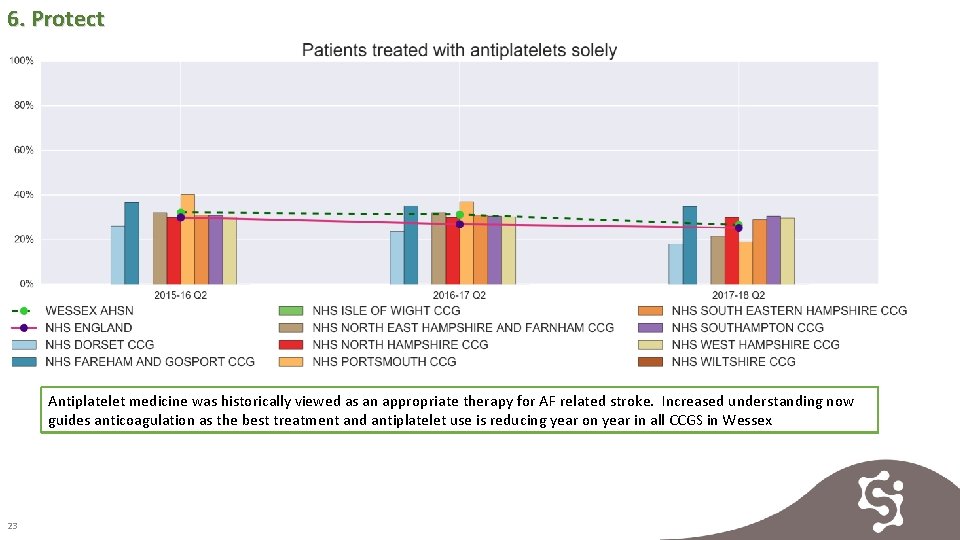
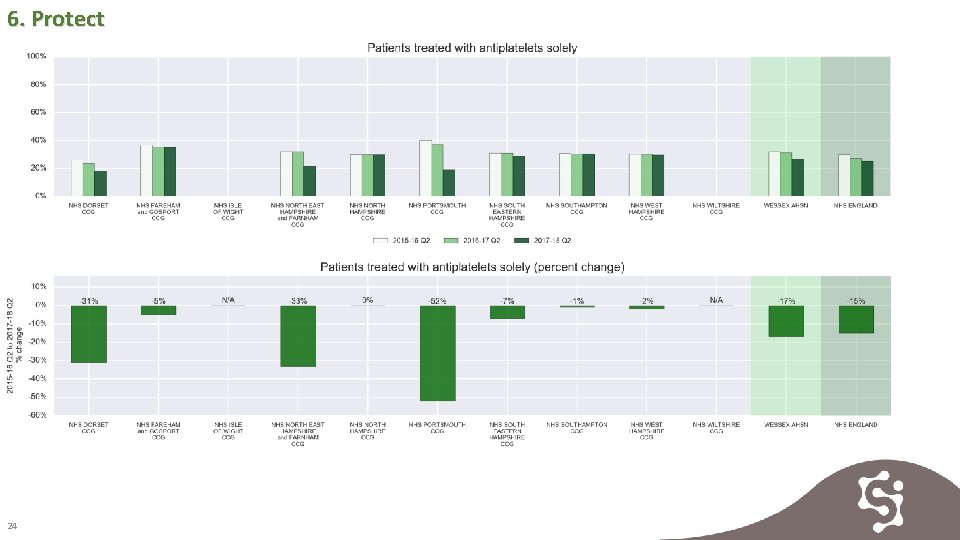
6. Protect Antiplatelet medicine was historically viewed as an appropriate therapy for AF related stroke. Increased understanding now guides anticoagulation as the best treatment and antiplatelet use is reducing year on year in all CCGS in Wessex 23

6. Protect 24

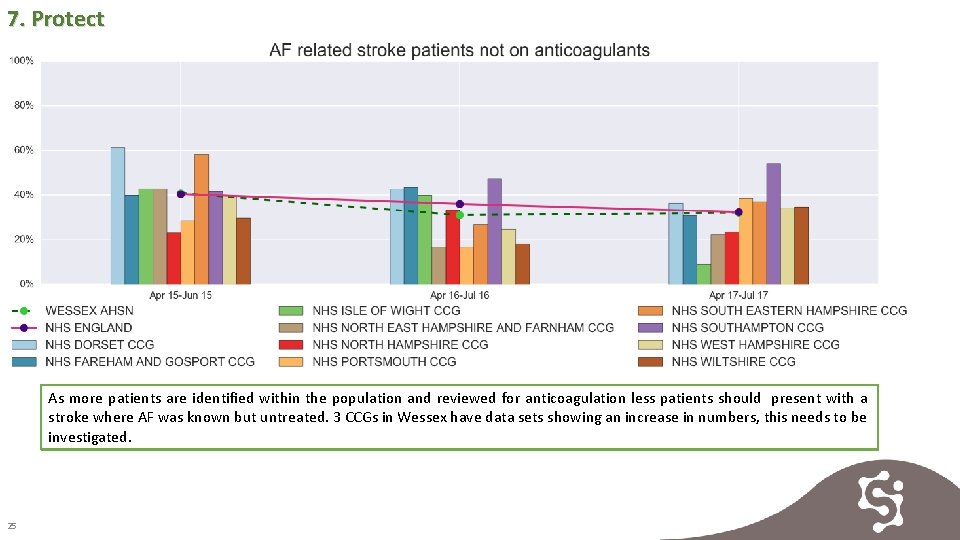
7. Protect As more patients are identified within the population and reviewed for anticoagulation less patients should present with a stroke where AF was known but untreated. 3 CCGs in Wessex have data sets showing an increase in numbers, this needs to be investigated. 25

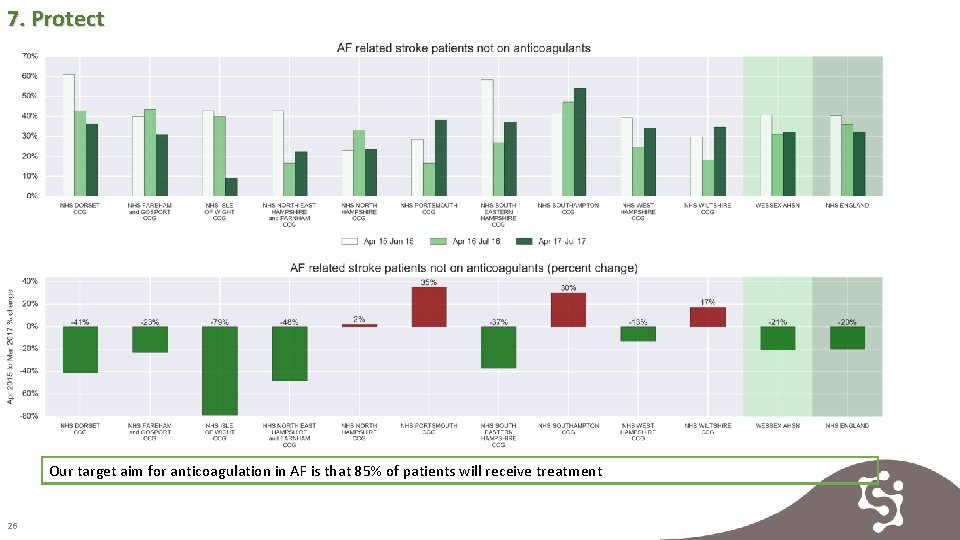
7. Protect Our target aim for anticoagulation in AF is that 85% of patients will receive treatment 26

PERFECT Perfection- Key Findings Between 30 -50% of patients do not take their medicines as intended. This results in up to £ 150 million or avoidable medicines waste in the NHS and poor patient outcomes. The New Medicines Service (NMS) provides support for people with long-term conditions newly prescribed anticoagulation to help improve medicines adherence. Increasing NMS discussions will increase adherence by at least 10% Evidence 1. Anticoagulant or antiplatelet MUR 2. Anticoagulant or antiplatelet NMS 3. Treated patients without adequate anticoagulation 27

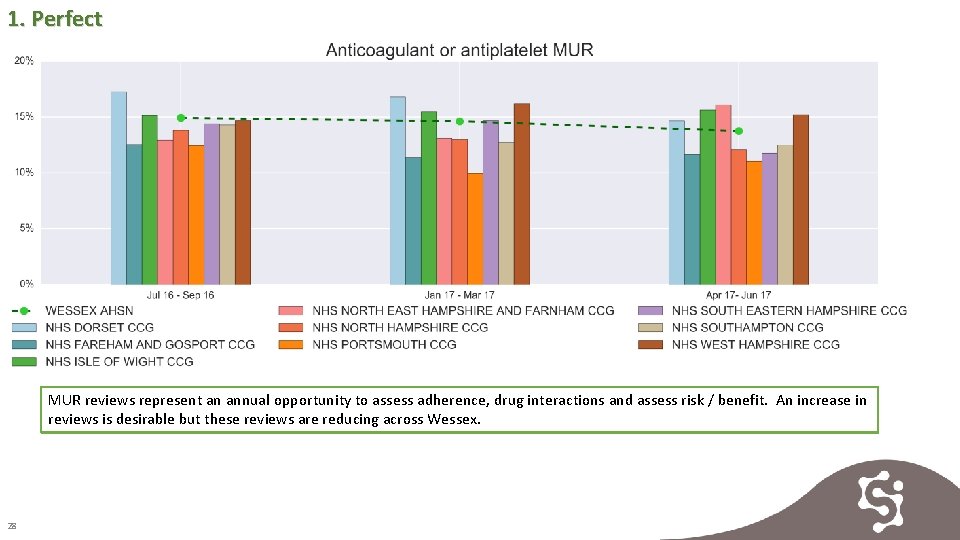
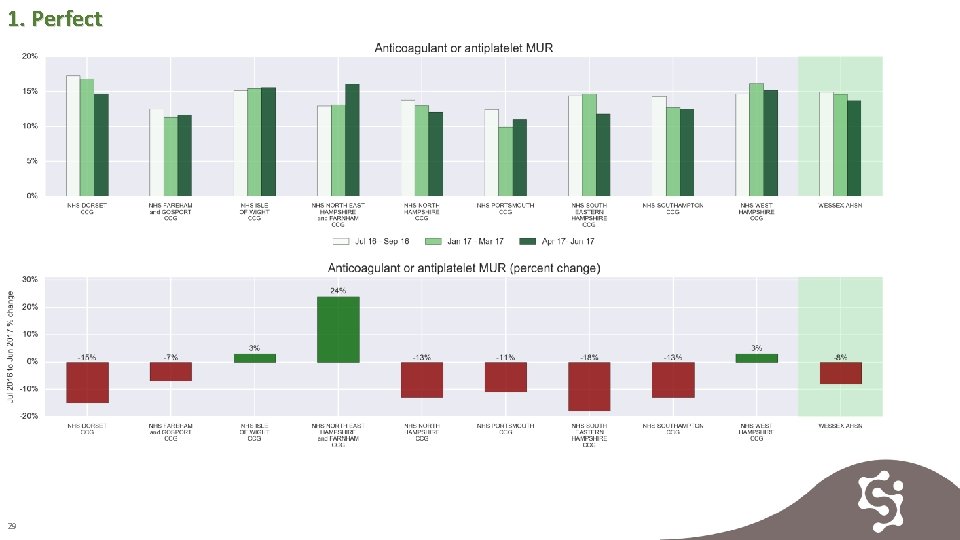
1. Perfect MUR reviews represent an annual opportunity to assess adherence, drug interactions and assess risk / benefit. An increase in reviews is desirable but these reviews are reducing across Wessex. 28

1. Perfect 29

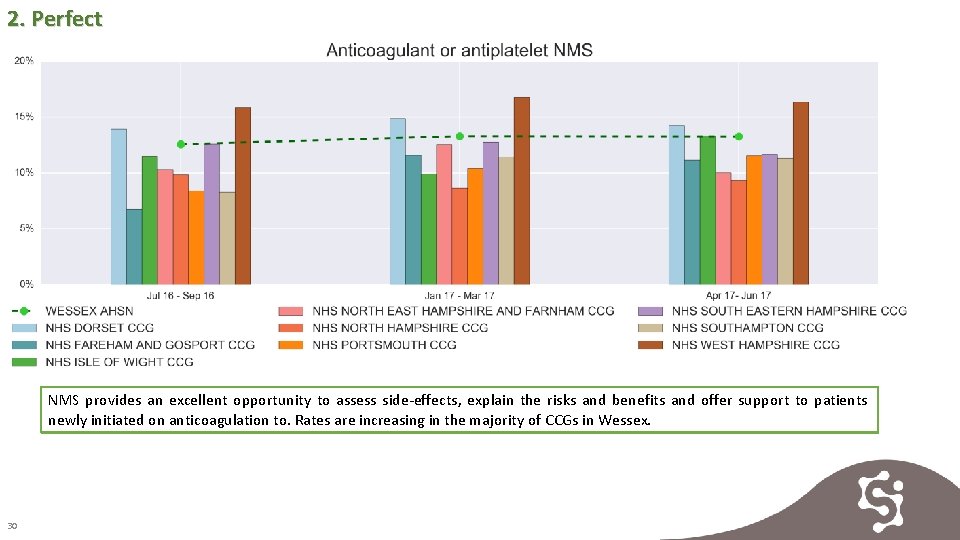
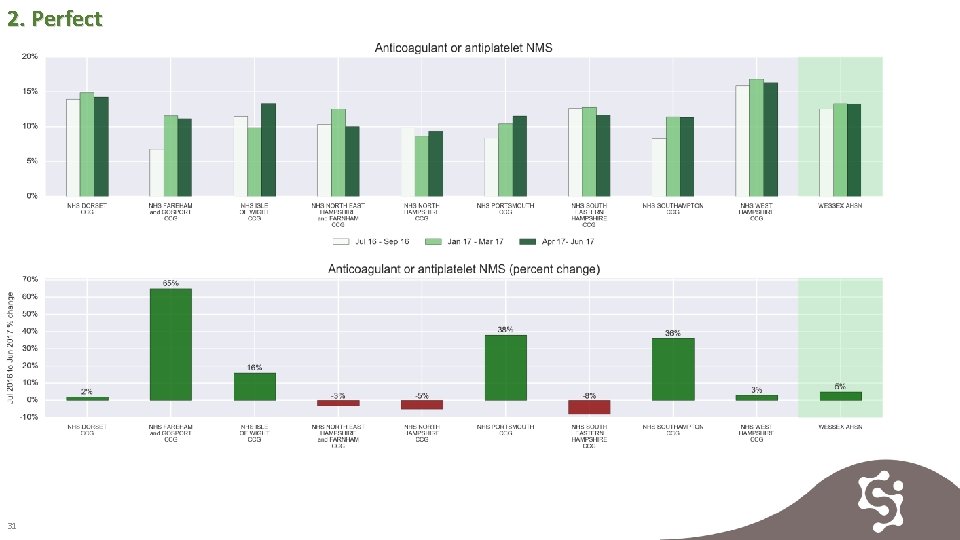
2. Perfect NMS provides an excellent opportunity to assess side-effects, explain the risks and benefits and offer support to patients newly initiated on anticoagulation to. Rates are increasing in the majority of CCGs in Wessex. 30

2. Perfect 31

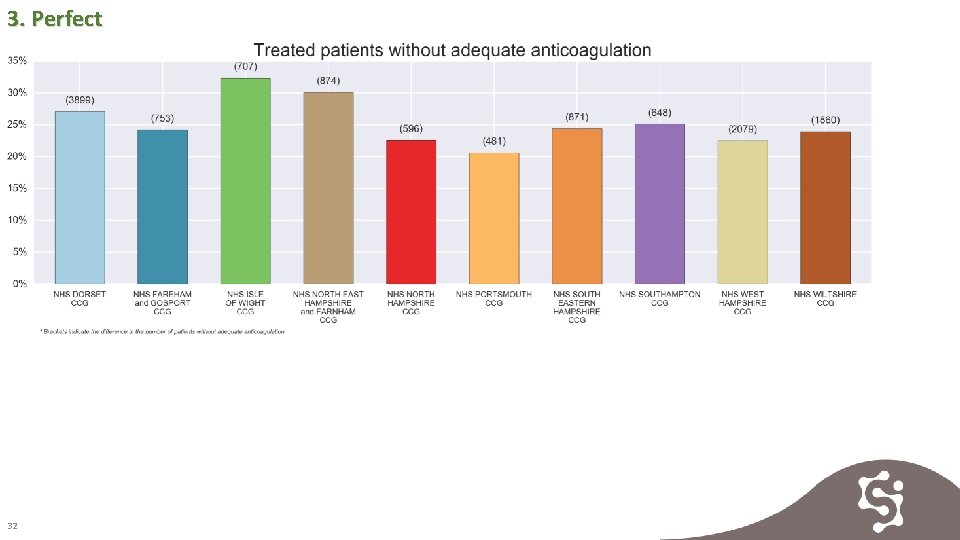
3. Perfect 32

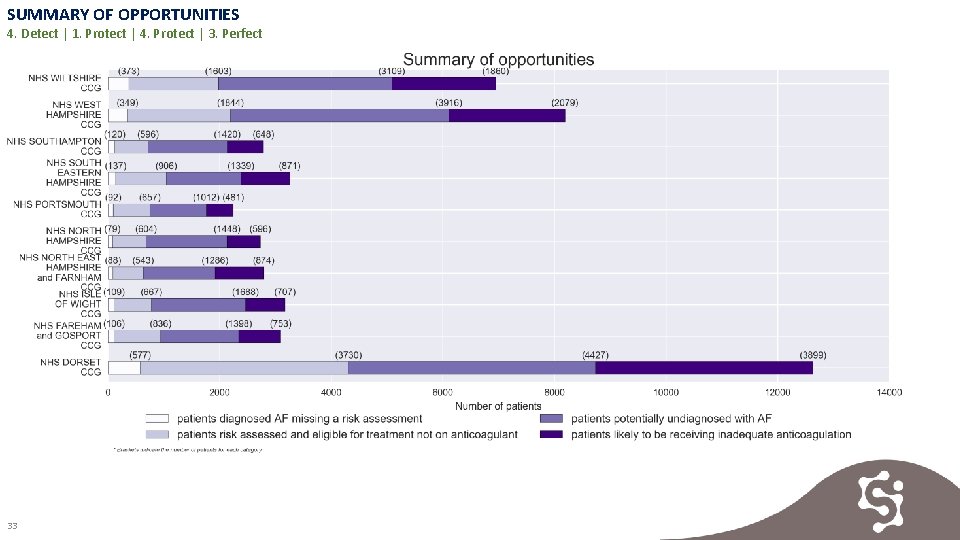
SUMMARY OF OPPORTUNITIES 4. Detect | 1. Protect | 4. Protect | 3. Perfect 33

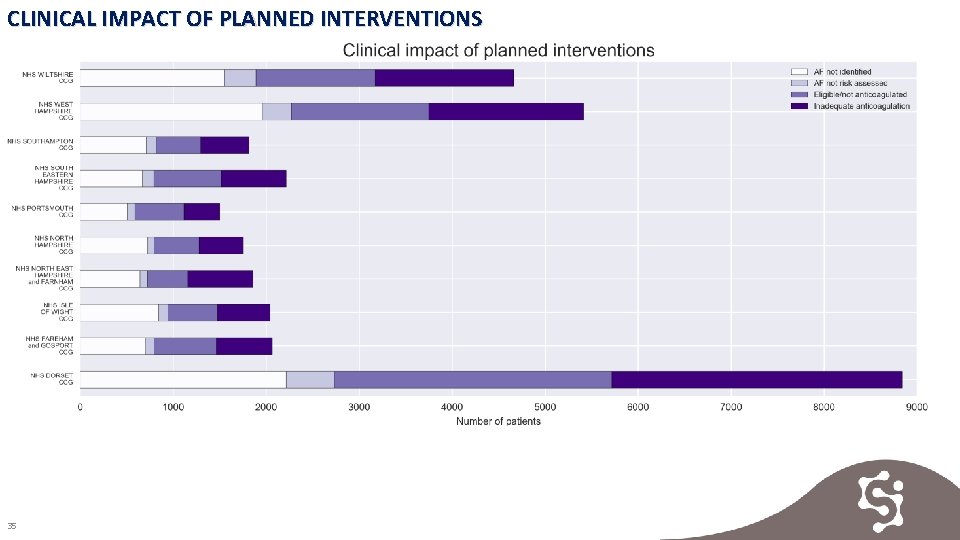
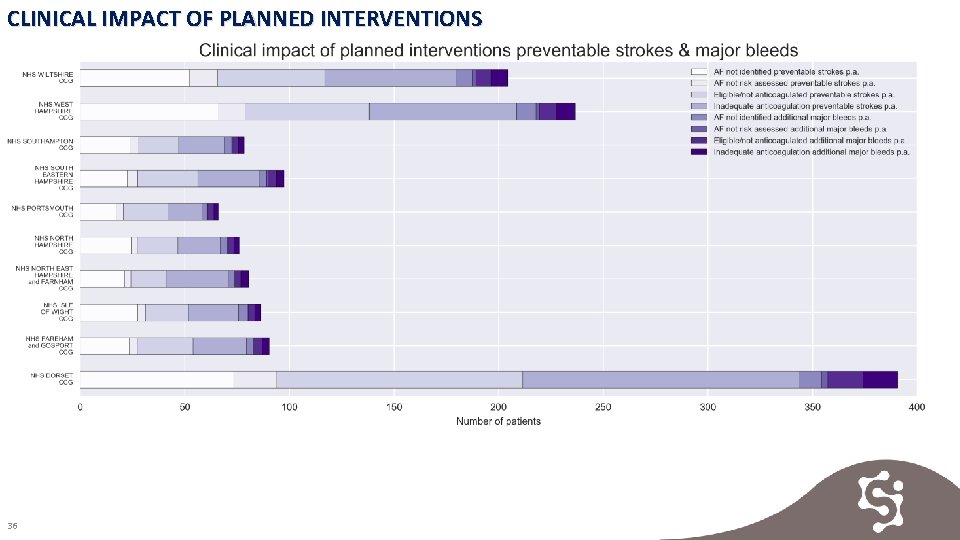
CLINICAL IMPACT OF PLANNED INTERVENTIONS Key Findings A potential total number of 32, 214 patients should be identified and treated across Wessex: • 10, 522 patients potentially undiagnosed with AF • 1, 829 patients diagnosed AF missing a risk assessment • 9, 589 patients assessed and eligible for treatment not on anticoagulant • 10, 274 patients likely to be receiving inadequate anticoagulation Evidence 1. Clinical impact of planned interventions 2. Clinical impact of planned interventions preventable strokes & major bleeds 34

CLINICAL IMPACT OF PLANNED INTERVENTIONS 35

CLINICAL IMPACT OF PLANNED INTERVENTIONS 36

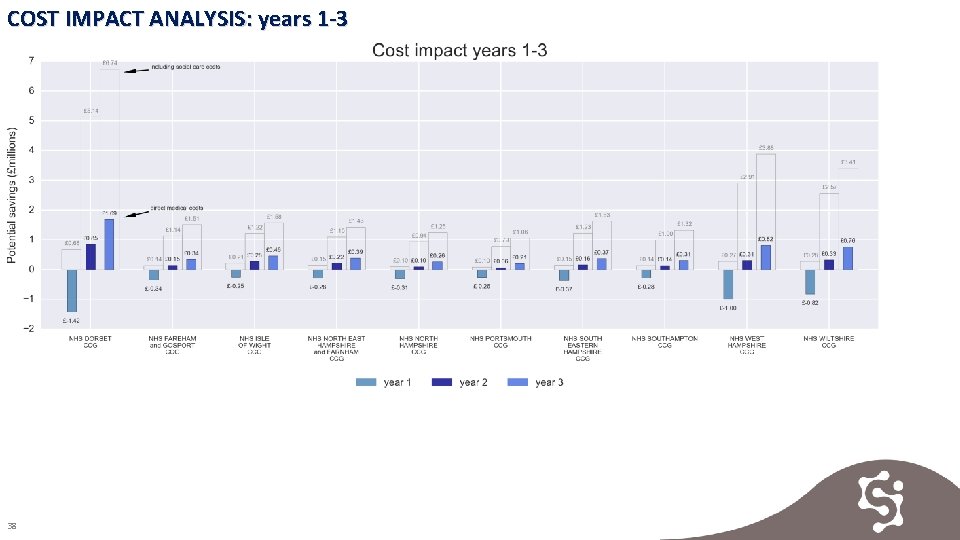
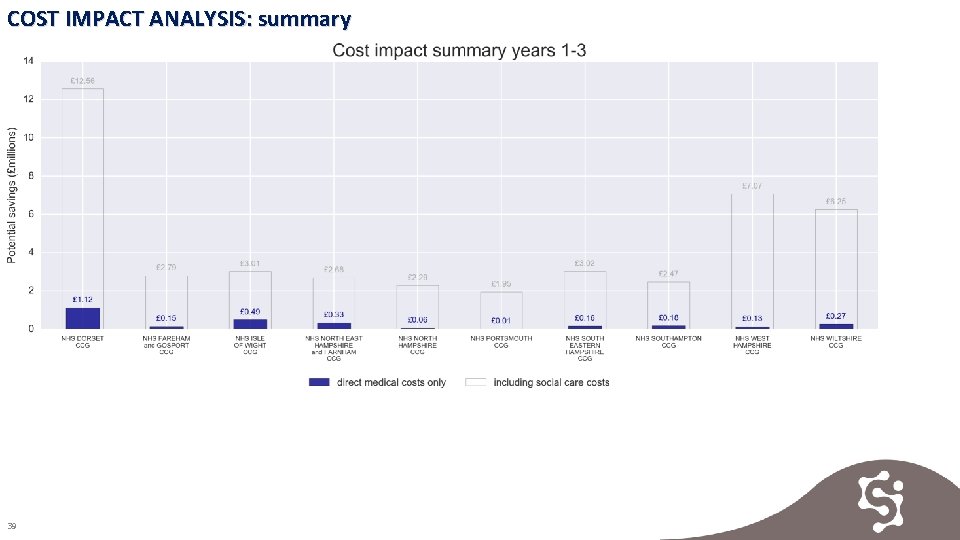
COST IMPACT ANALYSIS Key Findings Total potential 3 -year savings in direct medical costs of approximately £ 2. 9 m are achievable across Wessex. Total potential 3 -year savings including social care costs of approximately £ 44. 17 m are achievable across Wessex. Evidence 1. Cost impact analysis year 1 2. Cost impact analysis year 2 3. Cost impact analysis year 3 4. Cost impact analysis years 1 -3 5. Cost impact analysis summary 37

COST IMPACT ANALYSIS: years 1 -3 38

COST IMPACT ANALYSIS: summary 39

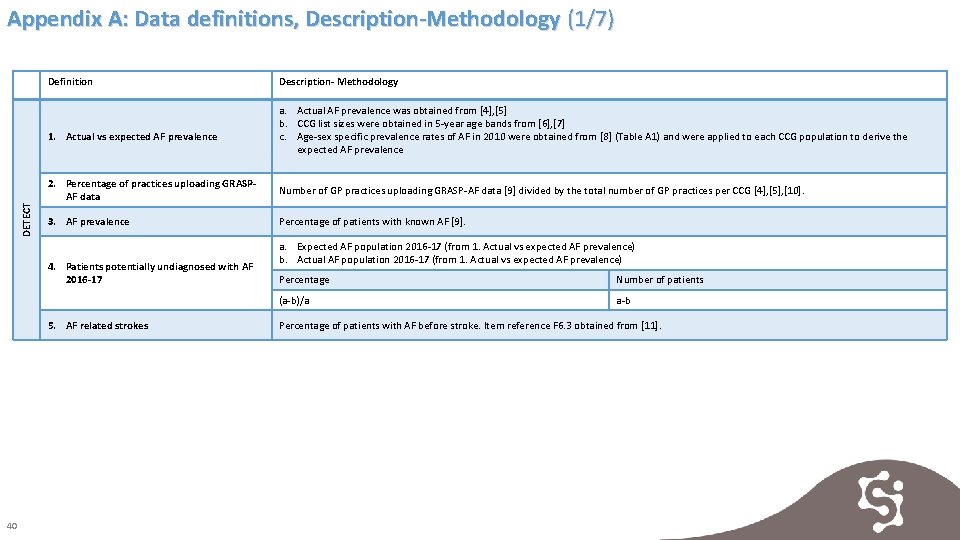
Appendix A: Data definitions, Description-Methodology (1/7) Definition DETECT 1. Actual vs expected AF prevalence a. Actual AF prevalence was obtained from [4], [5] b. CCG list sizes were obtained in 5 -year age bands from [6], [7] c. Age-sex specific prevalence rates of AF in 2010 were obtained from [8] (Table A 1) and were applied to each CCG population to derive the expected AF prevalence 2. Percentage of practices uploading GRASPAF data Number of GP practices uploading GRASP-AF data [9] divided by the total number of GP practices per CCG [4], [5], [10]. 3. AF prevalence Percentage of patients with known AF [9]. 4. Patients potentially undiagnosed with AF 2016 -17 5. AF related strokes 40 Description- Methodology a. Expected AF population 2016 -17 (from 1. Actual vs expected AF prevalence) b. Actual AF population 2016 -17 (from 1. Actual vs expected AF prevalence) Percentage Number of patients (a-b)/a a-b Percentage of patients with AF before stroke. Item reference F 6. 3 obtained from [11].

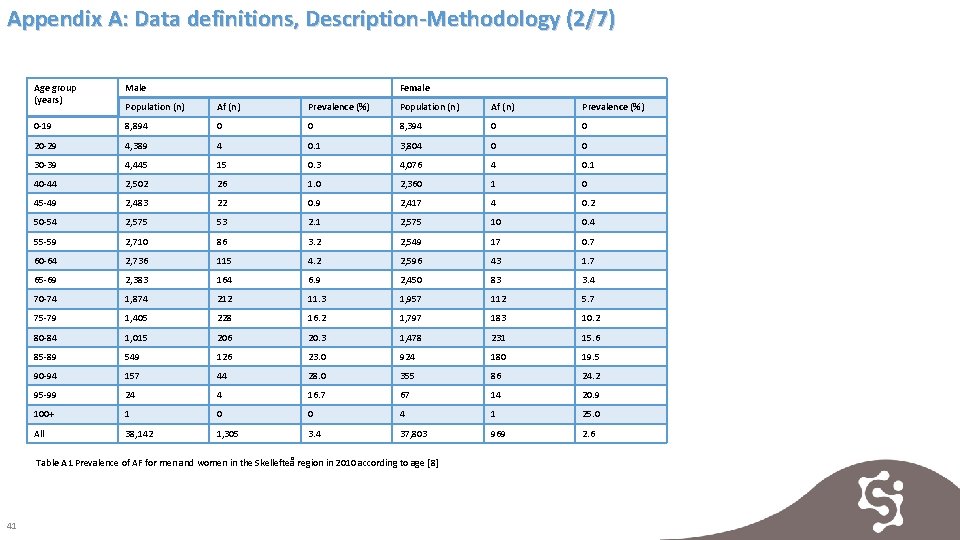
Appendix A: Data definitions, Description-Methodology (2/7) Age group (years) Male Female Population (n) Af (n) Prevalence (%) 0 -19 8, 894 0 0 8, 394 0 0 20 -29 4, 389 4 0. 1 3, 804 0 0 30 -39 4, 445 15 0. 3 4, 076 4 0. 1 40 -44 2, 502 26 1. 0 2, 360 1 0 45 -49 2, 483 22 0. 9 2, 417 4 0. 2 50 -54 2, 575 53 2. 1 2, 575 10 0. 4 55 -59 2, 710 86 3. 2 2, 549 17 0. 7 60 -64 2, 736 115 4. 2 2, 596 43 1. 7 65 -69 2, 383 164 6. 9 2, 450 83 3. 4 70 -74 1, 874 212 11. 3 1, 957 112 5. 7 75 -79 1, 405 228 16. 2 1, 797 183 10. 2 80 -84 1, 015 206 20. 3 1, 478 231 15. 6 85 -89 549 126 23. 0 924 180 19. 5 90 -94 157 44 28. 0 355 86 24. 2 95 -99 24 4 16. 7 67 14 20. 9 100+ 1 0 0 4 1 25. 0 All 38, 142 1, 305 3. 4 37, 803 969 2. 6 Table A 1 Prevalence of AF for men and women in the Skellefteå region in 2010 according to age [8] 41

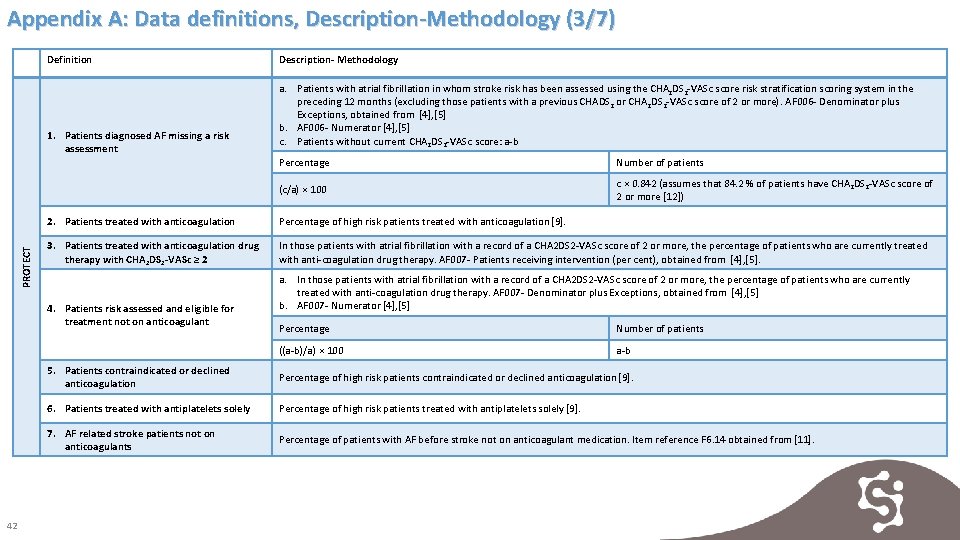
Appendix A: Data definitions, Description-Methodology (3/7) Definition PROTECT 1. Patients diagnosed AF missing a risk assessment a. Patients with atrial fibrillation in whom stroke risk has been assessed using the CHA 2 DS 2 -VASc score risk stratification scoring system in the preceding 12 months (excluding those patients with a previous CHADS 2 or CHA 2 DS 2 -VASc score of 2 or more). AF 006 - Denominator plus Exceptions, obtained from [4], [5] b. AF 006 - Numerator [4], [5] c. Patients without current CHA 2 DS 2 -VASc score: a-b Percentage Number of patients (c/a) × 100 c × 0. 842 (assumes that 84. 2 % of patients have CHA 2 DS 2 -VASc score of 2 or more [12]) 2. Patients treated with anticoagulation Percentage of high risk patients treated with anticoagulation [9]. 3. Patients treated with anticoagulation drug therapy with CHA 2 DS 2 -VASc ≥ 2 In those patients with atrial fibrillation with a record of a CHA 2 DS 2 -VASc score of 2 or more, the percentage of patients who are currently treated with anti-coagulation drug therapy. AF 007 - Patients receiving intervention (per cent), obtained from [4], [5]. 4. Patients risk assessed and eligible for treatment not on anticoagulant 42 Description- Methodology a. In those patients with atrial fibrillation with a record of a CHA 2 DS 2 -VASc score of 2 or more, the percentage of patients who are currently treated with anti-coagulation drug therapy. AF 007 - Denominator plus Exceptions, obtained from [4], [5] b. AF 007 - Numerator [4], [5] Percentage Number of patients ((a-b)/a) × 100 a-b 5. Patients contraindicated or declined anticoagulation Percentage of high risk patients contraindicated or declined anticoagulation [9]. 6. Patients treated with antiplatelets solely Percentage of high risk patients treated with antiplatelets solely [9]. 7. AF related stroke patients not on anticoagulants Percentage of patients with AF before stroke not on anticoagulant medication. Item reference F 6. 14 obtained from [11].

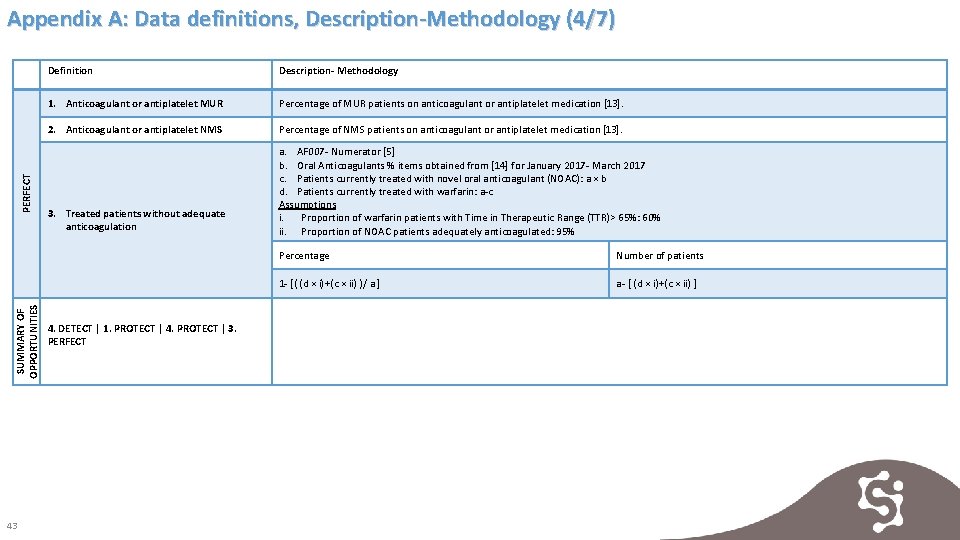
SUMMARY OF OPPORTUNITIES PERFECT Appendix A: Data definitions, Description-Methodology (4/7) 43 Definition Description- Methodology 1. Anticoagulant or antiplatelet MUR Percentage of MUR patients on anticoagulant or antiplatelet medication [13]. 2. Anticoagulant or antiplatelet NMS Percentage of NMS patients on anticoagulant or antiplatelet medication [13]. 3. Treated patients without adequate anticoagulation a. AF 007 - Numerator [5] b. Oral Anticoagulants % items obtained from [14] for January 2017 - March 2017 c. Patients currently treated with novel oral anticoagulant (NOAC): a × b d. Patients currently treated with warfarin: a-c Assumptions i. Proportion of warfarin patients with Time in Therapeutic Range (TTR)> 65%: 60% ii. Proportion of NOAC patients adequately anticoagulated: 95% 4. DETECT | 1. PROTECT | 4. PROTECT | 3. PERFECT Percentage Number of patients 1 - [( (d × i)+(c × ii) )/ a ] a- [ (d × i)+(c × ii) ]

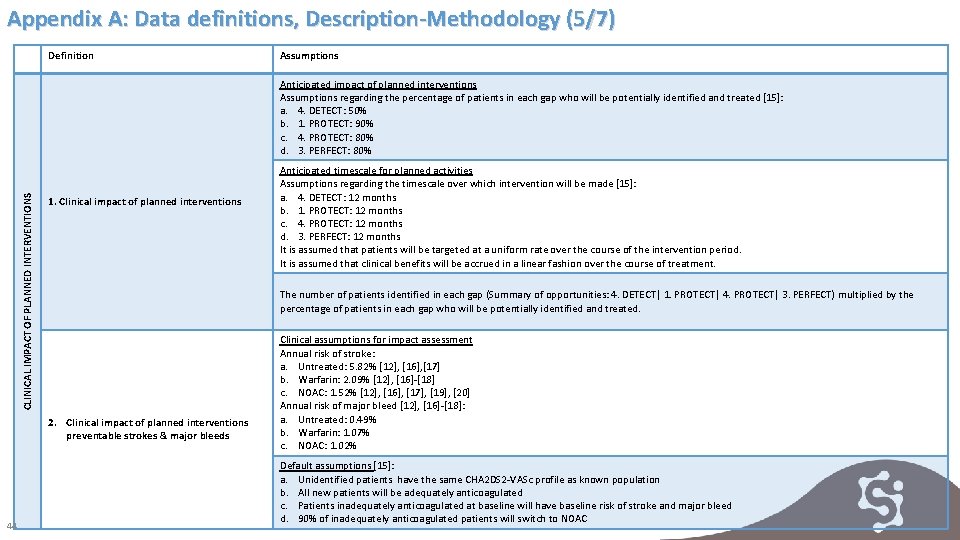
Appendix A: Data definitions, Description-Methodology (5/7) Definition Assumptions CLINICAL IMPACT OF PLANNED INTERVENTIONS Anticipated impact of planned interventions Assumptions regarding the percentage of patients in each gap who will be potentially identified and treated [15]: a. 4. DETECT: 50% b. 1. PROTECT: 90% c. 4. PROTECT: 80% d. 3. PERFECT: 80% 1. Clinical impact of planned interventions The number of patients identified in each gap (Summary of opportunities: 4. DETECT| 1. PROTECT| 4. PROTECT| 3. PERFECT) multiplied by the percentage of patients in each gap who will be potentially identified and treated. 2. Clinical impact of planned interventions preventable strokes & major bleeds 44 Anticipated timescale for planned activities Assumptions regarding the timescale over which intervention will be made [15]: a. 4. DETECT: 12 months b. 1. PROTECT: 12 months c. 4. PROTECT: 12 months d. 3. PERFECT: 12 months It is assumed that patients will be targeted at a uniform rate over the course of the intervention period. It is assumed that clinical benefits will be accrued in a linear fashion over the course of treatment. Clinical assumptions for impact assessment Annual risk of stroke: a. Untreated: 5. 82% [12], [16], [17] b. Warfarin: 2. 09% [12], [16]-[18] c. NOAC: 1. 52% [12], [16], [17], [19], [20] Annual risk of major bleed [12], [16]-[18]: a. Untreated: 0. 49% b. Warfarin: 1. 07% c. NOAC: 1. 02% Default assumptions [15]: a. Unidentified patients have the same CHA 2 DS 2 -VASc profile as known population b. All new patients will be adequately anticoagulated c. Patients inadequately anticoagulated at baseline will have baseline risk of stroke and major bleed d. 90% of inadequately anticoagulated patients will switch to NOAC

Appendix A: Data definitions, Description-Methodology (6/7) COST IMPACT ANALYSIS Cost-inputs Cost inputs for impact assessment Year 1 cost of stroke care: £ 12, 228 [12], [17], [21] Year 2+ cost of stroke care: £ 2, 430 [12], [17], [21] Cost of major bleed: £ 1, 173 [12], [17] Cost of screening for AF (per patient screened): £ 16. 34 [22] Annual cost of treatment for Warfarin (drug cost): £ 41. 32 [12] Annual cost of treatment for Warfarin (monitoring): £ 242 [12], [17] Annual cost of treatment for NOAC: £ 664. 06 [14], [23] Social care cost estimation Stroke savings including social care costs calculations were based on the ratio of direct care costs to social care costs as reported in [21]. Assumptions- Limitations Planned future changes to NOAC use [15] Projected percentage of NOAC use for year 1, year 2 and year 3 for new patients was assumed to be equal to the Oral Anticoagulants % items for each CCG obtained from [14] for January 2017 - March 2017. Projected percentage of NOAC use for each CCG for year 1, year 2 and year 3 for patients inadequately anticoagulated was assumed to be 90%. The impact of switching therapy for existing stable patients is not taken into consideration. Year 1, year 2 and year 3 cost impact analysis Expected AF not identified costs are subject to considerable uncertainty due to the fact that expected AF was calculated using age-sex specific prevalence rates of AF in 2010 [8] (Table A 1). 45

Appendix A: Data definitions, Description-Methodology (7/7) COST IMPACT ANALYSIS Cost impact calculation 46 4. Detect. Patients potentially undiagnosed with AF are used to evaluate [15]: a. The cost of screening for patients in the current population (>65 years of age) b. The additional cost of treating the newly identified patients at year 1, year 2 and year 3, based on the current warfarin/NOAC use c. The total cost of a major bleed in patients started on anticoagulant d. The cost impact on improved stroke prevention at year 1, year 2 and year 3, if all patients were to receive effective anticoagulation 1. Protect. Patients diagnosed AF missing a risk assessment [15]: a. The additional cost of treating the newly risk-assessed patients who qualify for anticoagulation b. The total cost of a major bleed in patients started on anticoagulant c. The cost impact on improved stroke prevention at year 1, year 2 and year 3, if all patients were to receive effective anticoagulation, and continue on treatment with adequate compliance 4. Protect. Patients assessed and eligible for treatment not on anticoagulant [15]: a. The anticipated additional year 1 cost of treating patients who are not currently anticoagulated. This assumes warfarin and NOAC treatment patterns remain consistent with the currently treated local AF population. The initiation of NOAC therapies in patients who have previously declined warfarin are accounted for in the population b. The total anticipated cost of a major bleed in patients started on anticoagulant c. The potential cost impact on improved stroke prevention at years 1, 2 and 3, if all patients were to receive effective anticoagulation and continue on treatment with adequate compliance. 3. Perfect. Treated patients without adequate anticoagulation [15]: a. The anticipated additional cost of changing treatment in inadequately treated patients. This assumes 25% of warfarin-treated patients will switch to a NOAC and 20% will increase their warfarin costs for the subsequent year to reflect a higher dose b. The total anticipated cost of a major bleed in patients started on adequate anticoagulation. It is assumed that inadequately treated patients will have had the same risk of bleed as an untreated patient c. The potential cost impact on improved stroke prevention at years 1, 2 and 3, if all patients were to receive effective anticoagulation, and continue on treatment with adequate compliance. It is assumed that inadequately treated patients will have the same risk of stroke as an untreated patient

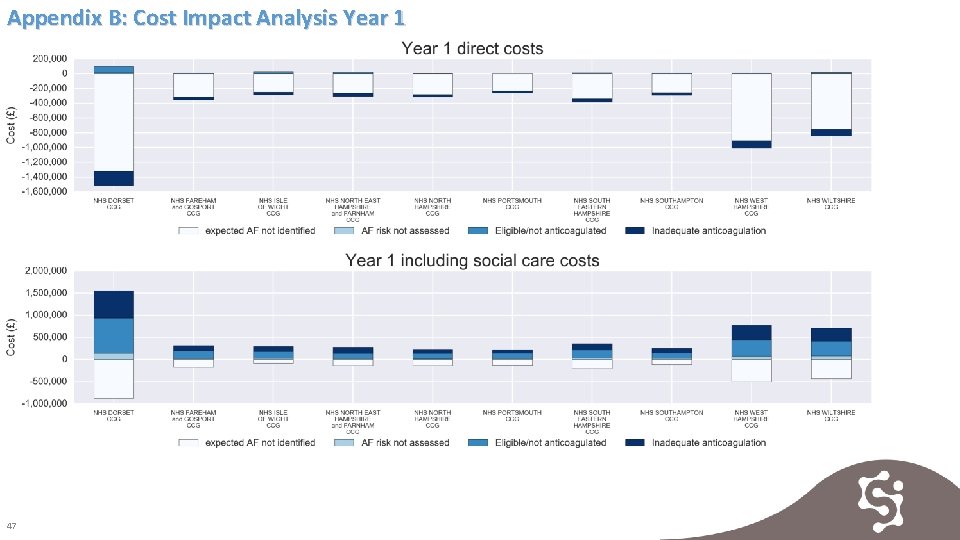
Appendix B: Cost Impact Analysis Year 1 47

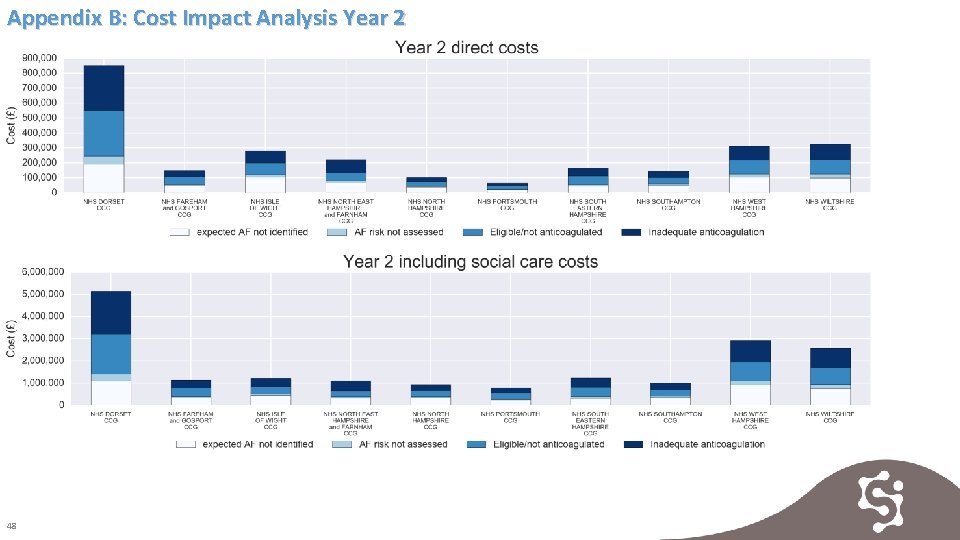
Appendix B: Cost Impact Analysis Year 2 48

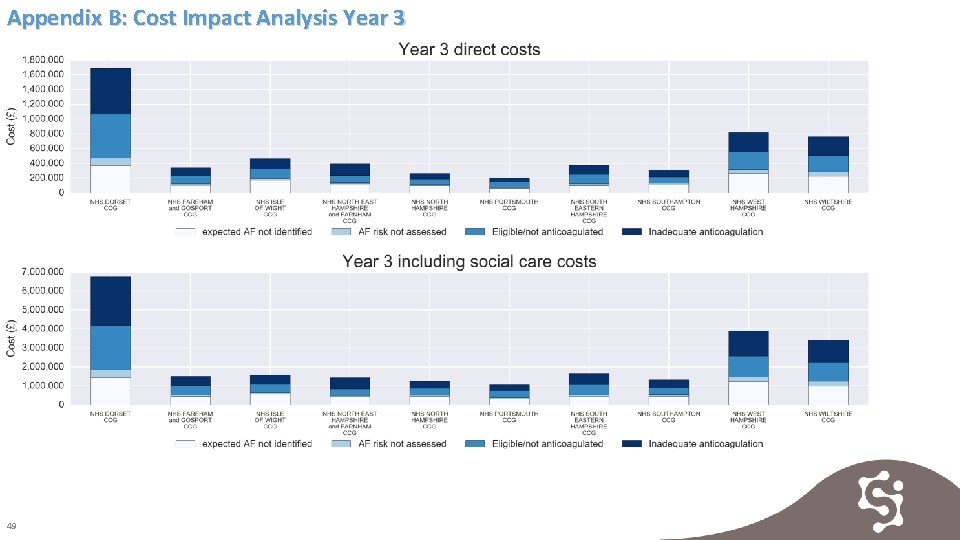
Appendix B: Cost Impact Analysis Year 3 49
![References 1 NHS Improving Quality Costs and benefits of Antithrombotic Therapy in Atrial Fibrillation References [1] NHS Improving Quality. Costs and benefits of Antithrombotic Therapy in Atrial Fibrillation](https://slidetodoc.com/presentation_image/124960b6bfc8b8e62e333c9944261ec1/image-51.jpg)
References [1] NHS Improving Quality. Costs and benefits of Antithrombotic Therapy in Atrial Fibrillation in England: An economic Analysis Based on Grasp AF. March 2015. Available from: http: //webarchive. nationalarchives. gov. uk/20150317173220/http: //www. nhsiq. nhs. uk/media/2566025/af_economic_analysis_final. pdf [2] Wessex Academic Health Science Network. Atrial Fibrillation Programme Newsletter. January 2017. Available from: http: //wessexahsn. org. uk/img/programmes/Atrial%20 Fibrillation%20 Programme%20 Winter%20 Newsletter%20 for%20 circulation. pdf [3] Anticoagulation Europe (UK), Atrial Fibrillation Association. The AF Report-Atrial Fibrillation: Preventing A Stroke Crisis. 2011. Available from: http: //www. preventafstrokecrisis. org/files/The%20 AF%20 Report%2014%20 April%202012. pdf [4] NHS Digital. Quality and Outcomes Framework (QOF)-2015 -2016. Accessed November 2017. Available from: http: //digital. nhs. uk/catalogue/PUB 22266 [5] NHS Digital. Quality and Outcomes Framework (QOF)-2016 -2017. Accessed November 2017. Available from: http: //digital. nhs. uk/catalogue/PUB 30124 [6] NHS Digital. Number of Patients Registered at a GP Practice (practice level, 5 year age groups) - April 2016. Accessed November 2017. Available from: http: //digital. nhs. uk/catalogue/PUB 20480 [7] NHS Digital. Number of Patients Registered at a GP Practice (practice level, 5 year age groups) - April 2017. Accessed November 2017. Available from: http: //digital. nhs. uk/catalogue/PUB 23475 [8] Norberg J, Bäckström S, Jansson J-H, Johansson L. Estimating the prevalence of atrial fibrillation in a general population using validated electronic health data. Clinical Epidemiology. 2013; 5: 475 -481. doi: 10. 2147/CLEP. S 53420. [9] PRIMIS and NHS England. Guidance on Risk Assessment and Stroke Prevention in Atrial Fibrilation (GRASP-AF) tool. Data extract received in September 2017: http: //www. nottingham. ac. uk/primis/tools-audits/toolsaudits/grasp-af. aspx [10] NHS Digital. Quality and Outcomes Framework (QOF)-2014 -2015. Accessed November 2017. Available from: http: //digital. nhs. uk/catalogue/PUB 18887 [11] Royal College of Physicians. Sentinel Stroke National Audit Programme (SSNAP), Clinical Audit. Accessed November 2017. Available from: https: //www. strokeaudit. org/results/Clinical-audit/Clinical-CCG-LHB-LCG. aspx [12] National Institute for Health and Care Excellence. Atrial fibrillation: management - Clinical guideline (CG 180) - costing template. June 2014. Available from: https: //www. nice. org. uk/guidance/cg 180/resources/costingtemplate-243732205 [13] NHS Business Authority. Medicines Use Review (MUR)/New Medicine Services (NMS) quarterly submission. Data extract received in August 2017. Data submission details available at: https: //www. nhsbsa. nhs. uk/pharmacies-gp-practices-and-appliance-contractors/dispensing-contractors-information/medicines-use [14] NHS Business Services Authority. Medicines Optimisation CCG Dashboard. Accessed November 2017. Available from: https: //apps. nhsbsa. nhs. uk/MOD/Atlas. CCGMeds. Op/atlas. html [15] Greater Manchester Academic Health Science Network, JB Medical, Public Health England. Atrial Fibrillation Budget Impact Model. Accessed November 2017. Available from: http: //www. gmahsn. org/documents/23650/0/AF+Business+Case+Model+2016/94 ed 3 df 3 -d 2 be-66 dd-a 1 d 4 -0 a 9442 e 8 e 8 f 8 [16] National Institute for Health and Care Excellence. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation (TA 249) - costing template. March 2012. Available at: https: //www. nice. org. uk/guidance/ta 249/resources/costing-template-424946701 [17] National Institute for Health and Care Excellence. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation (TA 256) - costing template. March 2012. Available at: https: //www. nice. org. uk/guidance/ta 256/resources/costing-template-425082781 [18] Hart R, Pearce L, Aquilar M. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857 -67 [19] Patel M, Mahaffey K, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883 -91 [20] Connolly S, Ezekowitz M, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139 -51 [21] Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003; 21 Suppl 1: 43 -50 [22] Hobbs F, Fitzmaurice D, Mant J et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005; 9: 1 -74 [23] British Medical Association and Royal Pharmaceutical Society. BNF 72 September 2016 -March 2017
 Detect protect perfect
Detect protect perfect Detect protect perfect
Detect protect perfect Factors of care that patients can expect to receive
Factors of care that patients can expect to receive Postoperative nursing diagnosis of cataract
Postoperative nursing diagnosis of cataract Nursing diagnosis for undescended testis
Nursing diagnosis for undescended testis Sengstaken blakemore tube nursing care
Sengstaken blakemore tube nursing care Chapter 58 care of patients with liver problems
Chapter 58 care of patients with liver problems Chapter 55 care of patients with stomach disorders
Chapter 55 care of patients with stomach disorders Levels of care primary secondary tertiary
Levels of care primary secondary tertiary Past perfect present perfect future perfect
Past perfect present perfect future perfect Peptídeo natriurético atrial
Peptídeo natriurético atrial Lvh criteria cornell
Lvh criteria cornell Dc cardioversion
Dc cardioversion Tatalaksana atrial fibrilasi
Tatalaksana atrial fibrilasi Atrial fibrilation ecg
Atrial fibrilation ecg Peptídeo natriurético atrial
Peptídeo natriurético atrial Ventricular escape rhythm
Ventricular escape rhythm Klasifikasi chf menurut nyha
Klasifikasi chf menurut nyha 300 150 100 ekg
300 150 100 ekg Chapter 6 atrial dysrhythmias
Chapter 6 atrial dysrhythmias Ecg tracing
Ecg tracing Left atrial hypertrophy ecg
Left atrial hypertrophy ecg Ekg axis
Ekg axis Atrial systole
Atrial systole Right atrial abnormality ecg
Right atrial abnormality ecg Atriclip mri safety
Atriclip mri safety Batimento de escape atrial
Batimento de escape atrial Tubular secretion in nephron
Tubular secretion in nephron Sobrecarga atrial direita
Sobrecarga atrial direita Atrial diastole
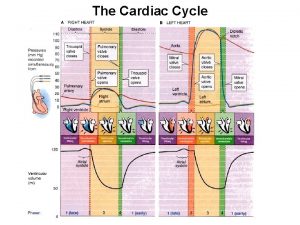
Atrial diastole Cardiac cycle class 11
Cardiac cycle class 11 Cardiac distension
Cardiac distension Atrial systole
Atrial systole Atrial fiberation
Atrial fiberation Na+ equilibrium potential
Na+ equilibrium potential Pathway hiperkalemia
Pathway hiperkalemia Pathway hiperkalemia
Pathway hiperkalemia Atrial repolarization
Atrial repolarization Valva mitrala inchidere incompleta
Valva mitrala inchidere incompleta Block av độ 1
Block av độ 1 Atrial systole
Atrial systole Atrial repolarization
Atrial repolarization Nursing management of atrial septal defect
Nursing management of atrial septal defect Wiggers diagram heart
Wiggers diagram heart Atrial septal defect
Atrial septal defect Ra rl la ll
Ra rl la ll Right atrial appendage
Right atrial appendage Cpt code for left atrial appendage ligation with atriclip
Cpt code for left atrial appendage ligation with atriclip Medical ppt blogspot
Medical ppt blogspot Endocrine parts
Endocrine parts Ccs afib guidelines
Ccs afib guidelines Acls ventricular tachycardia
Acls ventricular tachycardia