CHEMISTRY Is the study of the composition structure


















- Slides: 26

CHEMISTRY: Is the study of the composition, structure, and properties of matter.

MATTER: Anything with mass and volume. Fun- Fact: Matter can be changed by energy…

Properties of matter: Every substance has unique characteristic properties Extensive properties - depend on the amount of matter present. Intensive properties – DO NOT depend on the amount of matter present.

Extensive Properties: • • Mass Volume Length Amount of energy present

Intensive properties: • • • Solubility Texture Luster Taste Color Hardness Clarity Ductility Radioactivity Malleability Compressability • • • Melting point State of matter Density p. H Viscosity Magnetism Boiling point Cohesion Odor Electrical resistance

Physical properties Can be measured or observed without altering the composition of the material. Color, Shape, Size, density, melting, freezing or boiling point, malleability, ductility.

Chemical Properties: A characteristic of a substance that indicates whether it can undergo a certain chemical change, transforming it into a different substance. Example: flammability!

Change

Physical changes Do not change the identity or composition of the substance. Examples: melting, boiling, condensation, dissolving, deposition, sublimination

Chemical Changes A change that produces a new type of matter with properties different from the original. Ex: tarnishing, cooking, rusting Also called “Chemical reactions”

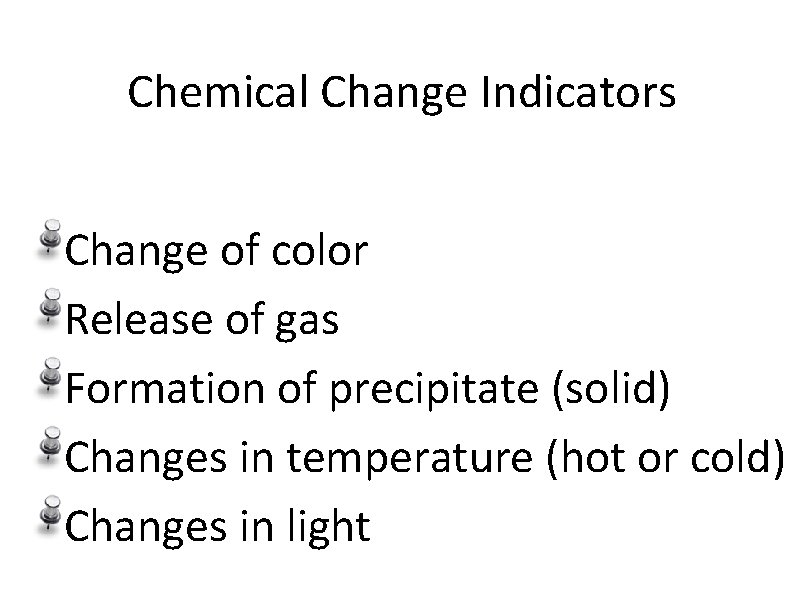
Chemical Change Indicators Change of color Release of gas Formation of precipitate (solid) Changes in temperature (hot or cold) Changes in light

Chemical Reactions Can be written as a chemical equation: Carbon + Oxygen Carbon Dioxide Reactants are the substances that react Carbon and Oxygen Products are what is formed (carbon dioxide) Carbon Dioxide

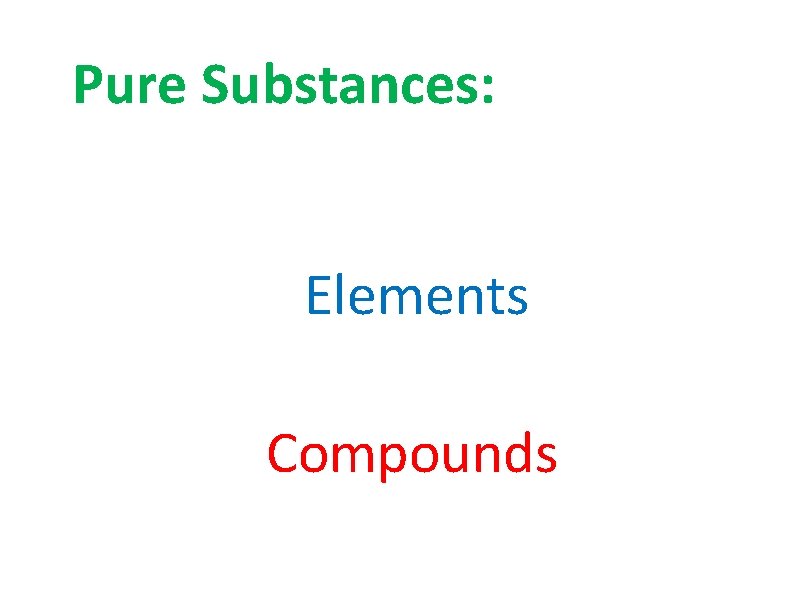
Pure Substances: Elements Compounds

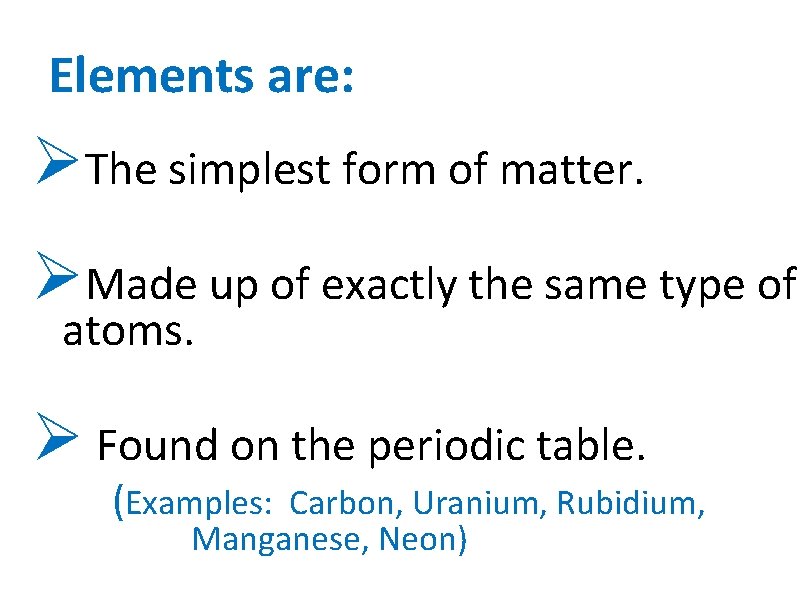
Elements are: ØThe simplest form of matter. ØMade up of exactly the same type of atoms. Ø Found on the periodic table. (Examples: Carbon, Uranium, Rubidium, Manganese, Neon)

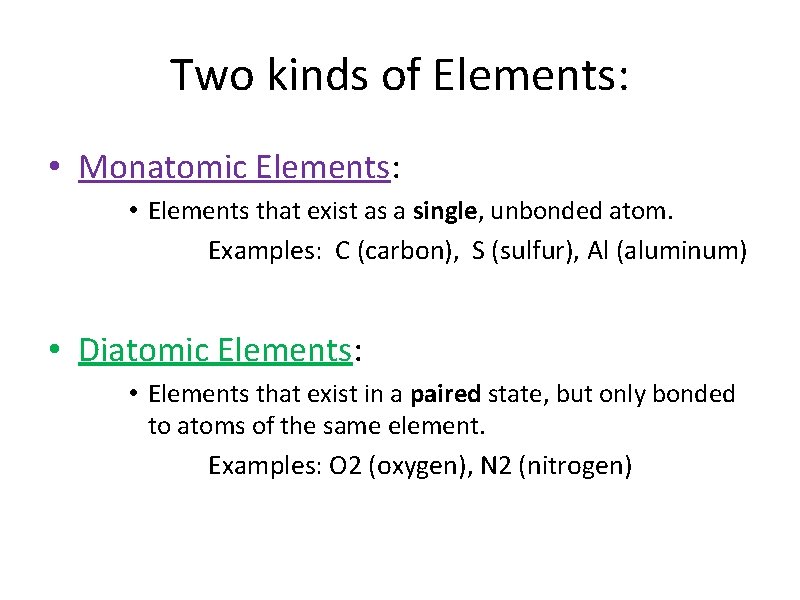
Two kinds of Elements: • Monatomic Elements: • Elements that exist as a single, unbonded atom. Examples: C (carbon), S (sulfur), Al (aluminum) • Diatomic Elements: • Elements that exist in a paired state, but only bonded to atoms of the same element. Examples: O 2 (oxygen), N 2 (nitrogen)

Compounds are: Ø Pure substances Ø Made from atoms of two or more different elements in a fixed ratio. Ex: sodium chloride (Na. Cl), water (H 20)

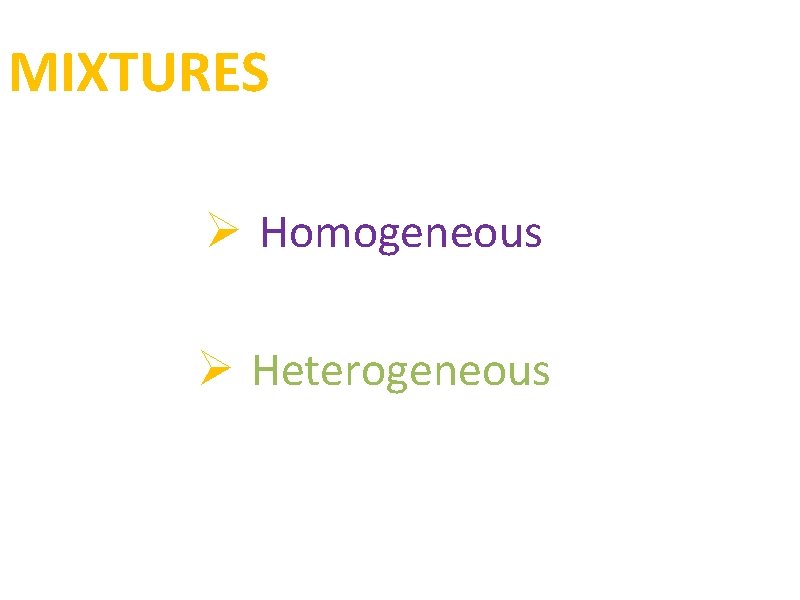
MIXTURES Ø Homogeneous Ø Heterogeneous

Mixtures are: Ø Made up of two or more substances that can be separated using physical means. Ø Each component retains its own characteristics.

Homogeneous mixtures ØThe composition & properties are uniform throughout the mixture. ØAlso called “Solutions”. Examples: sugar water, sea water, brass, air

Homogeneous mixtures ØParticles cannot be seen with a microscope. ØParticles can’t be separated using a centrifuge or filter.

Homogeneous mixtures ØAre clear solutions. ØThe light goes straight through it when using the Tyndall effect test.

Heterogeneous Mixtures ØAre not solutions. ØThe composition and properties are not uniform throughout. Examples: granite, blood, wood, milk

Heterogeneous Mixtures ØThe particles can be seen with a microscope. ØThe particles can be separated using a centrifuge or filter.

Heterogenous Mixtures Two main types: ØColloids ØSuspensions

Colloids Øare heterogeneous mixtures where the particles never settle out. ØTyndall Effect-particles scatter light. ex: gelatin, fog

Suspensions ØAre heterogeneous mixtures containing a liquid in which visible particles settle out upon standing. Examples: Muddy water, cough syrup or anything that says “shake well” before using.