Clinical Research and Regulations Marilyn Marshall QAO Office





























![Terms Case Report Form [CRF] A printed, optical or electronic document designed to record Terms Case Report Form [CRF] A printed, optical or electronic document designed to record](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-31.jpg)
![Terms Adverse Event [AE] Any untoward medical occurrence in a patient or clinical investigational Terms Adverse Event [AE] Any untoward medical occurrence in a patient or clinical investigational](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-32.jpg)
![Adverse Event [AE] • Any unfavorable and unintended sign, including an abnormal laboratory findings Adverse Event [AE] • Any unfavorable and unintended sign, including an abnormal laboratory findings](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-33.jpg)


![Adverse Event Reporting Investigators must report • All AEs [routine and serious] in the Adverse Event Reporting Investigators must report • All AEs [routine and serious] in the](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-36.jpg)



















- Slides: 59

Clinical Research and Regulations Marilyn Marshall QAO Office of the Vice President of Research


International Conference on Harmonization E 6: Good Clinical Practice: Consolidated Guideline


FDA’s Role in Clinical Research • A new drug cannot be marketed in the United States until there is “substantial evidence” as to its safety and effectiveness

FDA’s Role in Clinical Research • Regulation 21 CFR 314. 126: “substantial evidence” is collected in “adequate and well-controlled trials”

Characteristics of Adequate and Well-controlled Clinical Trials • Protocol contains a clear statement of objects of the study • Study design permits a valid comparison with a control to provide a quantitative assessment of the drug effect • Methods of assessment of subjects’ responses [efficacy and safety] are well -defined and reliable

Characteristics of Adequate and Well- controlled Clinical Trials • Study report provides sufficient details of study design, conduct and analysis • Patients were assigned to treatment and control groups in a way that minimizes bias [e. g. , randomization] • Adequate measures were used to minimize bias of subjects, observers, and data analysts [e. g. , blinding]

FDA’s Role in Clinical Research Reports of adequate and well-controlled investigations provide the primary basis for determining whethere is “substantial evidence” to support the claims of effectiveness for new drugs and antibiotics. 21 CFR 314. 126

FDA’s Role in Clinical Research The purpose of conducting clinical investigations of a drug is to distinguish the effect of a drug from other influences such as … • Spontaneous change in the course of the disease • Placebo effect • Biased observation 21 CRF 314. 126

Phases of Clinical Research: • Phase 0: Exploratory • Phase I: Pharmacokinetics and safety in humans • Phase II: Dose finding and efficacy • Phase III: Pivotal studies • Phase IV: Post approval commitments

21 CFR Part 11 (overview) Electronic Records, Electronic Signatures ß 21 CFR Part 11 March of 2003 the FDA withdrew the Guidance document on Part 11. However, this does not mean that we you should ignore the regulation.

21 CFR Part 11 (cont. ) ßPart 11 applies to Any Records that you choose to created, modified, maintained, archived, retrieved or transmitted in electronic form.

21 CRF Part 11 Key Requirements ß Validation ß Audit Trails ß Security ß Record retention and protection

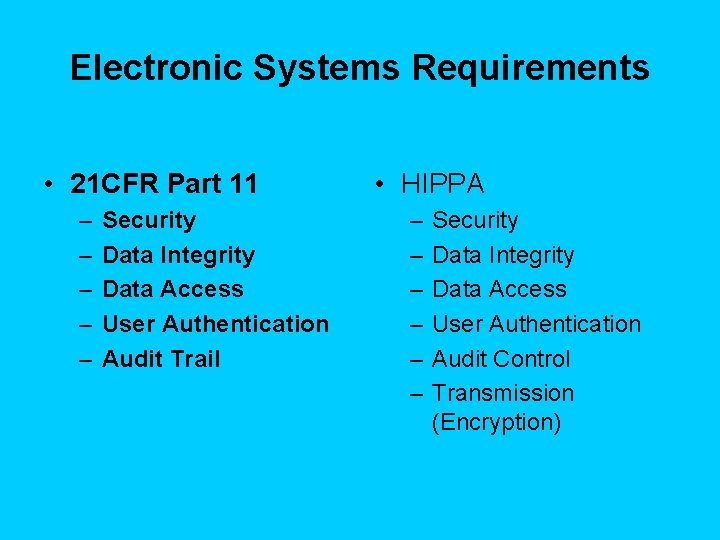
Electronic Systems Requirements • 21 CFR Part 11 – – – Security Data Integrity Data Access User Authentication Audit Trail • HIPPA – – – Security Data Integrity Data Access User Authentication Audit Control Transmission (Encryption)

21 CFR 50. 20 (overview) General Requirements for Informed Consent – …no investigator may involve a human being as a subject in research…unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative. – The information…shall be in a language understandable to the subject or the representative.

21 CFR 50. 20 (cont. ) General Requirements for Informed Consent – No informed consent, … may include any exculpatory language… which the subject or the representative is to waive or appear to waive any of the subject’s legal rights, – or release or appear to release the investigator, the sponsor, the institution, or its agents from liability for negligence.

21 CFR 56. 109 (overview) IRB Review of Research ßAn IRB shall: – review and have the authority to approve, require modifications in…or disapprove all research activities covered by these regulations…notify investigators and the institution in writing of its decision to approve or disapprove… – require that information given to the subjects as part of the informed consent is in accordance with § 50. 25. – conduct continuing review of research…

21 CFR 312. 56 (overview) Review of Ongoing Investigations The sponsor shall monitor the progress of all ongoing investigations being conducted under its IND. A sponsor who discovers that an investigator is not complying with the signed agreement (Form FDA-1572), …shall either secure compliance, or discontinue shipments of the investigational new drug…and shall notify the FDA.

21 CFR 312. 60 (overview) General Responsibilities of Investigators ßAn investigator is responsible for: – ensuring that investigation is conducted according to the signed investigator statement… – protecting the rights, safety, and welfare of the subjects… – Obtain the informed consent of each human subject… – control of drugs under investigation

ICH 5. 1 (overview) ßICH 5. 1 Quality Assurance and Quality Control – 5. 1. 1 The sponsor is responsible for implementing and maintaining quality assurance and quality control systems with written SOPs to ensure that trials are conducted and data are generated, documented (recorded), and reported in compliance with the protocol, GCP, and the applicable regulatory requirement(s).

Investigator-Initiated Clinical Research • Form 1571 • Sponsor is the individual investigator and therefore assumes all responsibilities of both Sponsor an Investigator


Sponsor-Investigator Obligations SI is obligated to monitor the clinical study • SI is responsible to IRB • SI is required to keep accurate and complete records of study

Purpose of Clinical Study Monitoring • Protect the interests of subjects involved in the clinical research • Protect the interests of the sponsor or academic institution • Adhere to federal and international requirements

Role of the Monitor 1. Verify subjects have been properly consented prior to any study related procedure 2. Verify eligible subjects are entered in the study and no eligible subjects excluded 3. Verify protocol is being followed as written

Role of the Monitor 4. Verify that adverse events are being recorded and reported to Sponsor in timely manner 5. Verify that violations and deviations are recorded and deviations are preapproved where possible 6. Verify that the data are accurately and consistently recorder in CRF’s

Role of the Monitor 7. Verify that CRF data are supported by source documents 8. Verify that Principle Investigator is actively overseeing the conduct of the clinical study 9. Verify that all IRB approvals are in place before changes are implemented

Role of the Monitor 10. Verify that Regulatory binder is being maintained 11. Verify that drug study medication is returned as required and accounted for 12. Verify that only authorized study personnel are involved in conduct of study

Role of the Monitor 14. Verify that study is being conducted honestly and with no fraud 15. Ensure data will withstand FDA and other regulatory scrutiny for completeness and accuracy
![Terms Case Report Form CRF A printed optical or electronic document designed to record Terms Case Report Form [CRF] A printed, optical or electronic document designed to record](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-31.jpg)
Terms Case Report Form [CRF] A printed, optical or electronic document designed to record all protocol-required information to be reported to the sponsor on each trial subject
![Terms Adverse Event AE Any untoward medical occurrence in a patient or clinical investigational Terms Adverse Event [AE] Any untoward medical occurrence in a patient or clinical investigational](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-32.jpg)
Terms Adverse Event [AE] Any untoward medical occurrence in a patient or clinical investigational subject administered an investigational drug and which does not necessarily have a causal relationship with the treatment/usage.
![Adverse Event AE Any unfavorable and unintended sign including an abnormal laboratory findings Adverse Event [AE] • Any unfavorable and unintended sign, including an abnormal laboratory findings](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-33.jpg)
Adverse Event [AE] • Any unfavorable and unintended sign, including an abnormal laboratory findings • Symptom or disease temporally associated with the use of an investigational drug

Describing an Adverse Event • Adverse events should be described in terms of a change in the status of a subject’s health, NOT in the action taken or outcome

Serious Adverse Event (SAE) • Event that results in any one of the following… • Death • Life threatening • Inpatient hospitalization • Persistent/significant disability/incapacity • Leads to any congenital anomaly/birth defect
![Adverse Event Reporting Investigators must report All AEs routine and serious in the Adverse Event Reporting Investigators must report • All AEs [routine and serious] in the](https://slidetodoc.com/presentation_image/665a4a6687d29665a5c54541a230c66c/image-36.jpg)
Adverse Event Reporting Investigators must report • All AEs [routine and serious] in the CRF • SAEs to both the sponsor and IRB immediately • 7 -calendar day notification if SAE is death or life threatening…followed by a 15 day written report.

Source Documents: • Original documents, data, and records [e. g. , hospital records, clinical and office charts, laboratory notes, memo’s, subjects’ diaries or evaluation checklists, pharmacy dispensing records, recorded data from automated machines…. ]

Tools to Perform Audits Logs and Checklists

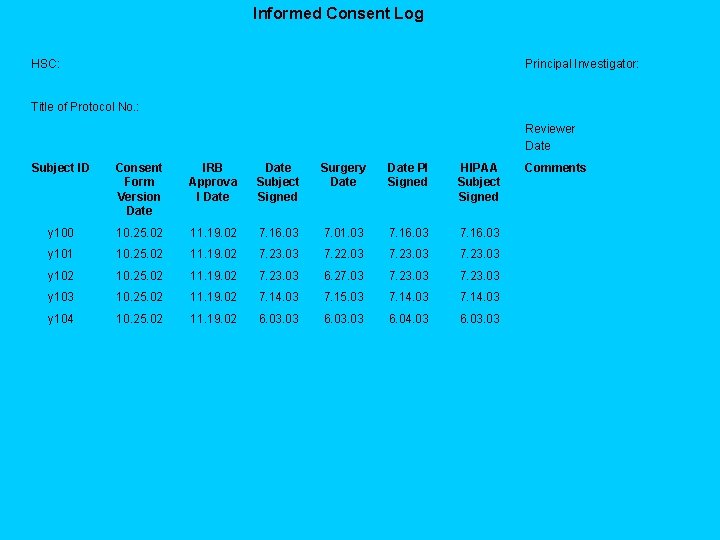
Tools to Perform Audits Log for Informed Consent Verification Informed Consent Log HSC: Principal Investigator: Title of Protocol No. : Reviewer Date Subject ID Consent Form Version Date IRB Approva l Date Subject Signed Surgery Date PI Signed HIPAA Subject Signed Comments

Informed Consent Log HSC: Principal Investigator: Title of Protocol No. : Reviewer Date Subject ID Consent Form Version Date IRB Approva l Date Subject Signed Surgery Date PI Signed HIPAA Subject Signed y 100 10. 25. 02 11. 19. 02 7. 16. 03 7. 01. 03 7. 16. 03 y 101 10. 25. 02 11. 19. 02 7. 23. 03 7. 22. 03 7. 23. 03 y 102 10. 25. 02 11. 19. 02 7. 23. 03 6. 27. 03 7. 23. 03 y 103 10. 25. 02 11. 19. 02 7. 14. 03 7. 15. 03 7. 14. 03 y 104 10. 25. 02 11. 19. 02 6. 03. 03 6. 04. 03 6. 03 Comments

Materials and Equipment: HSPP Location Project Approval Form Packet as approved by the IRB: IRB Approval Letter Date of approval____ Informed consent as approved by the IRB Approval dates checklists HIPAA Authorization Form as approved by the IRB Approval dates _______ Amendment Checklists Site Authorization Form Protocol Revision Checklists Brochure Revision Checklists Periodic Review Form Adverse Events documentation Copy of Verification Of Training Forms and log in checklist dates of all VOTF changes

Investigators’ Location Investigator’s File for clinical study and Patient Binders Review Study SOPs/Protocol Review Patient Informed consent/HIPPA and report on Consent Log Form Review Inclusion/Exclusion Signature pages and report on Patient I/E Form Review Case Review Forms for protocol compliance Review payments to patients per protocol/approved by IRB Review training records of personnel Review Regulatory Documents Review Drug or Device Accountability and Handing Other terms and conditions of IRB approval

Site Regulatory Binder Section 1: Protocols and Amendments Section 2: FDA Form 1572 Section 3: IRB Information and Approvals Section 4: Informed Consent & HIPAA Section 5: Personnel CV’s & Licensure Section 6: Investigator Brochure Section 7: SAEs and IND Safety Reports Section 8: Patient Screening & Enrollement

Site Regulatory Binder Section 9: Drug Accountability Section 10: Laboratory Information Section 11: Pharmacy Information Section 12: Monitoring Information Section 13: Site Signature Log Section 14: Case Report Forms Section 15: Correspondence Section 16: Other



Summary of GCP Requirements äWritten Procedures SOPs, Protocol ä Oversight IRB, Regulatory Agencies, Sponsor äInformed Consent Form (ICF) Subjects/ Patients

Summary of GCP Requirements (cont. ) ßQualification/ Training - Investigator, Nurses, Monitors, CRO, Lab, Auditors, etc ßDocumentation - Source, CRF, ICF, IDB,

Summary of GCP Requirements (cont. ) ß Sponsor responsible for implementing and maintaining quality systems ß Must be covered by written SOPs ß Must assure adherence to the protocol/SOPs/GCP/regulations ß Audit itself must be separate from routine monitoring and QC functions ß Must report any sites that are prematurely terminated from the study (ICH 5. 21, CFR 312. 56)

Summary of GCP Requirements (cont. ) ßAuditors must be: – Independent of clinical trial/data collection – Qualified by training and experience – Qualifications must be documented

FDA Findings Violations related to informed consent (21 CFR 50. 25, 21 CFR 50. 20, 21 CFR 312. 60) You failed to obtain proper informed consent in that the following essential elements of informed consent were not included in the consent form that was provided to the healthy volunteers. The consent form failed to disclose that the inhalation of XX was an experimental use of the drug.

FDA Findings Failure to inform the IRB of changes to the protocol [21 CFR 312. 66]. You failed to inform and obtain approval from [X] Institutional Review Board [X] for the changes in the protocols; …

FDA Findings Failure to conduct and investigation in accordance with the investigational plan 21 CFR Part 812. 110(b)). You failed to enroll subjects according to the inclusion/exclusion criteria. You also failed to perform all required tests at study visits. Your records contained instances where study procedures, including laboratory testing, were either not performed or were not consistently followed at scheduled examinations.

FDA Findings Your firm failed to ship investigational new drugs only to investigators participating in the investigation. [21 CFR 312. 53(b)] In May 2001, your firm shipped the wrong investigational vaccine to a clinical investigator who was not conducting a study for that test article. While the vial labels identified the test article that was shipped, it was not the test article that was intended for use with the type of cancer in the investigator’s protocol.

Fenster Warning Letter Public Health Service Food and Drug Administration 9200 Corporate Boulevard Rockville, Maryland 2085 March 21, 2006 WARNING LETTER VIA FEDERAL EXPRESS Paul Fenster, M. D. Associate Professor of Medicine University of Arizona 1501 North Campbell Ave. P. O. Box 245037 Tucson, AZ 85724 -5037 Dear Dr. Fenster: This Warning Letter is to inform you of objectionable conditions observed during the Food and Drug Administration (FDA) inspection conducted at your clinical site from October 3, 2005, through November 22, 2005, by an investigator from the FDA Los Angeles District Office. The purpose of this inspection was to determine whether activities and procedures related to your participation in the clinical study for the [redacted] , that ultimately resulted in approval under PMA [redacted] complied with applicable federal regulations. The [redacted] is a device as that term is defined in section 201(h) of the Federal Food, Drug, and Cosmetic Act (the Act), 21 U. S. C. 321(h). This letter also requests prompt corrective action to address the violations cited and discusses your written responses to the noted violations.

FDA Findings Failure to ensure that the investigation is conducted in accordance with the signed agreement with sponsor, the investigational plan, applicable FDA Regulations for protecting rights, safety and welfare of subjects under the investigator’s care and for control of devices under investigation, and any conditions of approval imposed by the FDA or IRB. [21 CFR 812. 100 and 21 CRF 812. 114(b). In addition, you failed to supervise device use. An investigator shall permit an investigational device to be sued only with subjects under the investigator’s supervision. [21 CRF 812. 110©].

Inspection Classifications NAI-No Action indicated. Minor or no objectionable practices VAI-Voluntary Action Indicated. Some objectionable conditions, but not serious enough for FDA to take action OAI-Official Action Indicated. FDA further action recommended.

Fraud in Clinical Studies Check for non-existent subjects and altered or fabricated data, personnel is as stated in the protocol If fraud is confirmed, FDA must be notified

 Marshall university financial aid office
Marshall university financial aid office Ohsu clinical trials office
Ohsu clinical trials office Marilyn monroe jesus
Marilyn monroe jesus Factory office layout
Factory office layout What is socra
What is socra Charlotte lemech
Charlotte lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Alcoac
Alcoac Role of statistician in clinical trials
Role of statistician in clinical trials Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Academic research organization
Academic research organization Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Asbmt clinical research training course
Asbmt clinical research training course Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Clinical research definition
Clinical research definition Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Drcr net
Drcr net Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Mrc clinical research training fellowship
Mrc clinical research training fellowship Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Pharmaceutical iwr applications
Pharmaceutical iwr applications Disadvantages of parliamentary law making
Disadvantages of parliamentary law making Death of marilyn monroe poem
Death of marilyn monroe poem Teori marilyn anne ray
Teori marilyn anne ray Marilyn monroe foto in rou gedig
Marilyn monroe foto in rou gedig Who is alex coopera boyfriend
Who is alex coopera boyfriend Marilyn burgess
Marilyn burgess Marilyn monroe taille 44
Marilyn monroe taille 44 Marilyn monroe murio por burlarse de dios
Marilyn monroe murio por burlarse de dios Marilyn burns fraction kit
Marilyn burns fraction kit Marilyn friend co teaching
Marilyn friend co teaching Marilyn monroe murio despues de burlarse de dios
Marilyn monroe murio despues de burlarse de dios Marilyn monroe billy graham
Marilyn monroe billy graham Utwr
Utwr Who did elton john wrote candle in the wind for
Who did elton john wrote candle in the wind for Marilyn monroe always gets her man in la county
Marilyn monroe always gets her man in la county Actual texture
Actual texture Marilyn carney
Marilyn carney Marilyn fy
Marilyn fy Marilyn monroe billy graham
Marilyn monroe billy graham Autumn rhythm 1950
Autumn rhythm 1950 A&m records v napster
A&m records v napster Marilyn green
Marilyn green Marilyn monroe
Marilyn monroe Bureaucratic caring theory
Bureaucratic caring theory Postmodern feminism
Postmodern feminism Dr marilyn king
Dr marilyn king Marshall and swift equipment cost index table
Marshall and swift equipment cost index table Presidential and radical reconstruction venn diagram
Presidential and radical reconstruction venn diagram Marshall plan and truman doctrine
Marshall plan and truman doctrine Health and safety regulations in engineering
Health and safety regulations in engineering Animal quarantine department
Animal quarantine department Six pack health and safety regulations
Six pack health and safety regulations Rules and regulations in table tennis
Rules and regulations in table tennis


















































































