A Chemistry study of the composition structure properties







- Slides: 26


A. Chemistry = study of the composition, structure, properties, and reactions of a substance. • Life depends on chemistry. . . Because chemical compounds are the building blocks of life.

ØNOTE: MASS= Mass vs. weight animati on The quantity of matter an object has. MASS AND WEIGHT ARE NOT THE SAME!

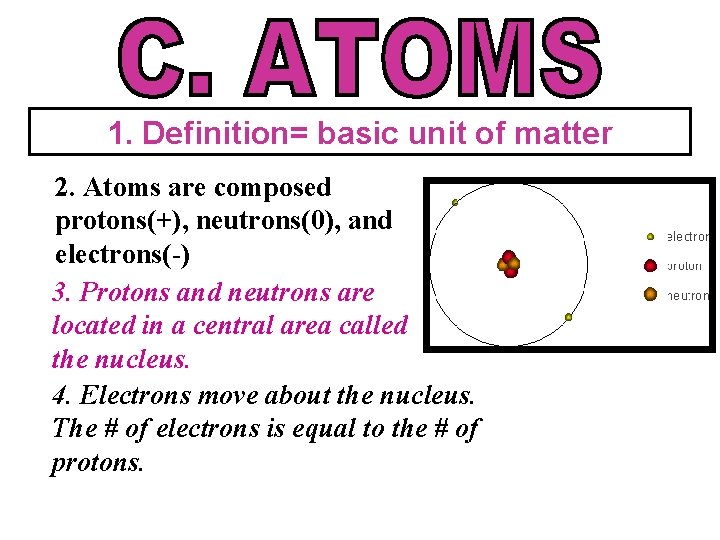
1. Definition= basic unit of matter 2. Atoms are composed protons(+), neutrons(0), and electrons(-) 3. Protons and neutrons are located in a central area called the nucleus. 4. Electrons move about the nucleus. The # of electrons is equal to the # of protons.

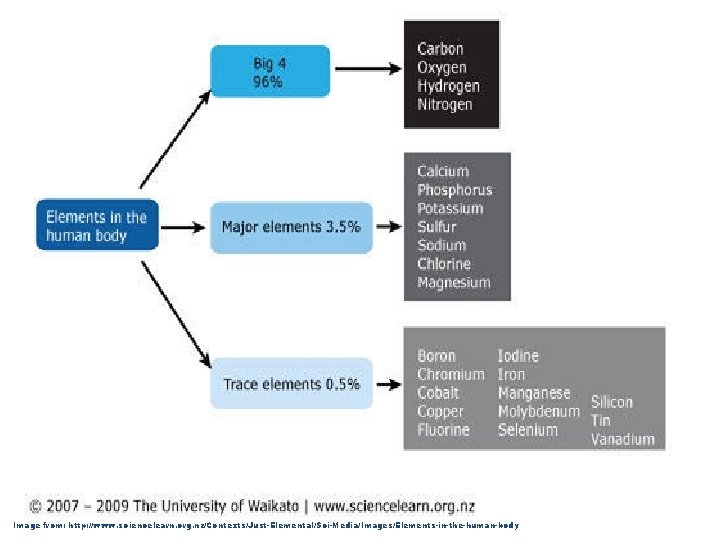
1. Definition = a pure substance that contains only one type of atom 2. About 96% of the mass of all kinds of living things is composed of a combination of just 4 elements…

Image from: http: //www. sciencelearn. org. nz/Contexts/Just-Elemental/Sci-Media/Images/Elements-in-the-human-body


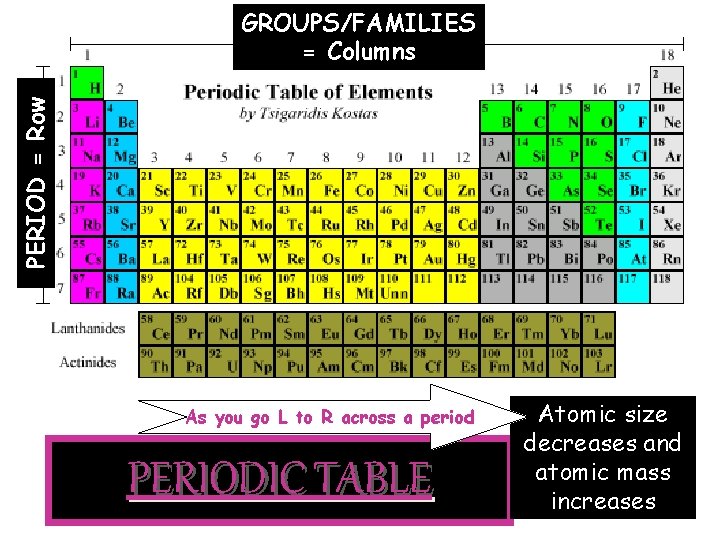
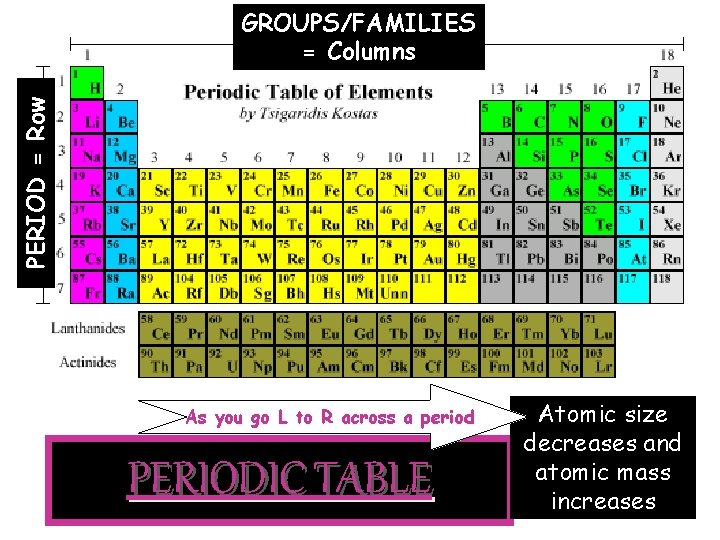
3. Periodic Table—developed as a way to organize elements Ø Elements found in order of atomic number (# of protons) ØAtomic weight (# of protons + # of neutrons) get bigger as you go across and down ØPeriods of elements = rows ---As you go across a row, elements get smaller in size BUT greater in mass ---Example: The go from a beach ball to a bowling ball Ø Groups of elements = columns Elements in the same group have similar properties and will bond in similar ways

PERIOD = Row GROUPS/FAMILIES = Columns As you go L to R across a period PERIODIC TABLE Atomic size decreases and atomic mass increases

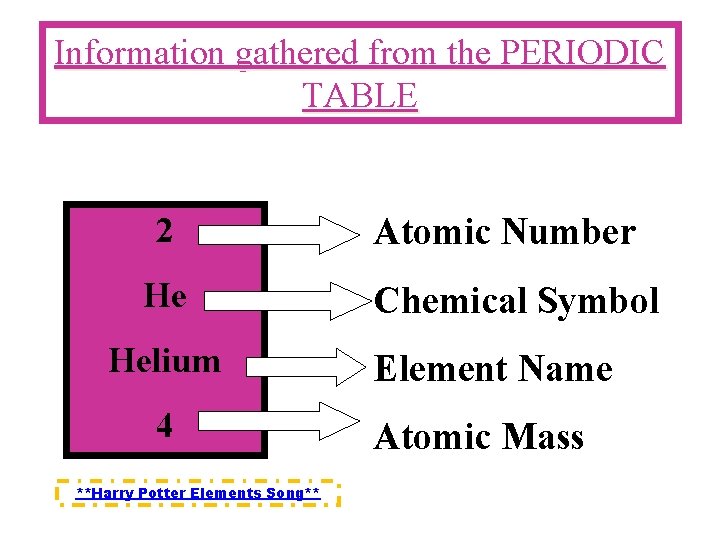
Information gathered from the PERIODIC TABLE 2 He Helium 4 **Harry Potter Elements Song** Atomic Number Chemical Symbol Element Name Atomic Mass

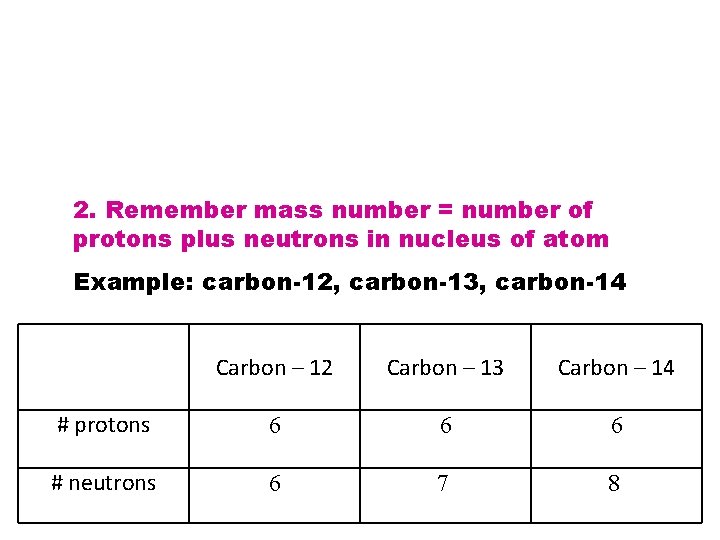
2. Remember mass number = number of protons plus neutrons in nucleus of atom Example: carbon-12, carbon-13, carbon-14 Carbon – 12 Carbon – 13 Carbon – 14 # protons 6 6 6 # neutrons 6 7 8

PERIOD = Row GROUPS/FAMILIES = Columns As you go L to R across a period PERIODIC TABLE Atomic size decreases and atomic mass increases

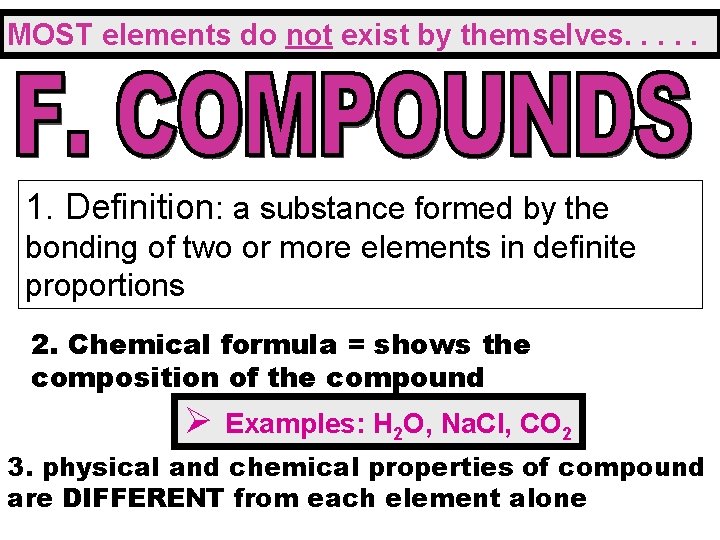
MOST elements do not exist by themselves. . . 1. Definition: a substance formed by the bonding of two or more elements in definite proportions 2. Chemical formula = shows the composition of the compound Ø Examples: H 2 O, Na. Cl, CO 2 3. physical and chemical properties of compound are DIFFERENT from each element alone

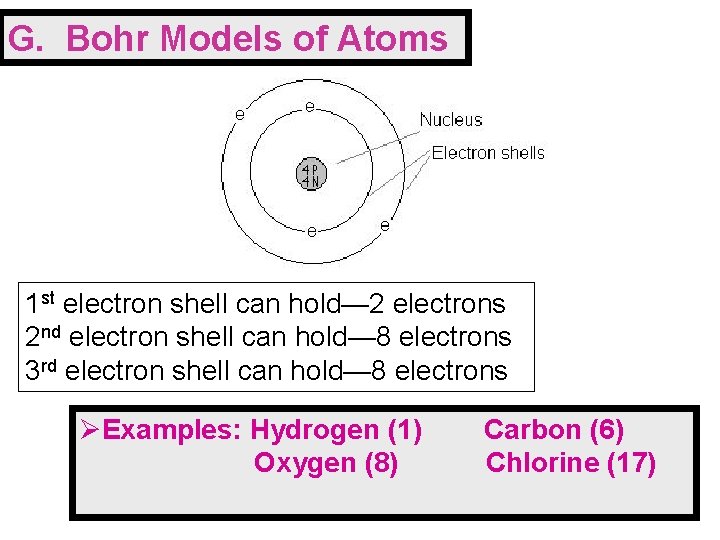
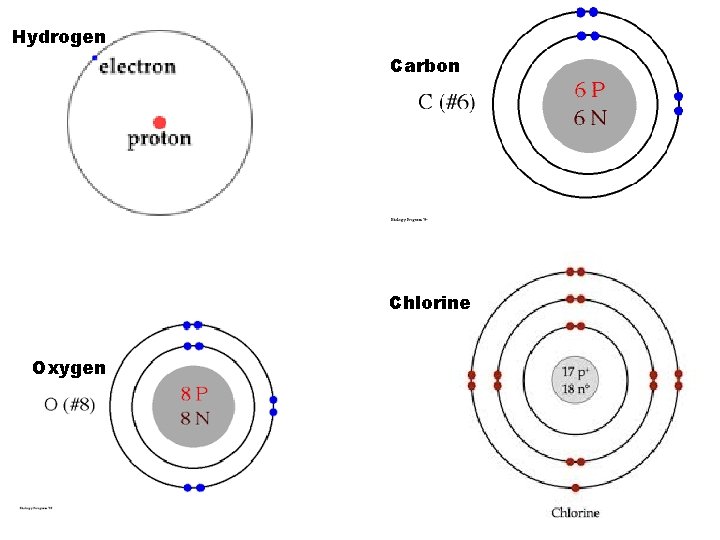
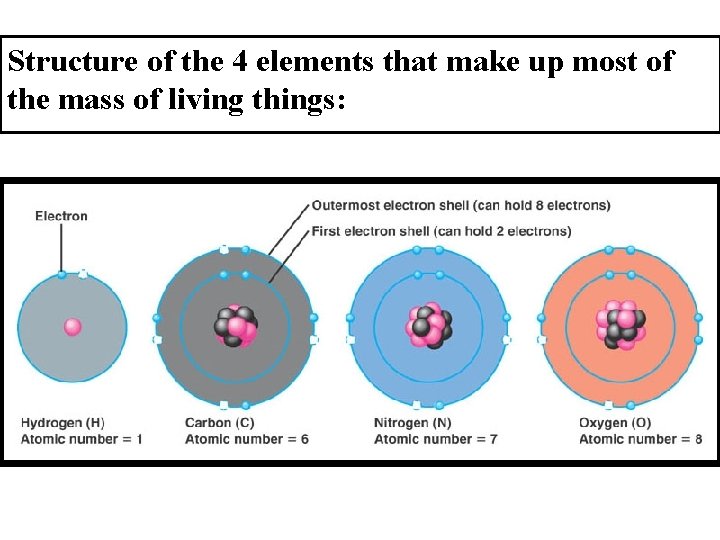
G. Bohr Models of Atoms 1 st electron shell can hold— 2 electrons 2 nd electron shell can hold— 8 electrons 3 rd electron shell can hold— 8 electrons ØExamples: Hydrogen (1) Oxygen (8) Carbon (6) Chlorine (17)

Hydrogen Carbon Chlorine Oxygen

1. Definition= the forces that hold together the atoms that make up compounds 2. Two main types of STRONG chemical bonds: 1= ionic bonds 2= covalent bonds. . . Let’s take a look at each type more closely. .

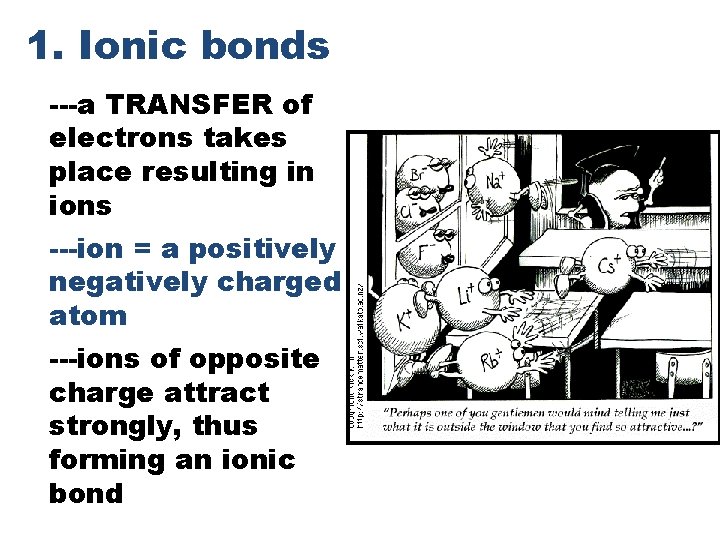
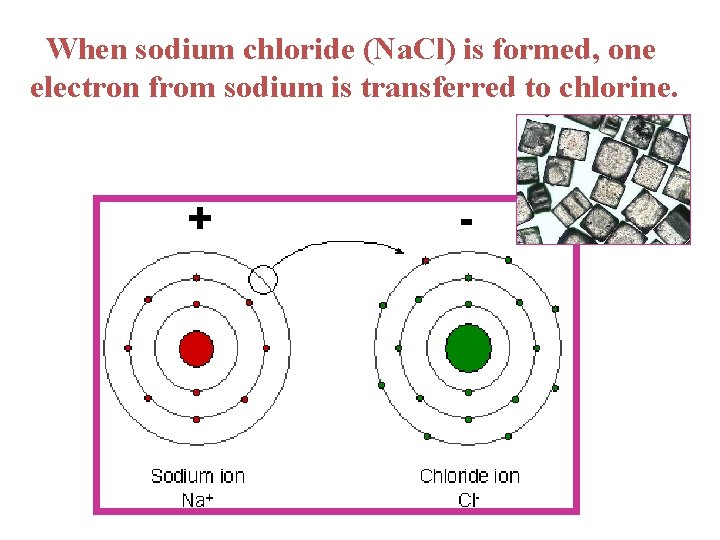
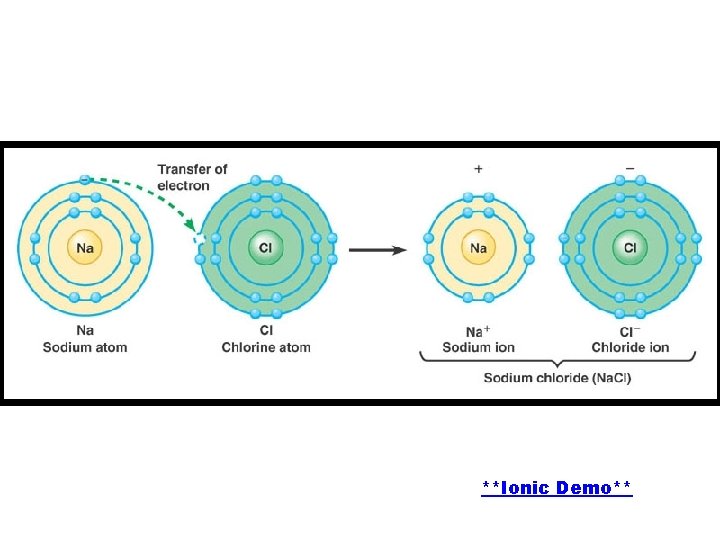
1. Ionic bonds ---a TRANSFER of electrons takes place resulting in ions ---ion = a positively or negatively charged atom ---ions of opposite charge attract strongly, thus forming an ionic bond

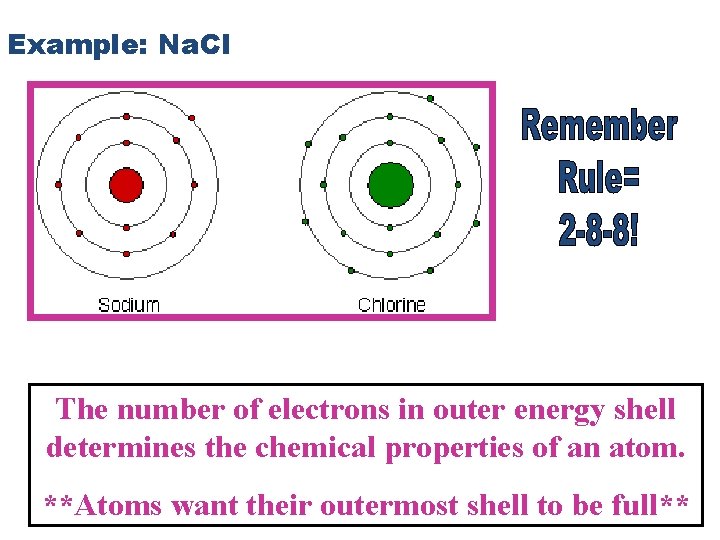
Example: Na. Cl The number of electrons in outer energy shell determines the chemical properties of an atom. **Atoms want their outermost shell to be full**

When sodium chloride (Na. Cl) is formed, one electron from sodium is transferred to chlorine.

**Ionic Demo**

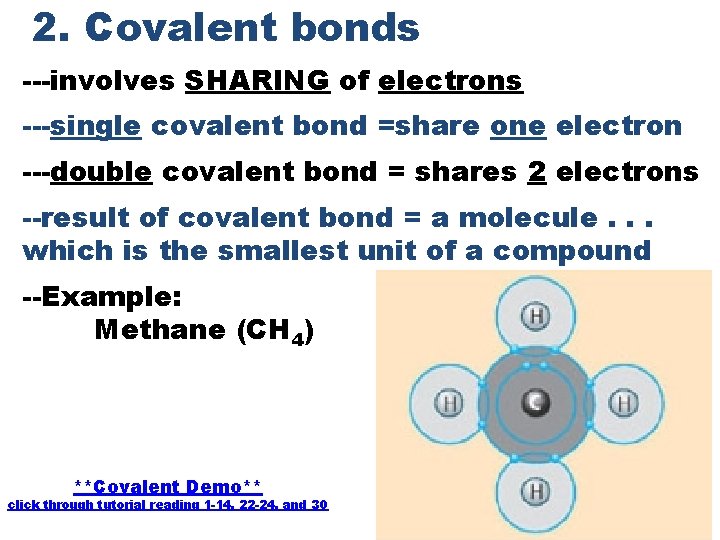
2. Covalent bonds ---involves SHARING of electrons ---single covalent bond =share one electron ---double covalent bond = shares 2 electrons --result of covalent bond = a molecule. . . which is the smallest unit of a compound --Example: Methane (CH 4) **Covalent Demo** click through tutorial reading 1 -14, 22 -24, and 30

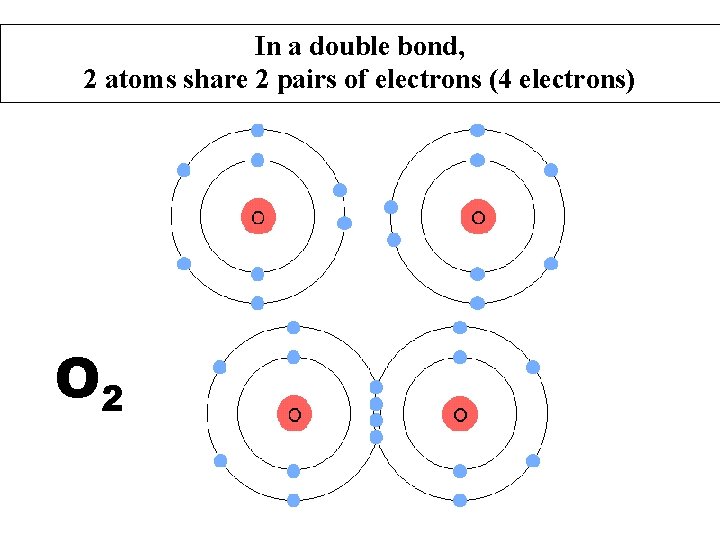
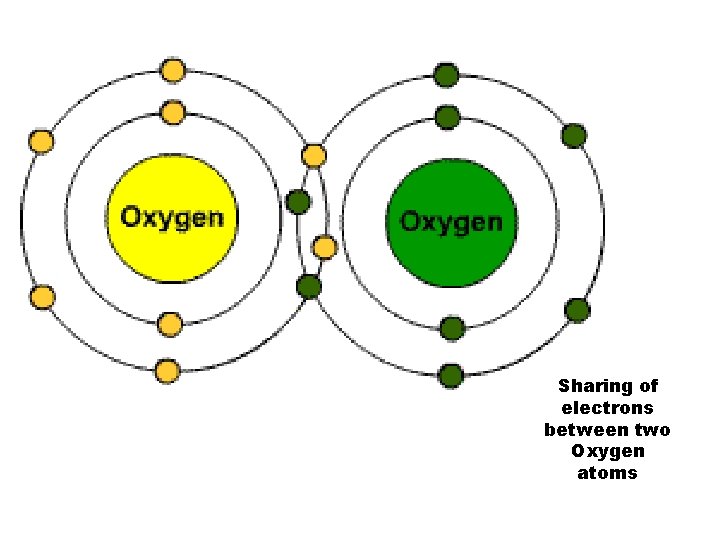
In a double bond, 2 atoms share 2 pairs of electrons (4 electrons) O 2

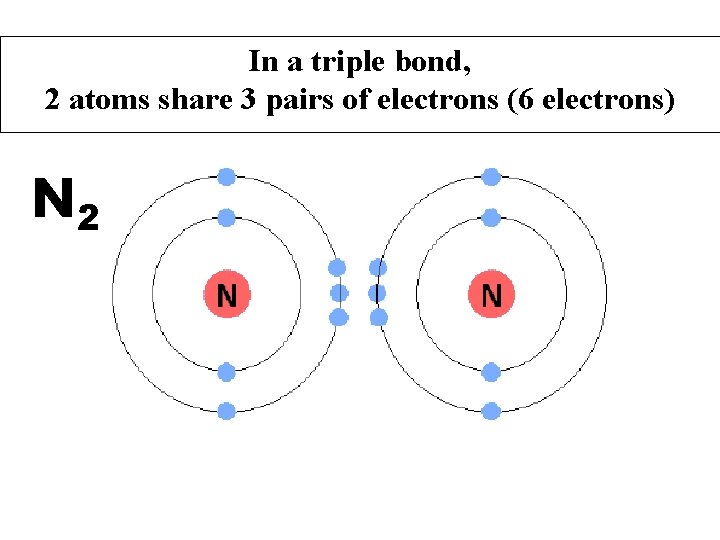
In a triple bond, 2 atoms share 3 pairs of electrons (6 electrons) N 2

Sharing of electrons between two Oxygen atoms

Structure of the 4 elements that make up most of the mass of living things:
