Compounds in Living Things Major elements in living







- Slides: 41

Compounds in Living Things

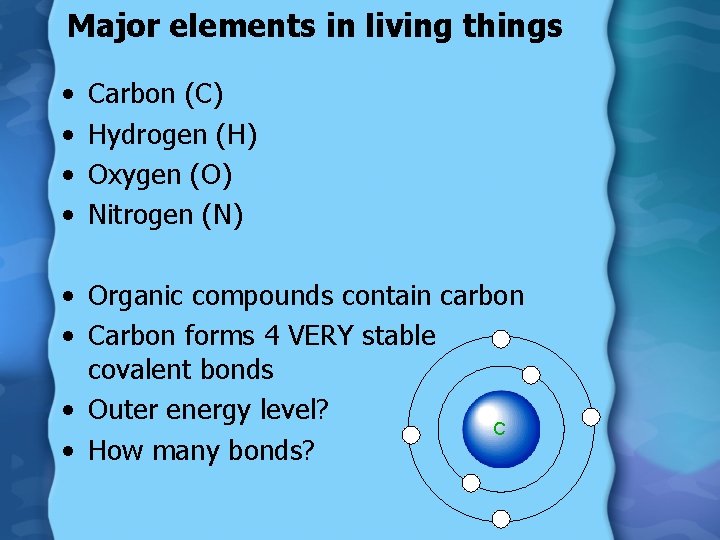
Major elements in living things • • Carbon (C) Hydrogen (H) Oxygen (O) Nitrogen (N) • Organic compounds contain carbon • Carbon forms 4 VERY stable covalent bonds • Outer energy level? C • How many bonds?

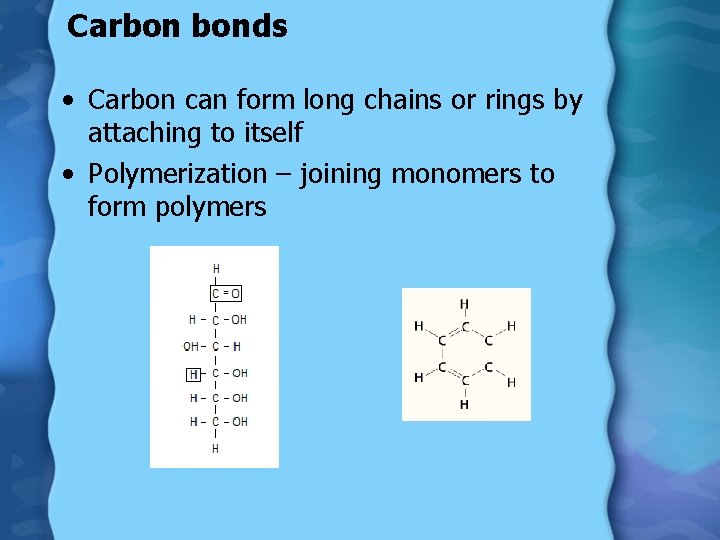
Carbon bonds • Carbon can form long chains or rings by attaching to itself • Polymerization – joining monomers to form polymers

What is a monomer? n n Monomer - smallest unit of a macromolecule Polymer - a large chain of monomers

What is an organic molecule? n n n Contains C and H May contain N, O, P, and/or S SPONCH = all possible elements

What types of organic molecules exist? n n Carbohydrates Lipids Proteins Nucleic Acids Carbs Lipids Proteins

Brainstorm n What do you know about these molecules: n n n Carbohydrates Lipids Proteins Nucleic Acids What do their names make you think of? Do you know anything about their structures?

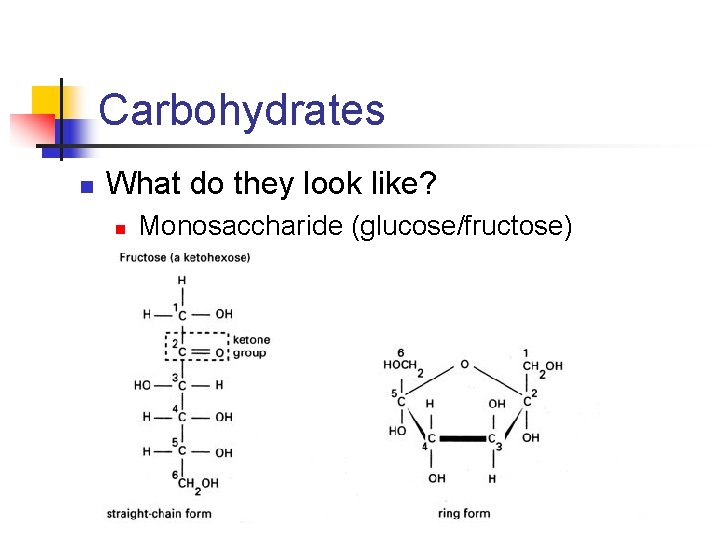
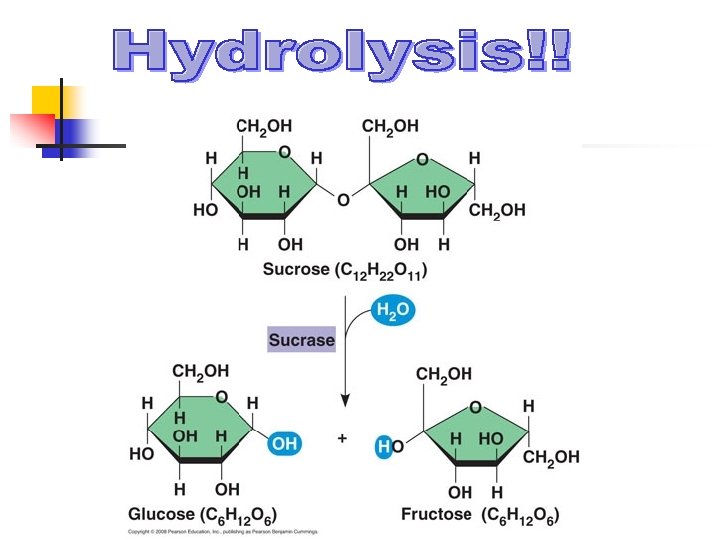
Carbohydrates n What are they in simple terms? n n n What do they do for us? n n Sugars Starches Energy storage!! (Both long and short term) What’s the monomer? n A monosaccharide (glucose, fructose, etc. )

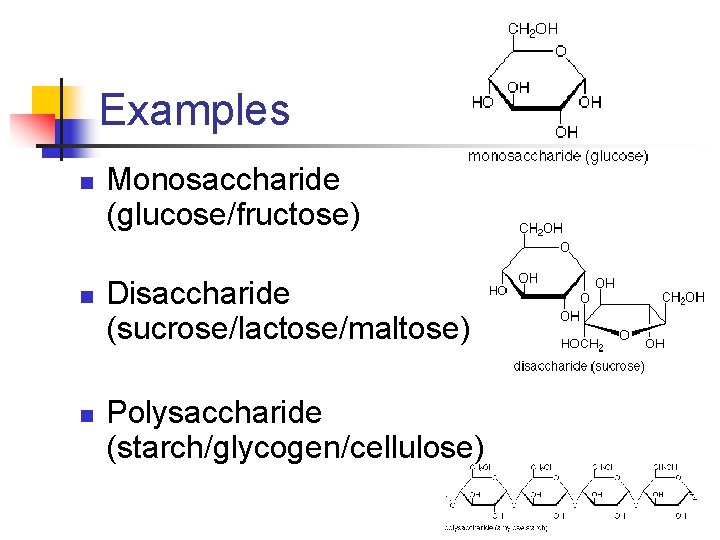
Carbohydrates n What do they look like? n Monosaccharide (glucose/fructose)



Animation: Polymers Right click on animation / Click play © 2012 Pearson Education, Inc.

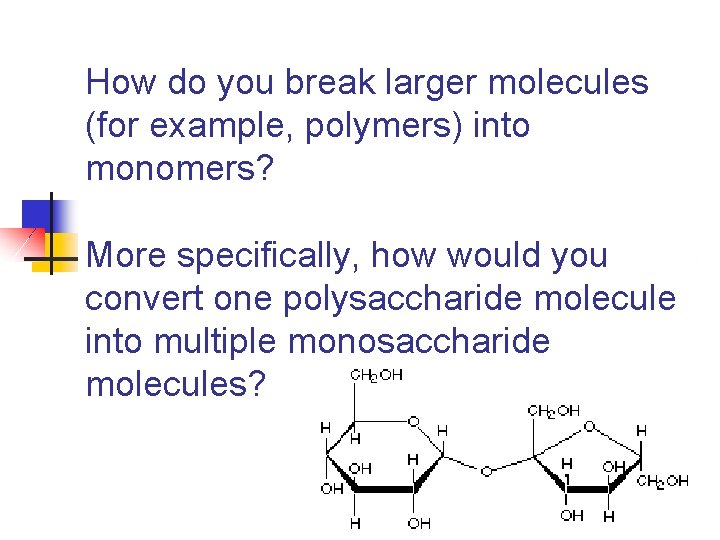
How do you break larger molecules (for example, polymers) into monomers? More specifically, how would you convert one polysaccharide molecule into multiple monosaccharide molecules?


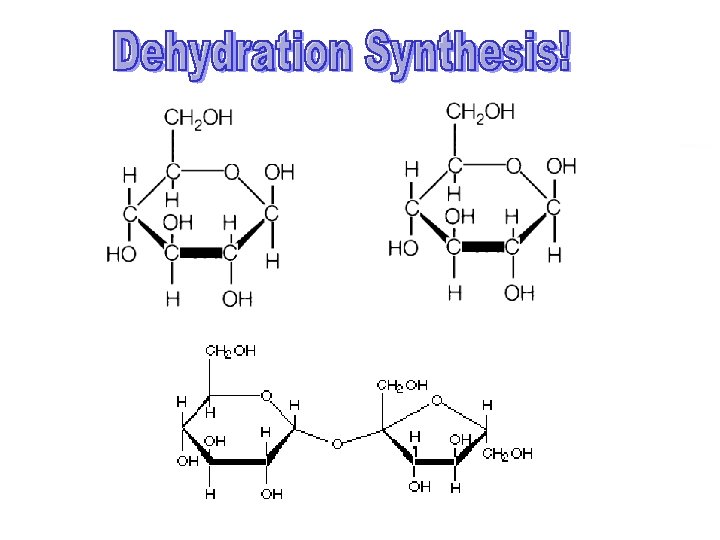
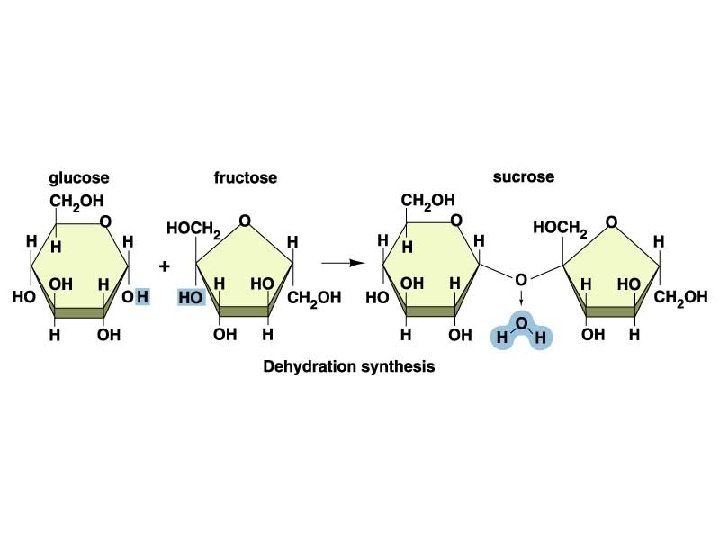
Examples n n n Monosaccharide (glucose/fructose) Disaccharide (sucrose/lactose/maltose) Polysaccharide (starch/glycogen/cellulose)

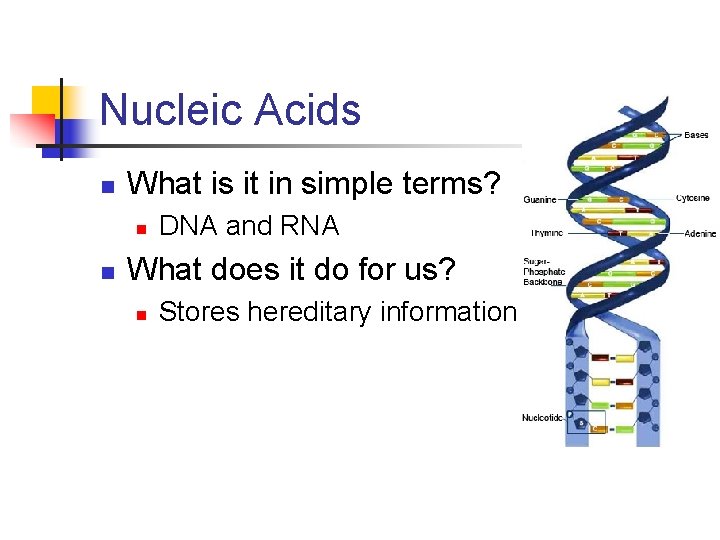
Nucleic Acids n What is it in simple terms? n n DNA and RNA What does it do for us? n Stores hereditary information

Lipids n What are they? n n Fats, oils, and waxes What do they do for us? n n n Store energy Act as chemical messengers (hormones) Form cell membranes

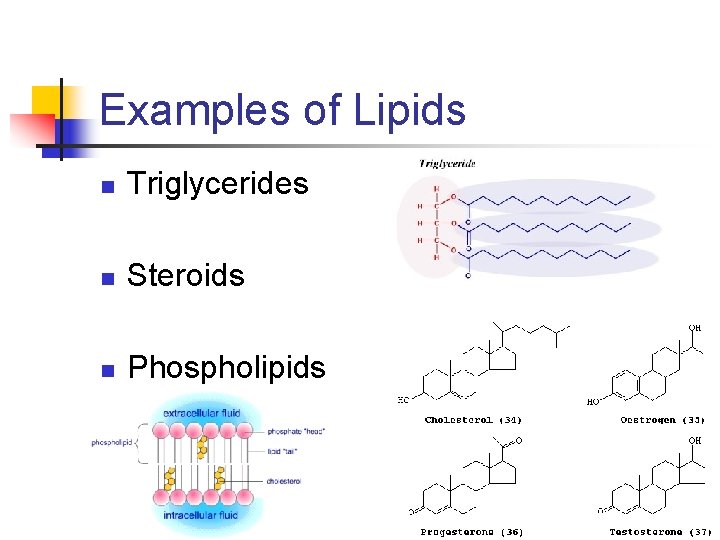
Examples of Lipids n Triglycerides n Steroids n Phospholipids

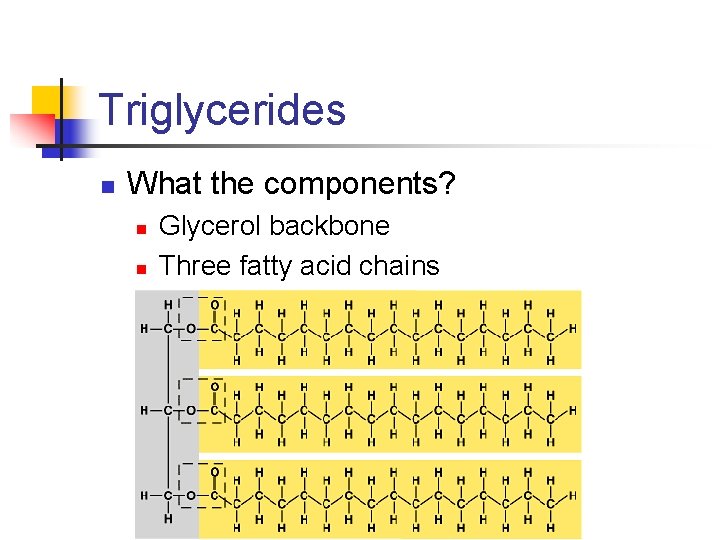
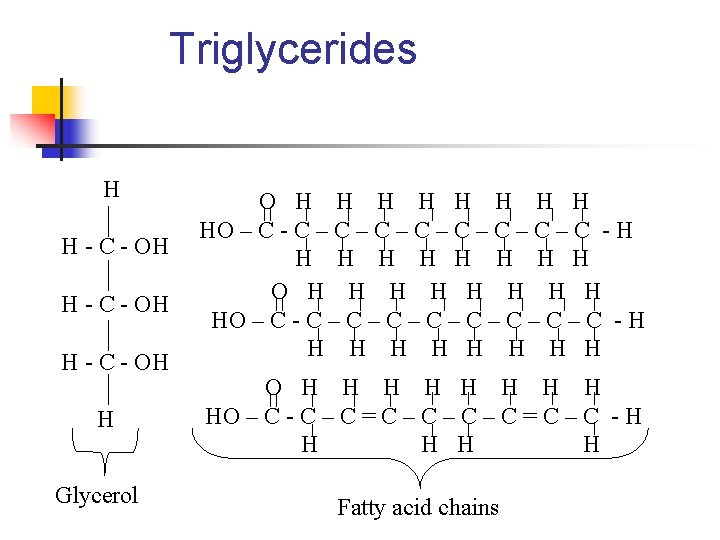
Triglycerides n What the components? n n Glycerol backbone Three fatty acid chains

Triglycerides H H - C - OH H Glycerol O H H H H HO – C - C – C – C – C – C - H H H H H O H H H H HO – C - C – C = C – C - H H H Fatty acid chains

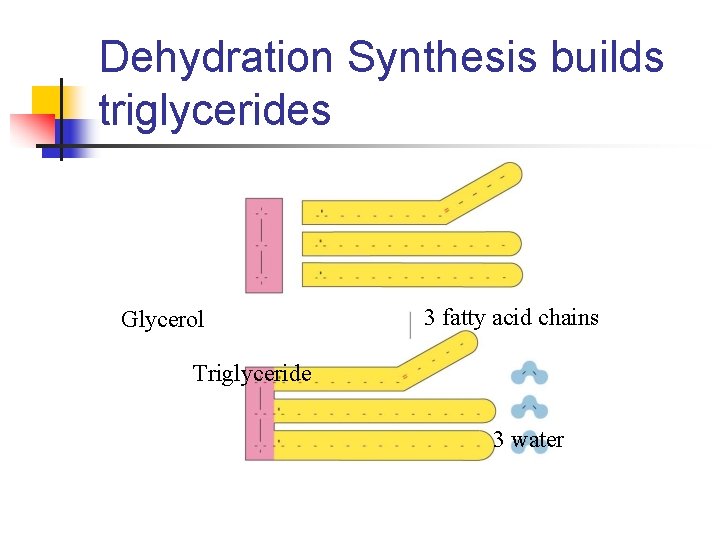
Dehydration Synthesis builds triglycerides Glycerol 3 fatty acid chains Triglyceride 3 water

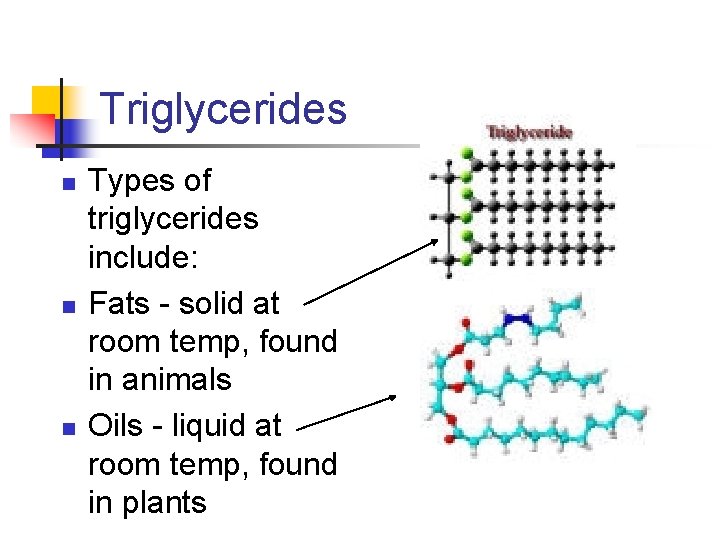
Triglycerides n n n Types of triglycerides include: Fats - solid at room temp, found in animals Oils - liquid at room temp, found in plants

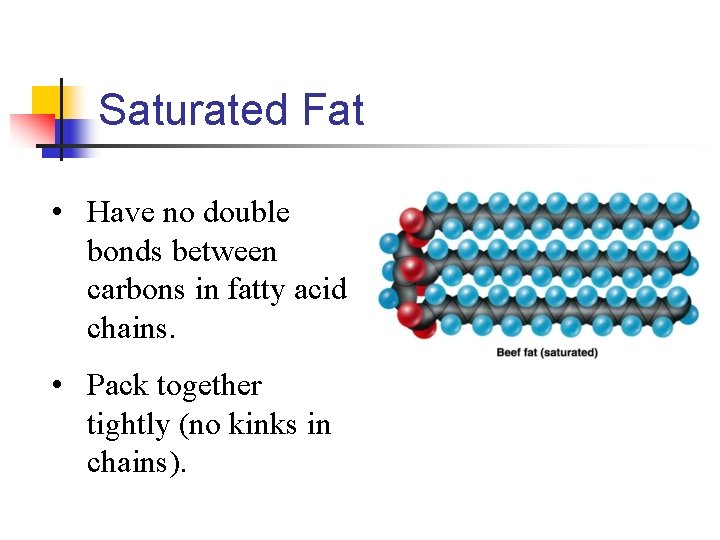
Saturated Fat • Have no double bonds between carbons in fatty acid chains. • Pack together tightly (no kinks in chains).

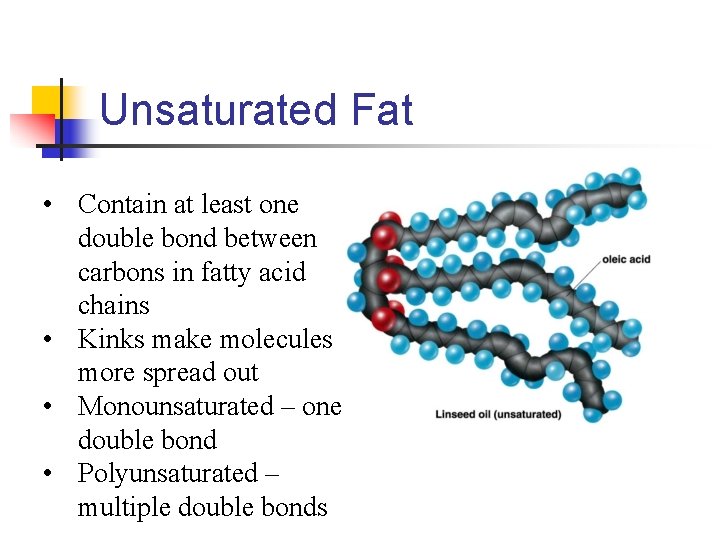
Unsaturated Fat • Contain at least one double bond between carbons in fatty acid chains • Kinks make molecules more spread out • Monounsaturated – one double bond • Polyunsaturated – multiple double bonds

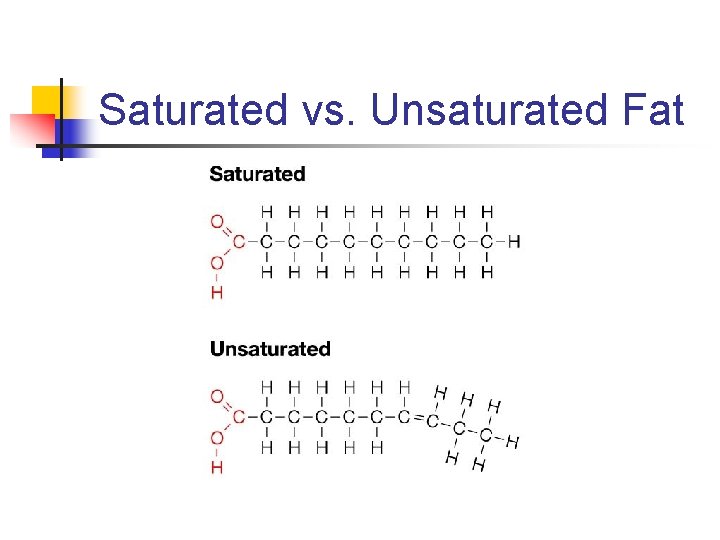
Saturated vs. Unsaturated Fat

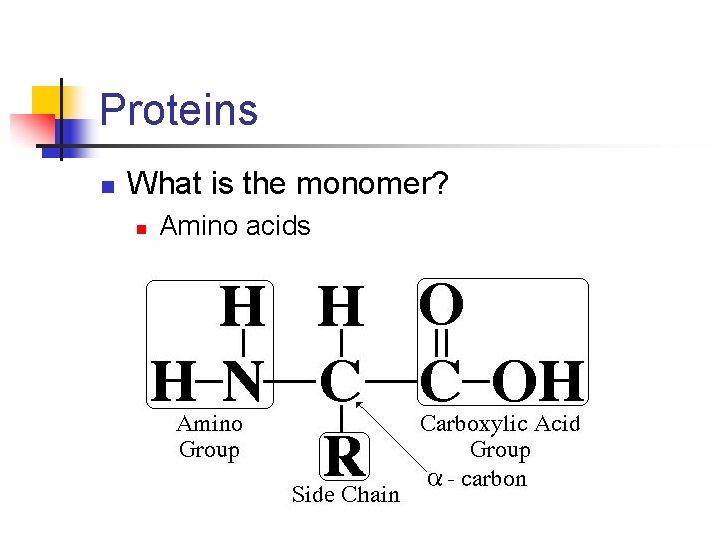
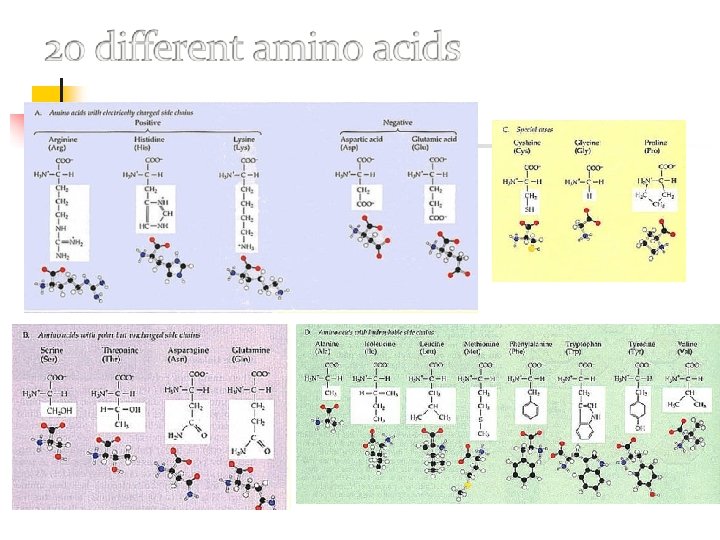
Proteins n What is the monomer? n Amino acids


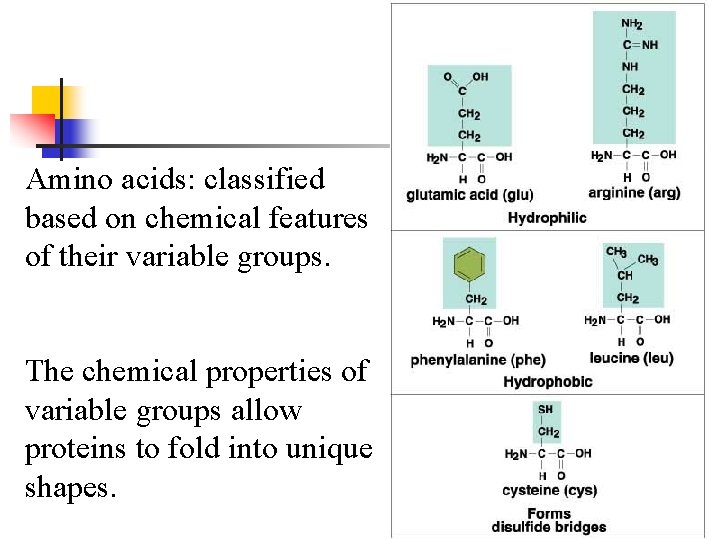
Amino acids: classified based on chemical features of their variable groups. The chemical properties of variable groups allow proteins to fold into unique shapes.

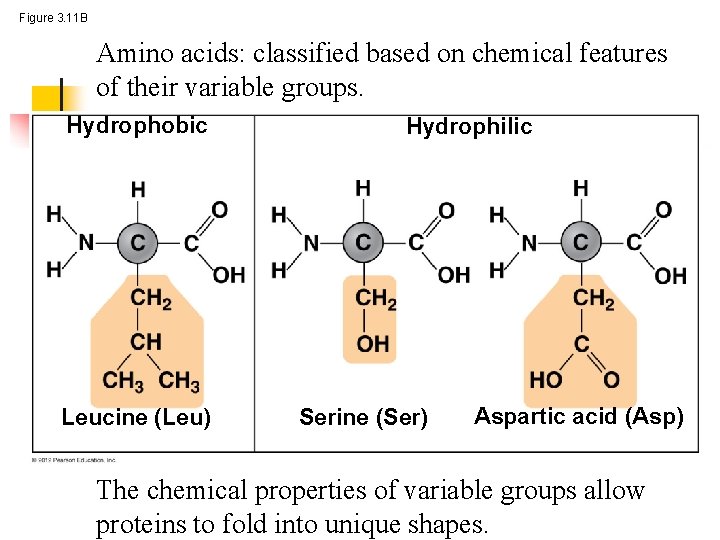
Figure 3. 11 B Amino acids: classified based on chemical features of their variable groups. Hydrophobic Leucine (Leu) Hydrophilic Serine (Ser) Aspartic acid (Asp) The chemical properties of variable groups allow proteins to fold into unique shapes.

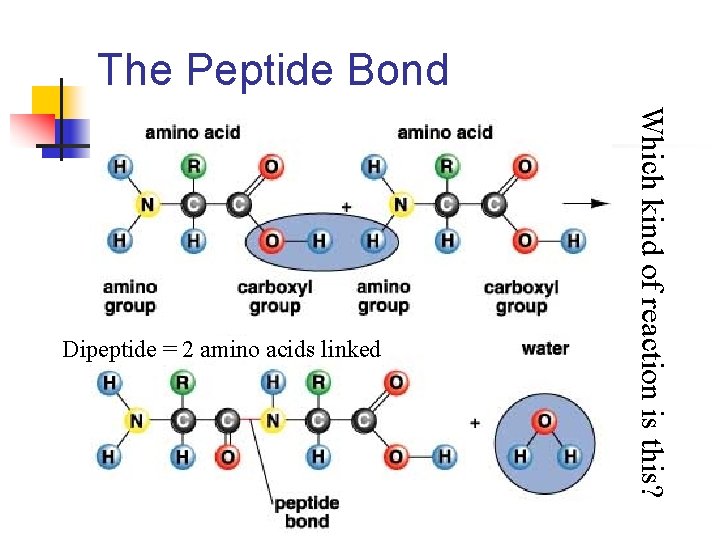
The Peptide Bond Which kind of reaction is this? Dipeptide = 2 amino acids linked

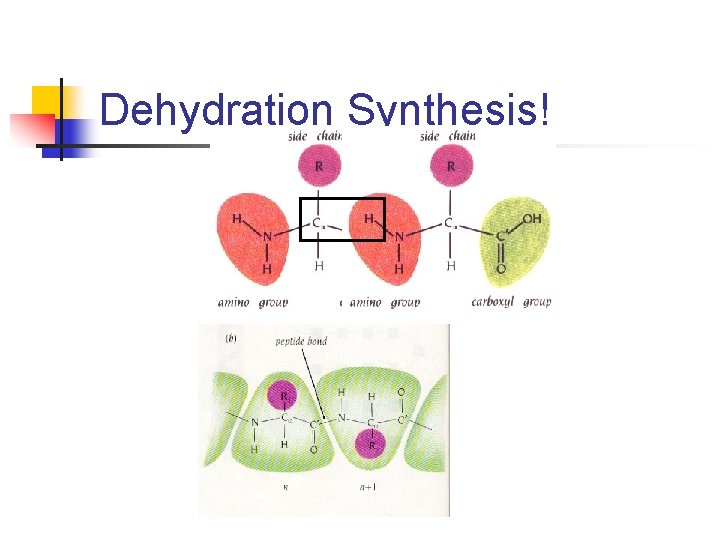
Dehydration Synthesis!

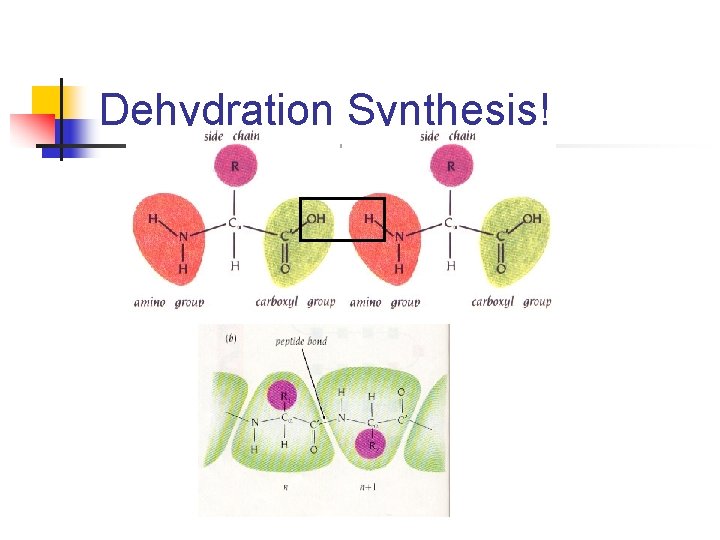
Dehydration Synthesis!

How can there be so many different proteins? n n Proteins can be 1000 s of amino acids long… Think about how many words that you can form with 26 letters… (and words aren’t even close to 1000 letters long)

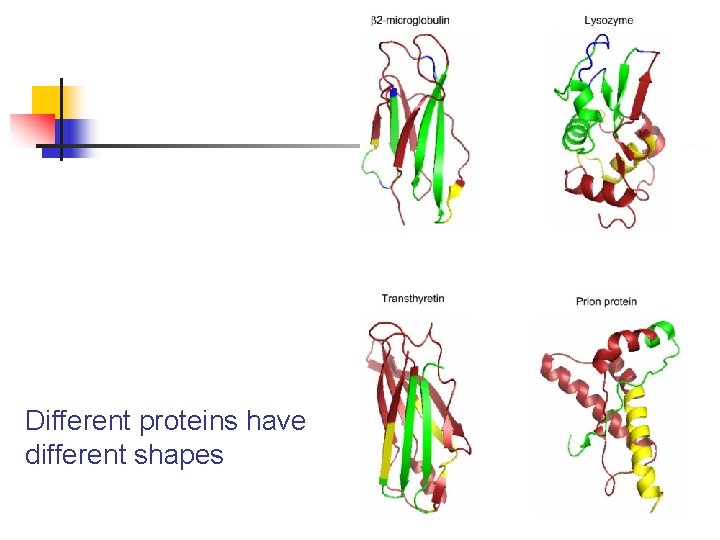
Different proteins have different shapes

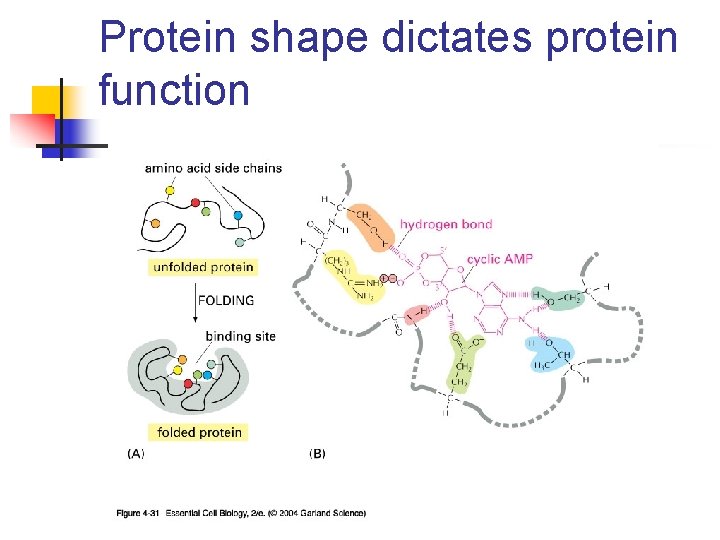
Protein shape dictates protein function

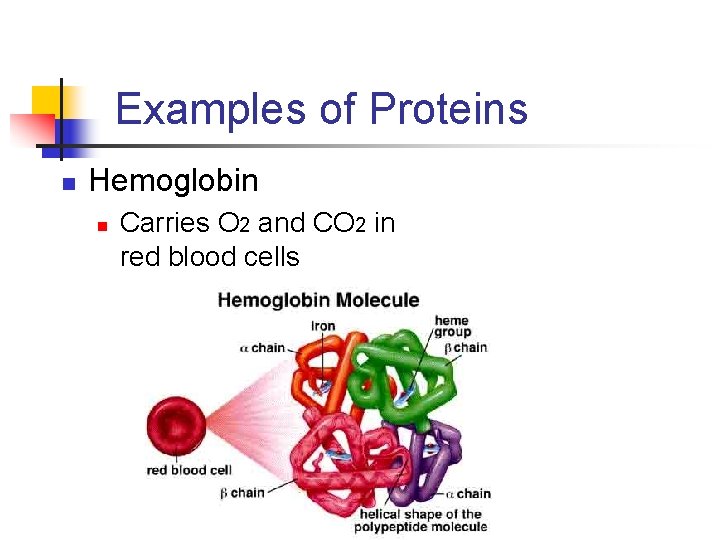
Examples of Proteins n Hemoglobin n Carries O 2 and CO 2 in red blood cells

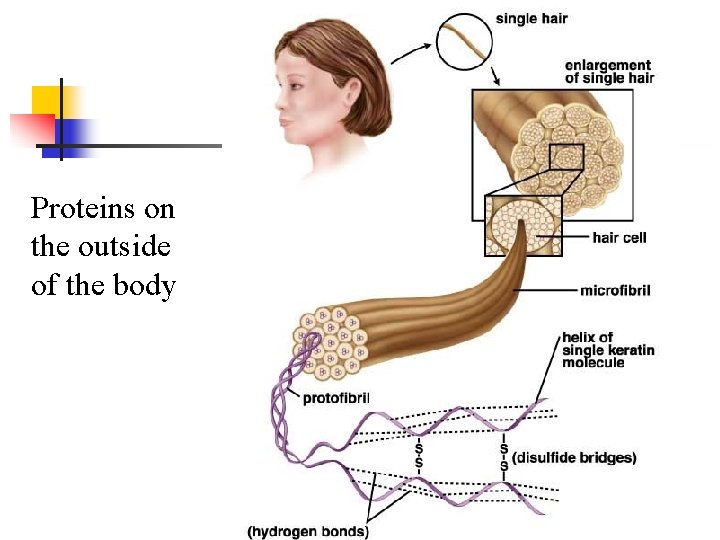
Examples of Proteins n Cell proteins: n n n Cell receptors (sit on the surface of a cell) that allow cells to “talk” to one another Cytoskeleton proteins – sit inside the cell and act like a skeleton to hold its shape Proteins on the surface of the body n Keratin, collagen, elastin n found in hair, fingernails, and skin

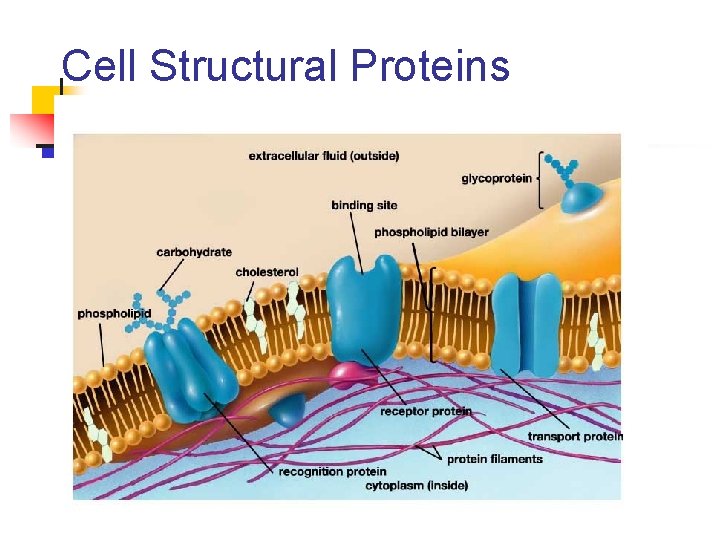
Cell Structural Proteins

Proteins on the outside of the body

Examples of Proteins n Some Hormones n n insulin, glucagon, growth hormone Enzymes n Speed up chemical reactions

Proteins - Summary n What do they do for us? n n n EVERYTHING! Structural components of cells and bodies Cell to cell communication Hemoglobin (Transport O 2 and CO 2) Chemical messengers (hormones) Enzymes (speed up chemical reactions)