Definitions Arrhenius In aqueous solution Acids increase hydrogen
![Definitions Arrhenius - In aqueous solution… Acids increase hydrogen ion concentration [H+] HCl(aq) H+ Definitions Arrhenius - In aqueous solution… Acids increase hydrogen ion concentration [H+] HCl(aq) H+](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-1.jpg)
![Definitions Arrhenius - In aqueous solution… Bases increase hydroxide ion concentration [OH-] KOH(aq) + Definitions Arrhenius - In aqueous solution… Bases increase hydroxide ion concentration [OH-] KOH(aq) +](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-2.jpg)
![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-16.jpg)


![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-19.jpg)

![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-22.jpg)

![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-24.jpg)
![Calculate the [H+] of a solution with a p. H of 4. 60 p. Calculate the [H+] of a solution with a p. H of 4. 60 p.](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-25.jpg)
![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-26.jpg)




- Slides: 39
![Definitions Arrhenius In aqueous solution Acids increase hydrogen ion concentration H HClaq H Definitions Arrhenius - In aqueous solution… Acids increase hydrogen ion concentration [H+] HCl(aq) H+](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-1.jpg)
Definitions Arrhenius - In aqueous solution… Acids increase hydrogen ion concentration [H+] HCl(aq) H+ + Cl(aq) H H Cl O H H – + O H (aq) Cl H HCl(aq) + H 2 O(l) H 3 + O (aq)+ Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Cl (aq)
![Definitions Arrhenius In aqueous solution Bases increase hydroxide ion concentration OH KOHaq Definitions Arrhenius - In aqueous solution… Bases increase hydroxide ion concentration [OH-] KOH(aq) +](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-2.jpg)
Definitions Arrhenius - In aqueous solution… Bases increase hydroxide ion concentration [OH-] KOH(aq) + K (aq)+ OH (aq) Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem (aq)

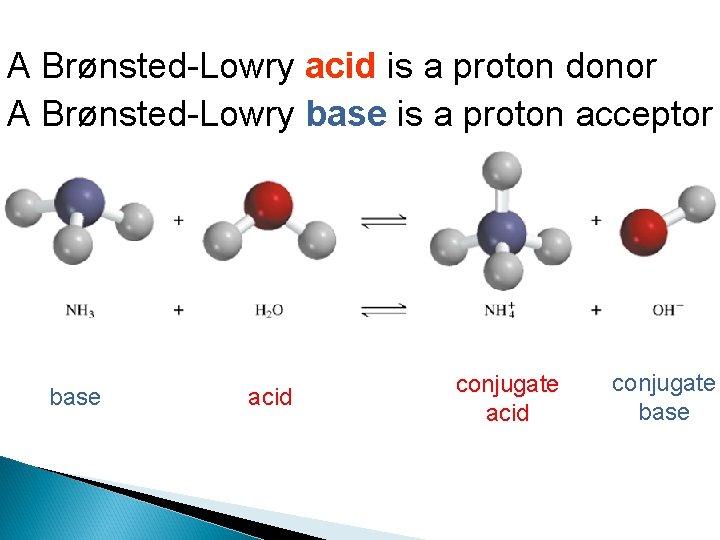
A Brønsted-Lowry acid is a proton donor A Brønsted-Lowry base is a proton acceptor base acid conjugate base

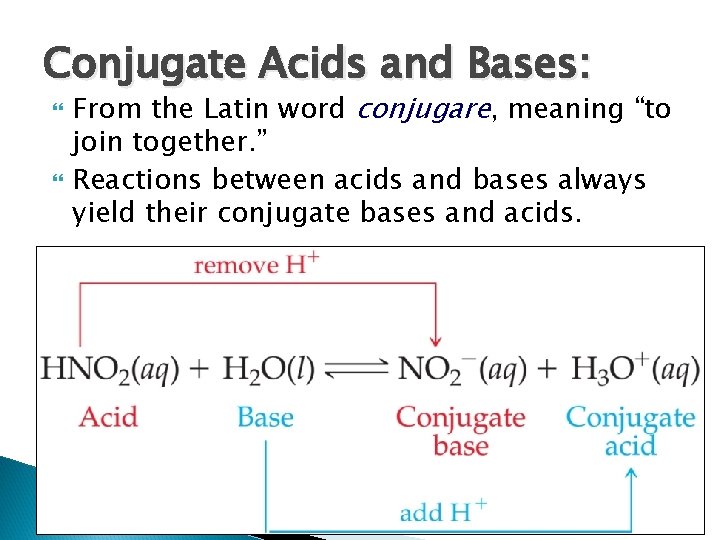
Conjugate Acids and Bases: From the Latin word conjugare, meaning “to join together. ” Reactions between acids and bases always yield their conjugate bases and acids.

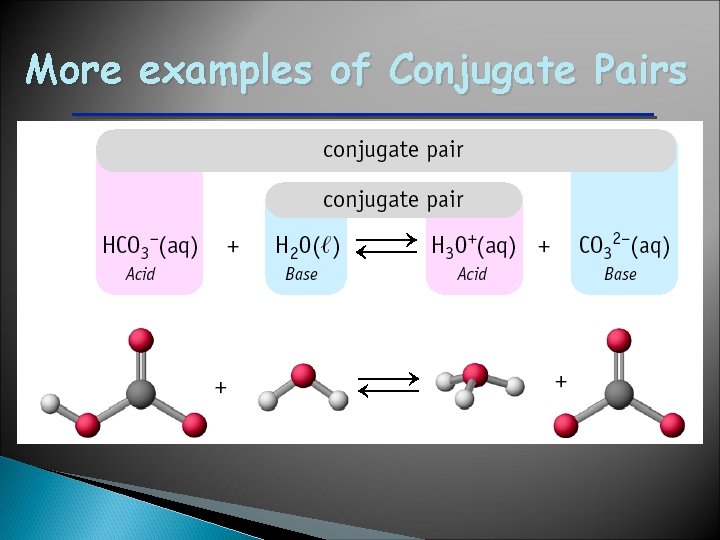
More examples of Conjugate Pairs

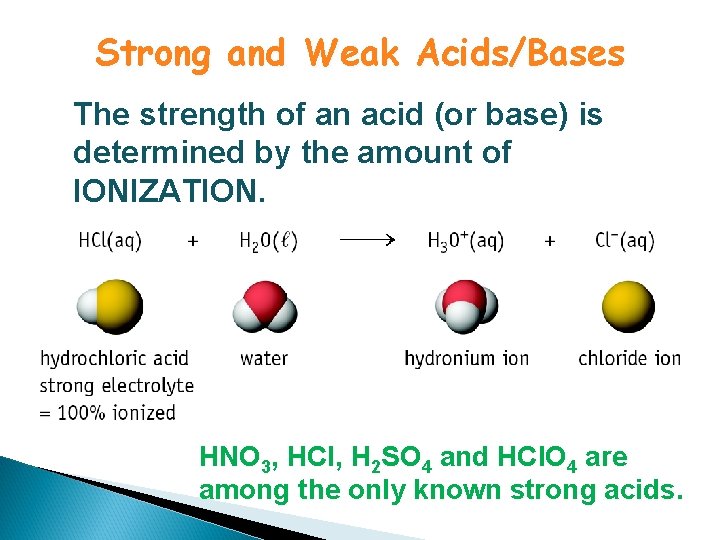
Strong and Weak Acids/Bases The strength of an acid (or base) is determined by the amount of IONIZATION. HNO 3, HCl, H 2 SO 4 and HCl. O 4 are among the only known strong acids.

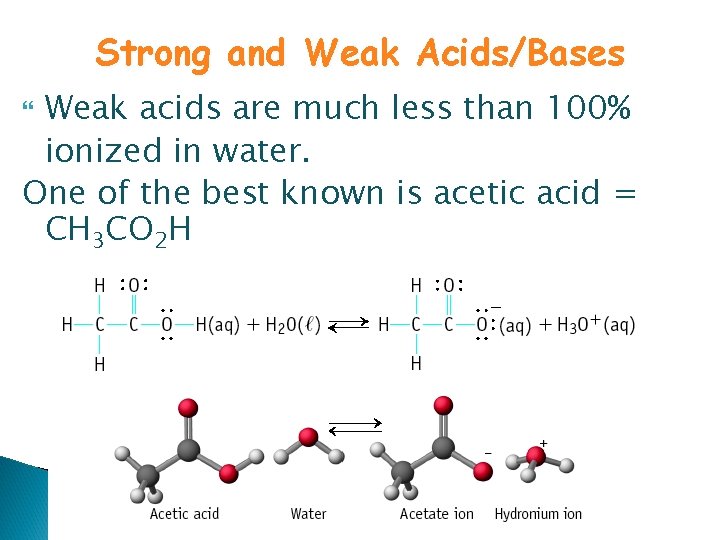
Strong and Weak Acids/Bases Weak acids are much less than 100% ionized in water. One of the best known is acetic acid = CH 3 CO 2 H

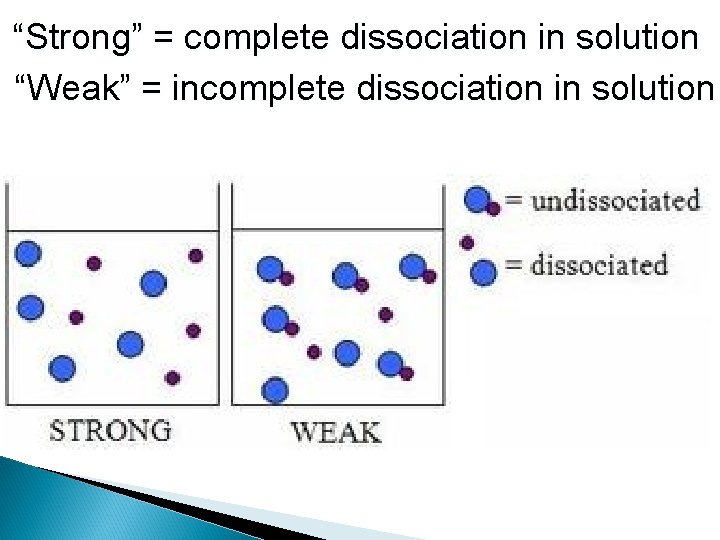
“Strong” = complete dissociation in solution “Weak” = incomplete dissociation in solution

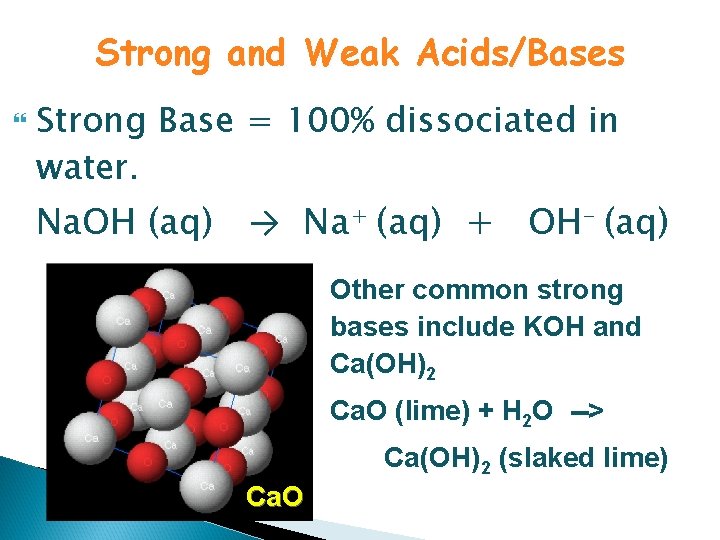
Strong and Weak Acids/Bases Strong Base = 100% dissociated in water. Na. OH (aq) → Na+ (aq) + OH- (aq) Other common strong bases include KOH and Ca(OH)2 Ca. O (lime) + H 2 O --> Ca(OH)2 (slaked lime) Ca. O

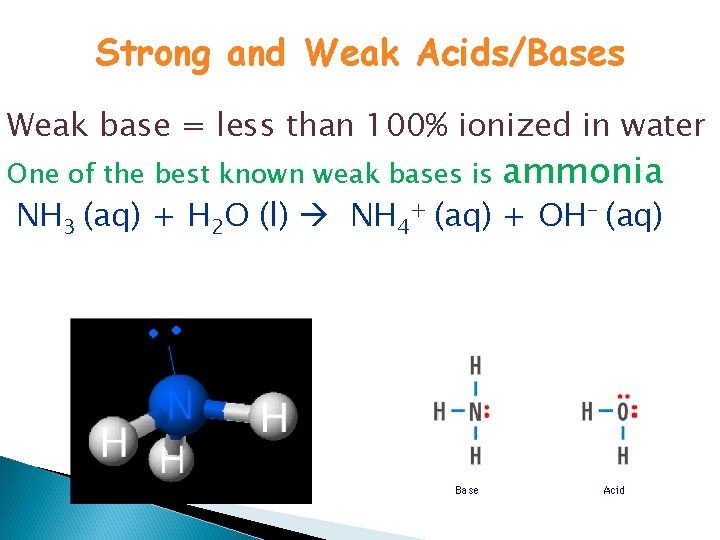
Strong and Weak Acids/Bases Weak base = less than 100% ionized in water One of the best known weak bases is ammonia NH 3 (aq) + H 2 O (l) NH 4+ (aq) + OH- (aq)

Extra (nontestable) information… Factors Affecting Acid Strength The more polar the H-X bond and/or the weaker the H-X bond, the more acidic the compound. Acidity increases from left to right across a row and from top to bottom down a group.

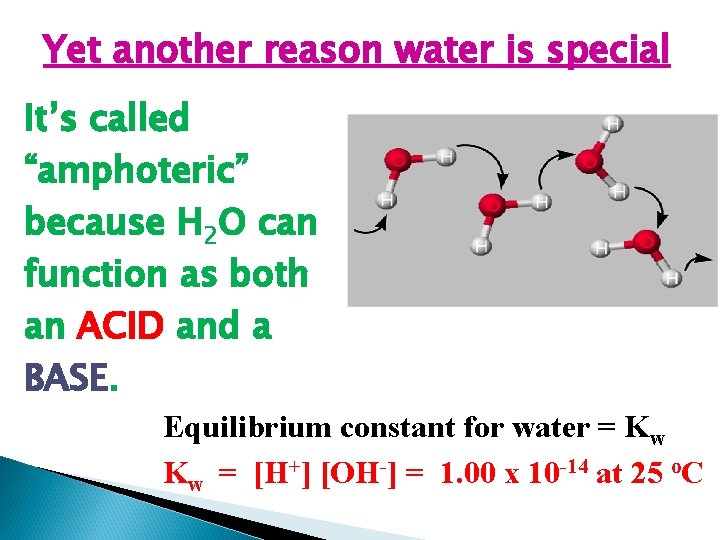
Yet another reason water is special It’s called “amphoteric” because H 2 O can function as both an ACID and a BASE. Equilibrium constant for water = Kw Kw = [H+] [OH-] = 1. 00 x 10 -14 at 25 o. C

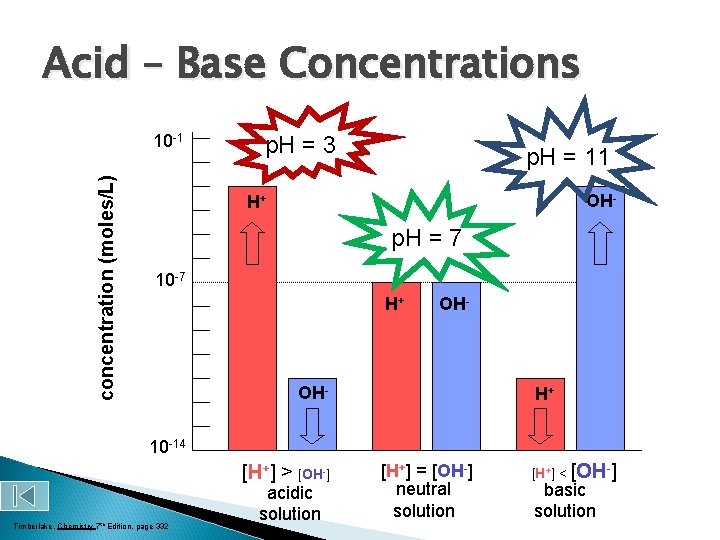
Acid – Base Concentrations concentration (moles/L) 10 -1 p. H = 3 p. H = 11 OH- H+ p. H = 7 10 -7 H+ OH- H+ 10 -14 [H+] > [OH-] Timberlake, Chemistry 7 th Edition, page 332 acidic solution [H+] = [OH-] neutral solution [H+] < [OH-] basic solution

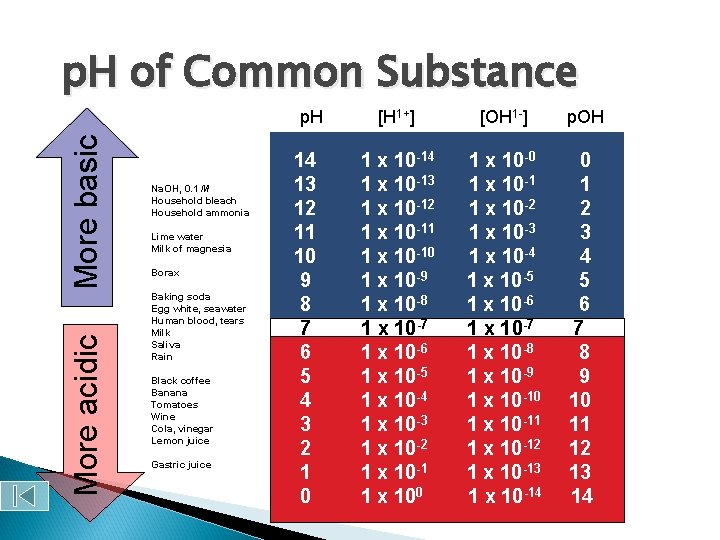
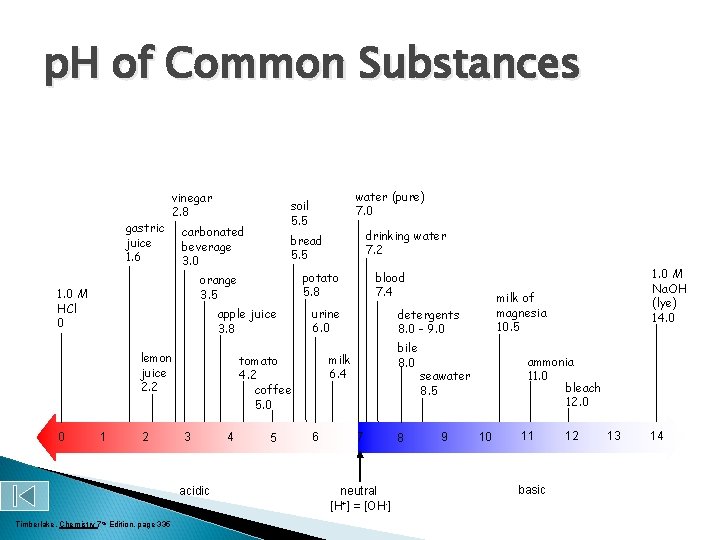
p. H of Common Substance More acidic More basic p. H Na. OH, 0. 1 M Household bleach Household ammonia Lime water Milk of magnesia Borax Baking soda Egg white, seawater Human blood, tears Milk Saliva Rain Black coffee Banana Tomatoes Wine Cola, vinegar Lemon juice Gastric juice 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 [H 1+] [OH 1 -] p. OH 1 x 10 -14 1 x 10 -13 1 x 10 -12 1 x 10 -11 1 x 10 -10 1 x 10 -9 1 x 10 -8 1 x 10 -7 1 x 10 -6 1 x 10 -5 1 x 10 -4 1 x 10 -3 1 x 10 -2 1 x 10 -1 1 x 100 1 x 10 -1 1 x 10 -2 1 x 10 -3 1 x 10 -4 1 x 10 -5 1 x 10 -6 1 x 10 -7 1 x 10 -8 1 x 10 -9 1 x 10 -10 1 x 10 -11 1 x 10 -12 1 x 10 -13 1 x 10 -14 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14

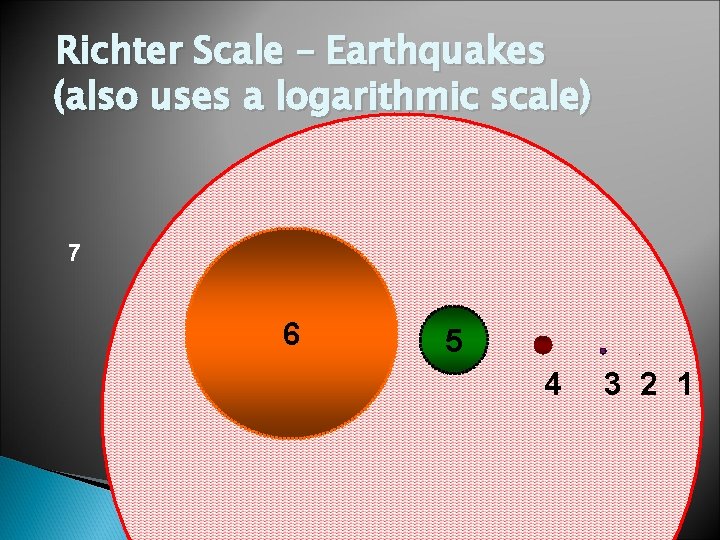
Richter Scale – Earthquakes (also uses a logarithmic scale) 7 6 5 . 4 3 2 1
![p H Calculations p H logH 10 p H p OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-16.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH Kw = [H+] [OH-] = 1 x 10 -14 p. OH = -log[OH-] = 10 -p. OH [OH-]

What is the p. H of a solution whose p. OH is 11. 09? Is this solution acidic or basic? p. H + p. OH = 14 p. H + 11. 09 = 14 p. H = 14 – 11. 09 = 2. 91 Solution is acidic (less acidic than vinegar, more than orange juice)

![p H Calculations p H logH 10 p H p OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-19.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH Kw = [H+] [OH-] = 1 x 10 -14 p. OH = -log[OH-] = 10 -p. OH [OH-]

Calculate the p. H of a 0. 240 M nitric acid solution. Is this solution acidic or basic? p. H = - log [H+] = -log [0. 240 M H+] p. H = 0. 620 Solution is acidic (less acidic than battery acid, more than stomach fluids)

p. H of Common Substances gastric juice 1. 6 vinegar 2. 8 carbonated beverage 3. 0 0 1 2 acidic Timberlake, Chemistry 7 th Edition, page 335 urine 6. 0 4 5 bile 8. 0 6 7 neutral [H+] = [OH-] 8 ammonia 11. 0 bleach 12. 0 seawater 8. 5 9 1. 0 M Na. OH (lye) 14. 0 milk of magnesia 10. 5 detergents 8. 0 - 9. 0 milk 6. 4 tomato 4. 2 coffee 5. 0 3 blood 7. 4 potato 5. 8 apple juice 3. 8 lemon juice 2. 2 drinking water 7. 2 bread 5. 5 orange 3. 5 1. 0 M HCl 0 water (pure) 7. 0 soil 5. 5 10 11 basic 12 13 14
![p H Calculations p H logH 10 p H p OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-22.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH Kw = [H+] [OH-] = 1 x 10 -14 p. OH = -log[OH-] = 10 -p. OH [OH-]

What is the hydroxide ion concentration of a solution with a hydronium ion concentration of 3. 3 x 10 -10 M? Is this solution acidic or basic? Kw = [H+] [OH-] = 1. 0 x 10 -14 = [3. 3 x 10 -10] [OH-] = 3. 0 x 10 -5 M Solution is basic (less basic than ammonia, more than sea water)
![p H Calculations p H logH 10 p H p OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-24.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH Kw = [H+] [OH-] = 1 x 10 -14 p. OH = -log[OH-] = 10 -p. OH [OH-]
![Calculate the H of a solution with a p H of 4 60 p Calculate the [H+] of a solution with a p. H of 4. 60 p.](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-25.jpg)
Calculate the [H+] of a solution with a p. H of 4. 60 p. H = - log [H+] 4. 60 = -log [H+] 2 nd log -4. 6 [H+] = 2. 51 x 10 -5 M 10 - 4. 60 You can check your answer by working backwards. p. H = - log [2. 51 x 10 -5 M] p. H = 4. 60
![p H Calculations p H logH 10 p H p OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](https://slidetodoc.com/presentation_image_h2/2c237769352112076e74eb2c917bb224/image-26.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH Kw = [H+] [OH-] = 1 x 10 -14 p. OH = -log[OH-] = 10 -p. OH [OH-]

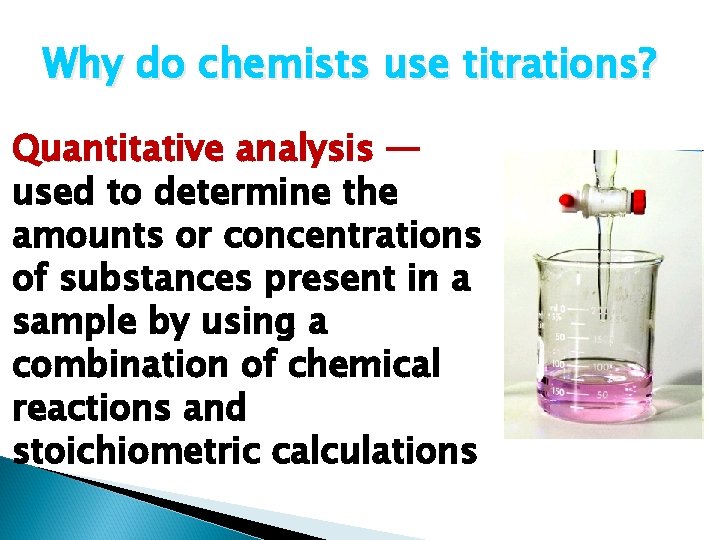
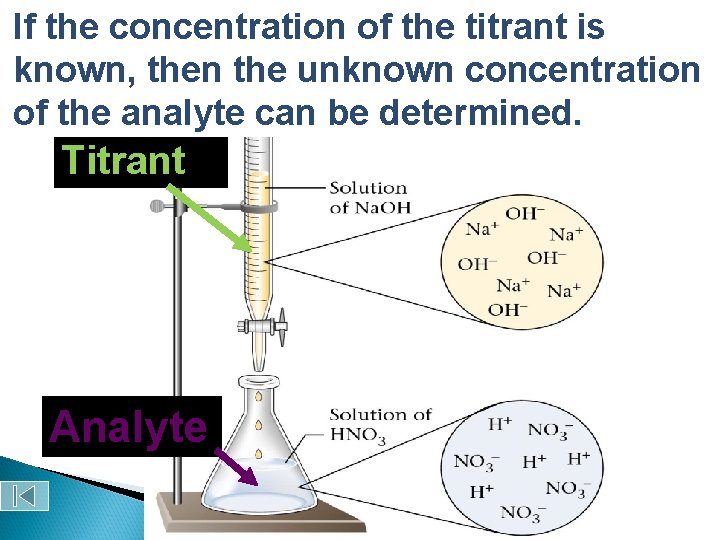
Why do chemists use titrations? Quantitative analysis — used to determine the amounts or concentrations of substances present in a sample by using a combination of chemical reactions and stoichiometric calculations

Titration standard solution Definition ◦ Analytical method in which a standard solution is used to determine the concentration of an unknown solution Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

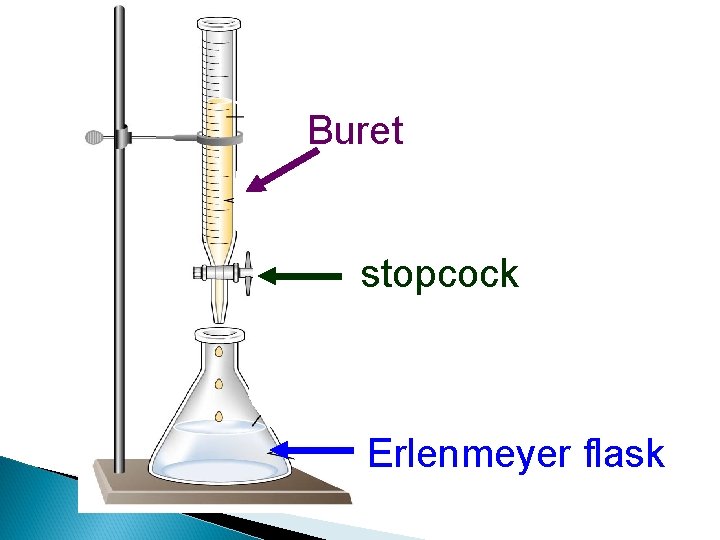
Buret stopcock Erlenmeyer flask

Titration Vocabulary Titrant Analyte Equivalence point ◦ The substance added to the analyte in a titration (a standard solution) ◦ The substance being analyzed ◦ The point in a titration at which the quantity of titrant is exactly sufficient for stoichiometric reaction with the analyte.

If Acid-Base the concentration of the titrant is Titration known, then the unknown concentration of the analyte can be determined. Titrant Analyte

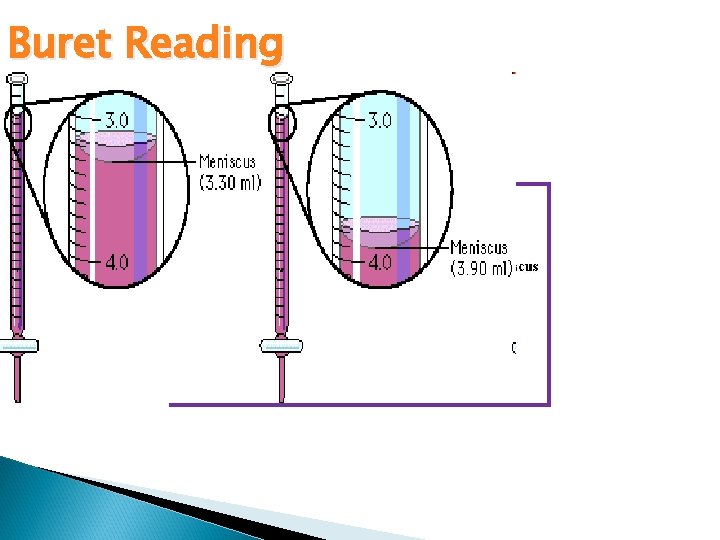
Buret Reading

Acidic, basic, or neutral? ?

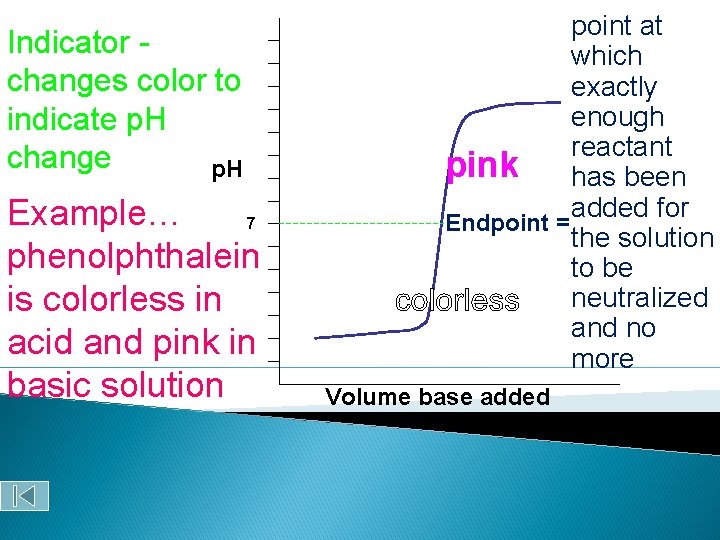
The “perfect pink” for a titration with phenolphthalein Phenolphthalein is clear in acidic solutions but pink in basic solutions!

Indicator changes color to indicate p. H change p. H Example… 7 phenolphthalein is colorless in acid and pink in basic solution point at which exactly enough reactant pink has been added for Endpoint = the solution to be neutralized and no more Volume base added

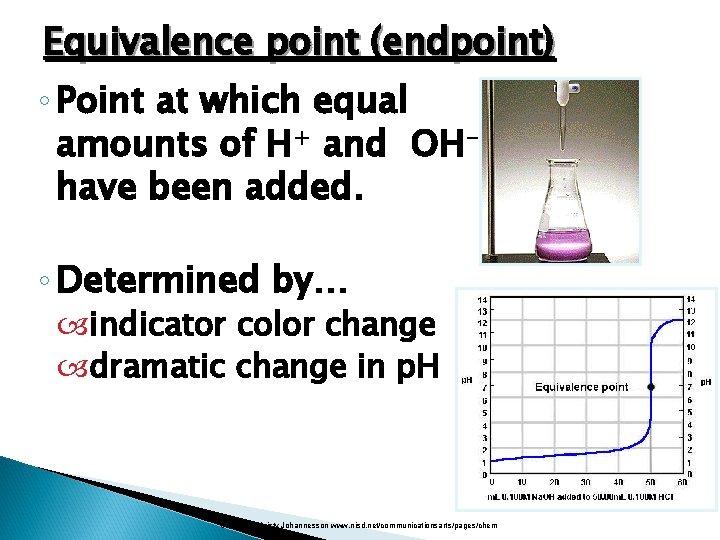
Equivalence point (endpoint) ◦ Point at which equal amounts of H+ and OHhave been added. ◦ Determined by… indicator color change dramatic change in p. H Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

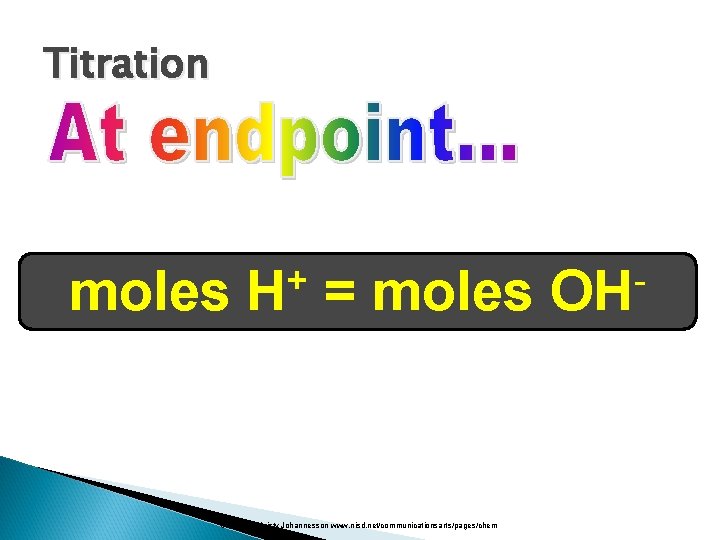
Titration moles + H = moles Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem OH

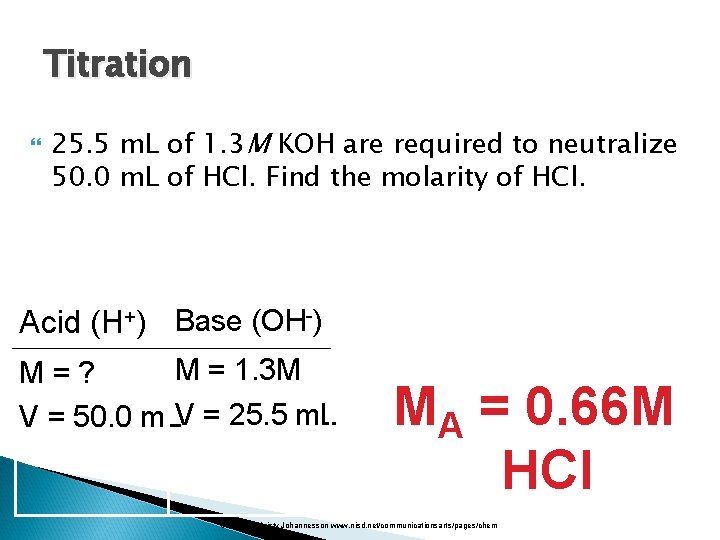
Titration 25. 5 m. L of 1. 3 M KOH are required to neutralize 50. 0 m. L of HCl. Find the molarity of HCl. Acid (H+) Base (OH-) M = 1. 3 M M=? V = 50. 0 m. LV = 25. 5 m. L MA = 0. 66 M HCl Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

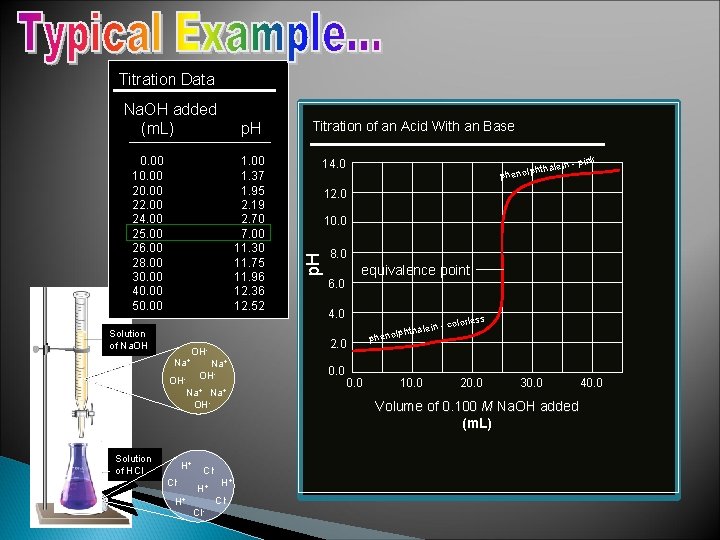
Titration Data p. H 0. 00 10. 00 22. 00 24. 00 25. 00 26. 00 28. 00 30. 00 40. 00 50. 00 1. 37 1. 95 2. 19 2. 70 7. 00 11. 30 11. 75 11. 96 12. 36 12. 52 Solution of Na. OH Na+ H+ Cl- Na+ OH- 25 m. L H+ Cl. H+ H+ Cl- 14. 0 htha phenolp ink lein - p 12. 0 10. 0 8. 0 6. 0 equivalence point 4. 0 s olorles -c hthalein lp o n e ph 2. 0 OH- OHNa+ OH- Solution of HCl Titration of an Acid With an Base p. H Na. OH added (m. L) 0. 0 10. 0 20. 0 30. 0 Volume of 0. 100 M Na. OH added (m. L) 40. 0