Chemistry 125 Lecture 61 March 26 2010 NMR



- Slides: 30

Chemistry 125: Lecture 61 March 26, 2010 NMR Spectroscopy Through-Space Coupling, Decoupling & Correlation This For copyright notice see final page of this file

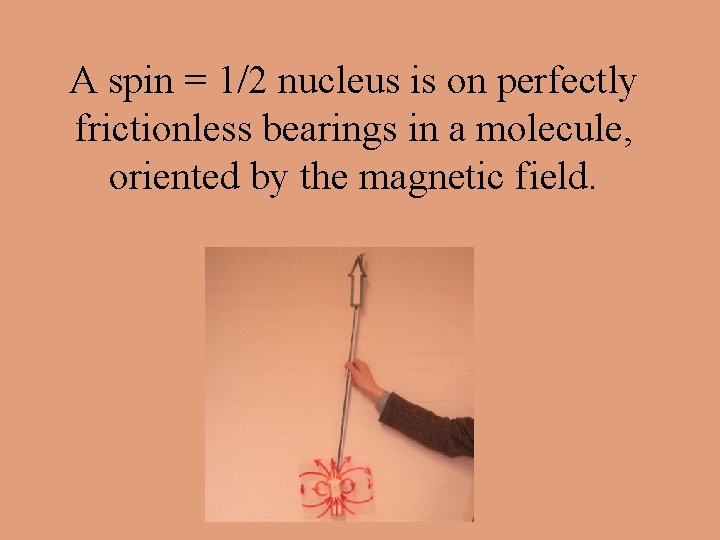
A spin = 1/2 nucleus is on perfectly frictionless bearings in a molecule, oriented by the magnetic field.

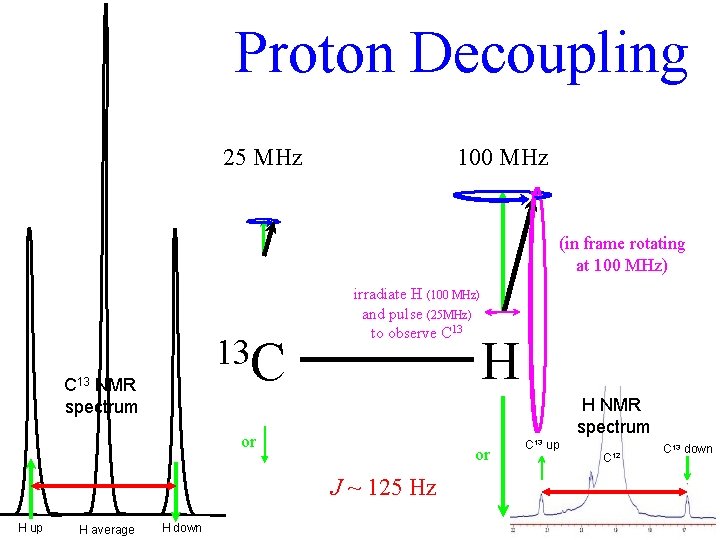
Proton Decoupling 25 MHz 100 MHz (in frame rotating at 100 MHz) 13 C C 13 NMR spectrum irradiate H (100 MHz) and pulse (25 MHz) to observe C 13 H NMR spectrum or or J ~ 125 Hz H up H average H down H C 13 up C 12 C 13 down

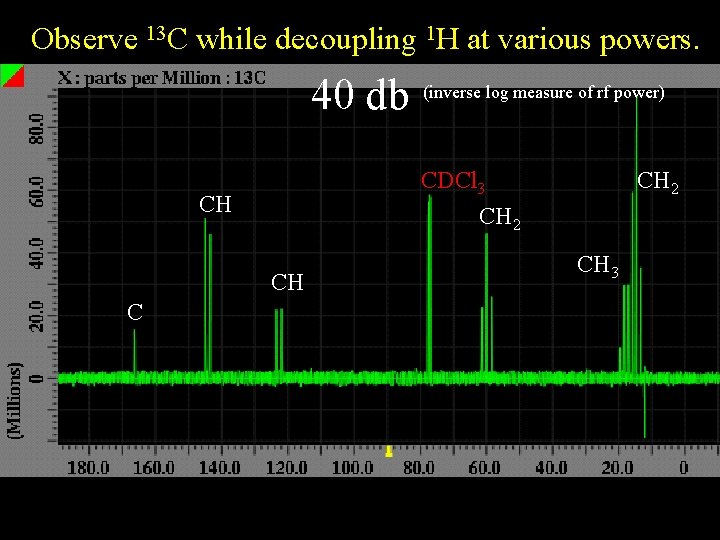
Observe 13 C while decoupling 1 H at various powers. 40 db CDCl 3 CH 2 CH CH C (inverse log measure of rf power) CH 2 CH 3

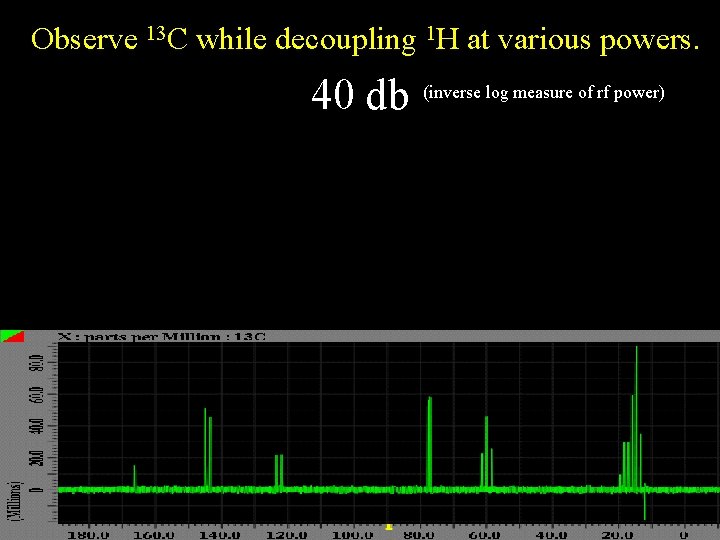
Observe 13 C while decoupling 1 H at various powers. 40 db (inverse log measure of rf power)

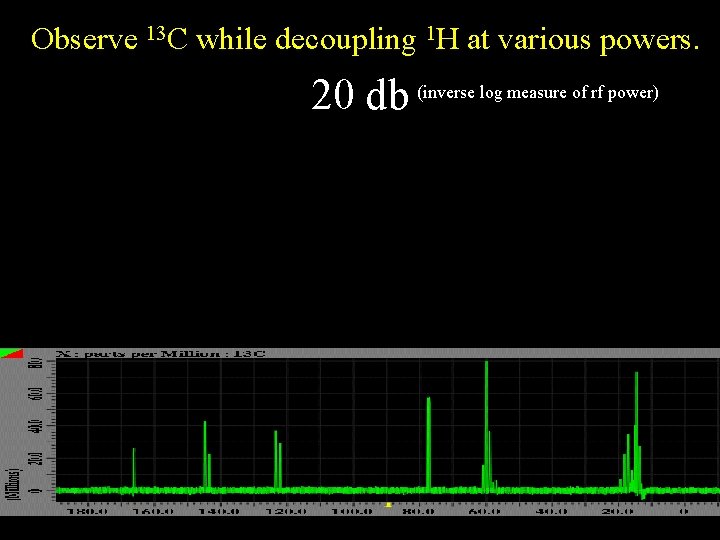
Observe 13 C while decoupling 1 H at various powers. 20 db (inverse log measure of rf power)

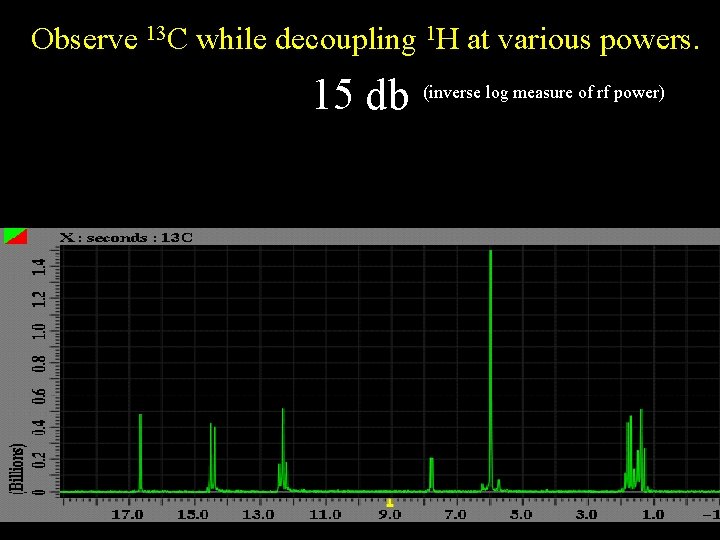
Observe 13 C while decoupling 1 H at various powers. 15 db (inverse log measure of rf power)

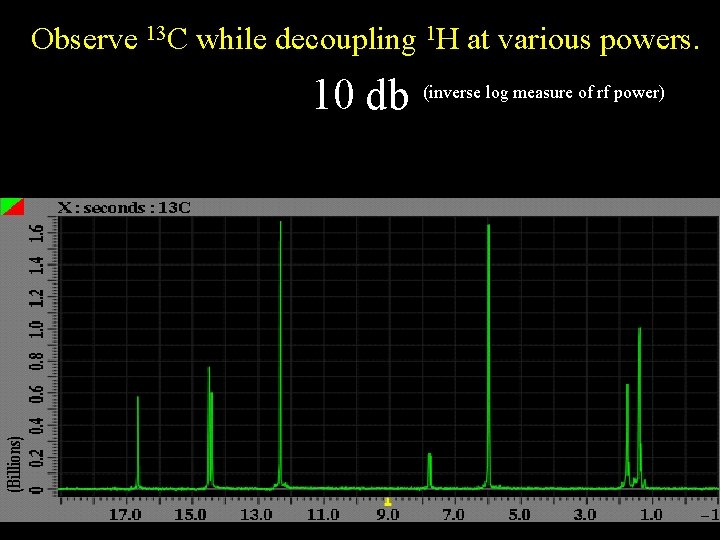
Observe 13 C while decoupling 1 H at various powers. 10 db (inverse log measure of rf power)

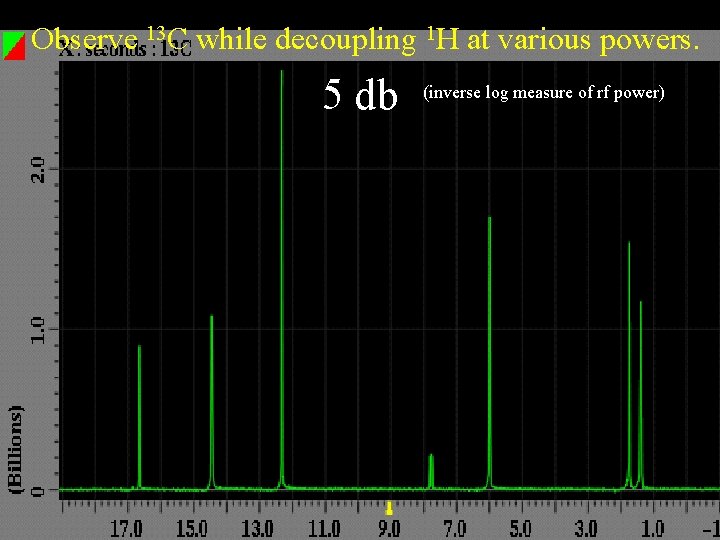
Observe 13 C while decoupling 1 H at various powers. 5 db (inverse log measure of rf power)

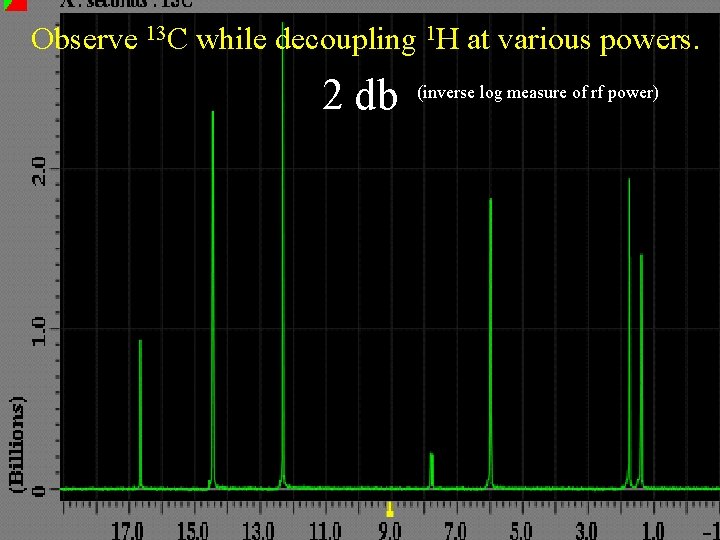
Observe 13 C while decoupling 1 H at various powers. 2 db (inverse log measure of rf power)

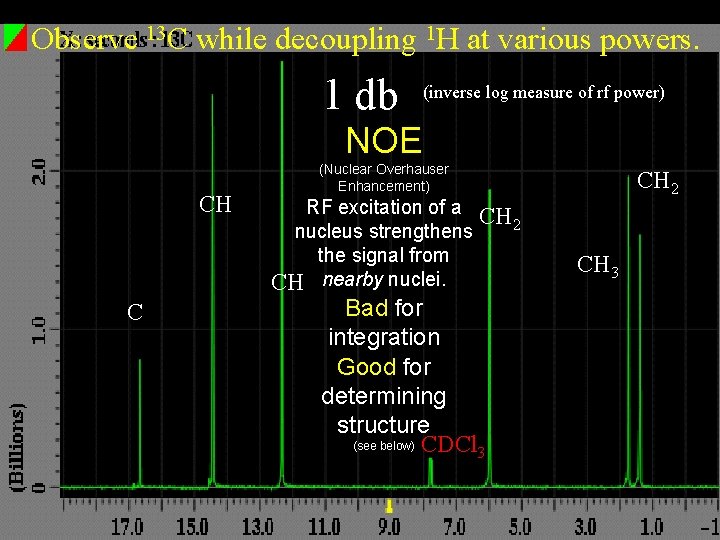
Observe 13 C while decoupling 1 H at various powers. 1 db (inverse log measure of rf power) NOE CH C (Nuclear Overhauser Enhancement) RF excitation of a CH 2 nucleus strengthens the signal from CH nearby nuclei. Bad for integration Good for determining structure (see below) CDCl 3 CH 2 CH 3

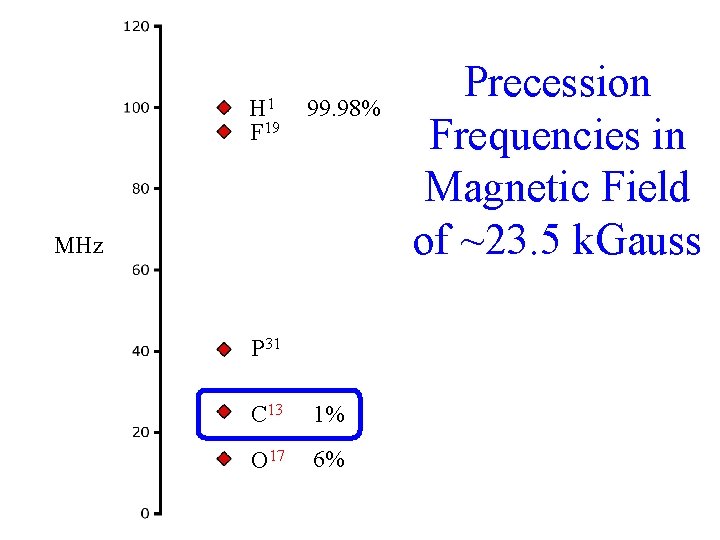
H 1 F 19 99. 98% MHz P 31 C 13 1% O 17 6% Precession Frequencies in Magnetic Field of ~23. 5 k. Gauss

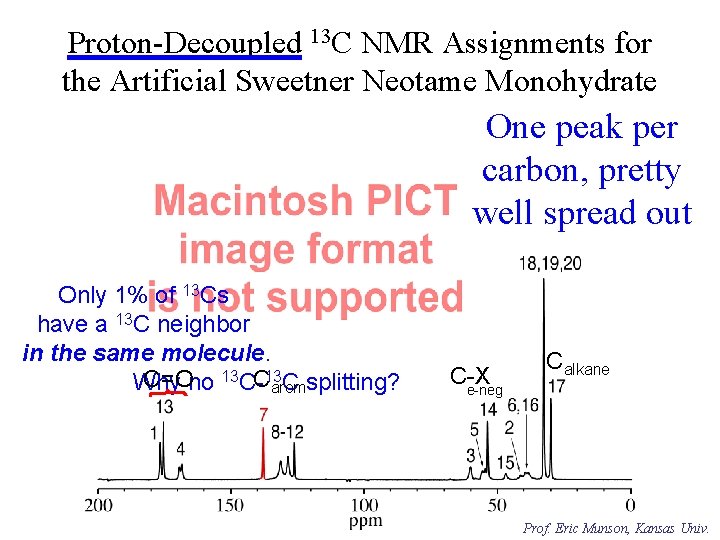
Proton-Decoupled 13 C NMR Assignments for the Artificial Sweetner Neotame Monohydrate One peak per carbon, pretty well spread out Only 1% of 13 Cs have a 13 C neighbor in the same molecule. C=Ono 13 C-C 13 arom Why C splitting? C-X e-neg Calkane Prof. Eric Munson, Kansas Univ.

Power of Correlation: Dilute 13 C Double Labeling 2 -D NMR 2 -D Chromatography

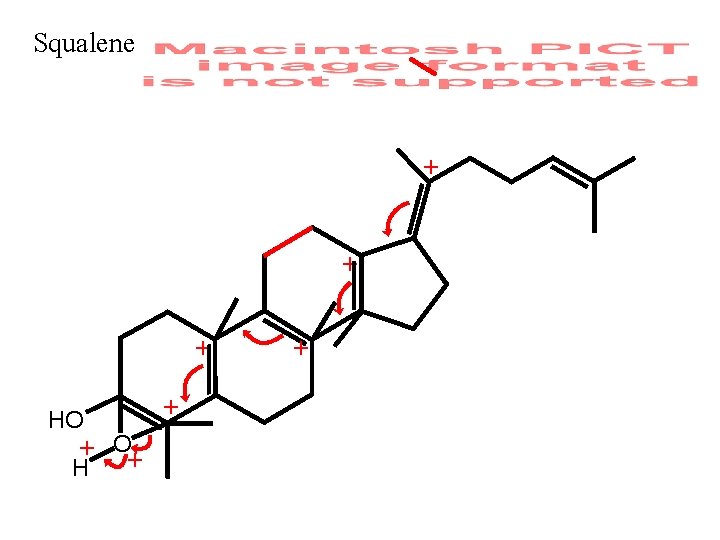
Double Labeling Introduction: Lanosterol Biogenesis Cf. Frames 6 -13 of Lecture 52 and Sec. 12. 13 pp. 554 -562

Squalene + + + HO + O+ H + +

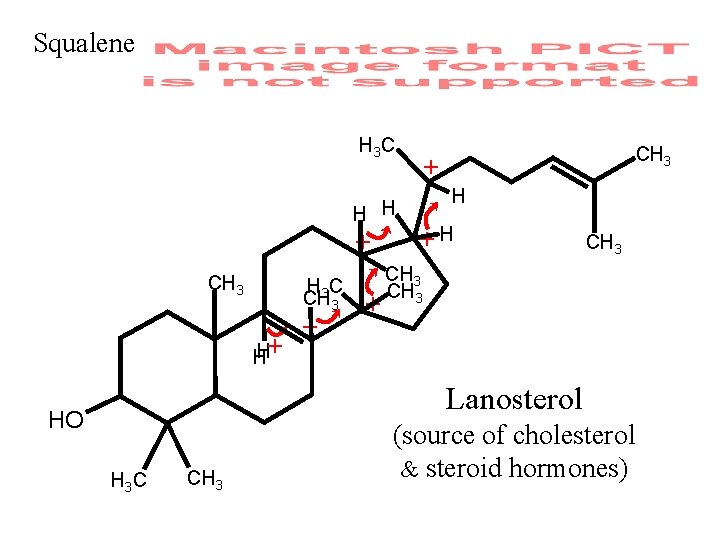
Squalene H 3 C H H + CH 3 C CH 3 H+ H + + CH 3 + H +H CH 3 Lanosterol HO H 3 C CH 3 (source of cholesterol & steroid hormones)

Squalene H 3 C Cute Story H H + CH 3 + 3° 3° H +H 3° CH 3 C CH 3 + + 3° 3° CH 3 Is it True? CH 3 H+ H Lanosterol (Wait for NMR) HO H 3 C CH 3 (source of cholesterol & steroid hormones)

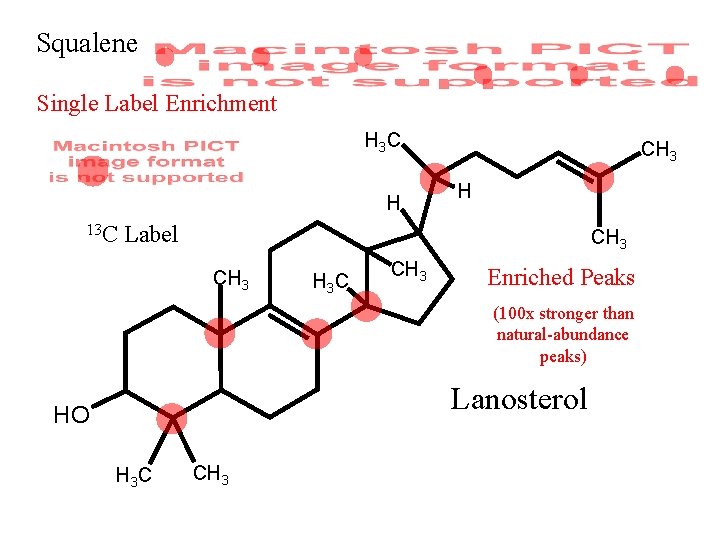
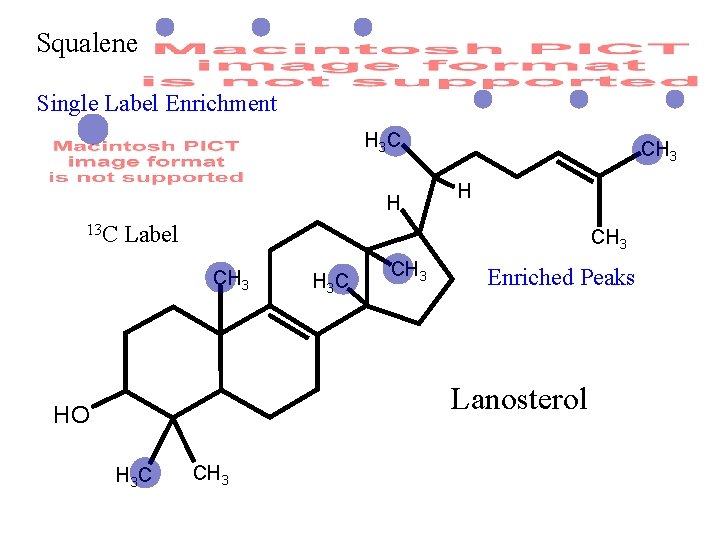
Squalene Single Label Enrichment H 3 C H 13 C CH 3 H Label CH 3 H 3 C CH 3 Enriched Peaks (100 x stronger than natural-abundance peaks) Lanosterol HO H 3 C CH 3

Squalene Single Label Enrichment H 3 C H 13 C CH 3 H Label CH 3 H 3 C CH 3 Enriched Peaks Lanosterol HO H 3 C CH 3

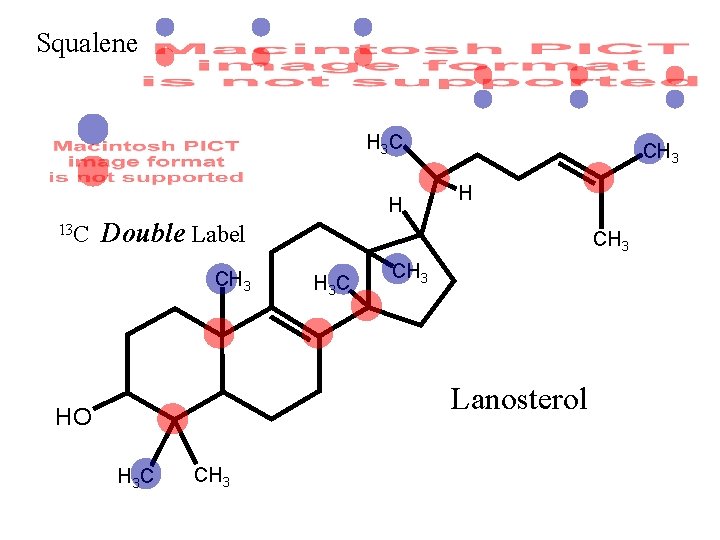
Squalene H 3 C H 13 C CH 3 H Double Label CH 3 H 3 C CH 3 Lanosterol HO H 3 C CH 3

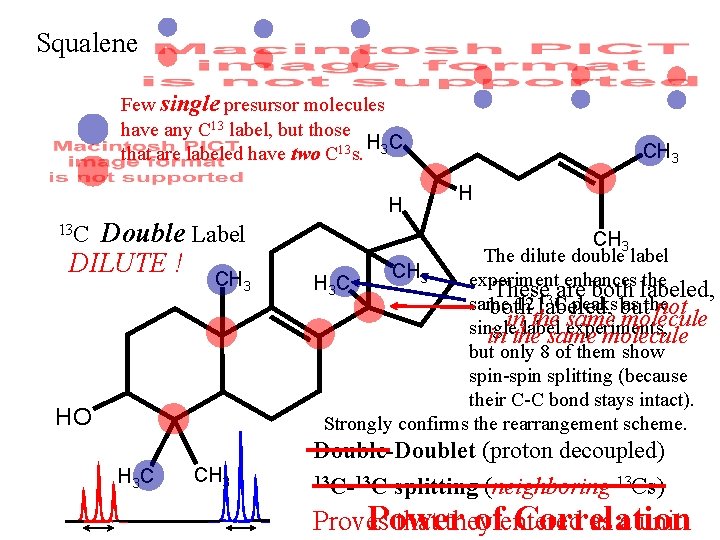
Squalene Few single presursor molecules have any C 13 label, but those HC that are labeled have two C 13 s. 3 H Double Label DILUTE ! 13 C CH 3 HO H 3 C CH 3 H CH 3 The dilute double label CH 3 experiment enhances the H 3 C These 13 are both labeled, same 12 labeled, C peaks but as the both not in the same molecule single label experiments, in the same molecule but only 8 of them show spin-spin splitting (because their C-C bond stays intact). Strongly confirms the rearrangement scheme. Double-Doublet (proton decoupled) 13 C-13 C splitting (neighboring 13 Cs) Proves that they as a unit. Power ofentered Correlation

Power of Correlation: Dilute 13 C Double Labeling 2 -D NMR 2 -D Chromatography

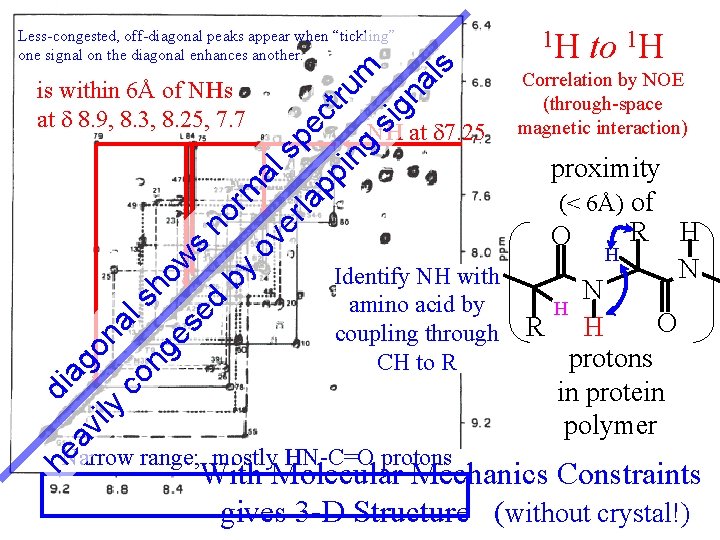
on co al s ng ho es w ed s n by orm ov al er sp la pp ect ru in m g si gn al s Less-congested, off-diagonal peaks appear when “tickling” one signal on the diagonal enhances another. av i ly di ag is within 6Å of NHs at 8. 9, 8. 3, 8. 25, 7. 7 NH at 7. 25 to 1 H Correlation by NOE (through-space magnetic interaction) proximity (< 6Å) of R H O H N Identify NH with N amino acid by H O H coupling through R protons CH to R in protein polymer Narrow range; mostly HN-C=O protons he 1 H With Molecular Mechanics Constraints gives 3 -D Structure (without crystal!)

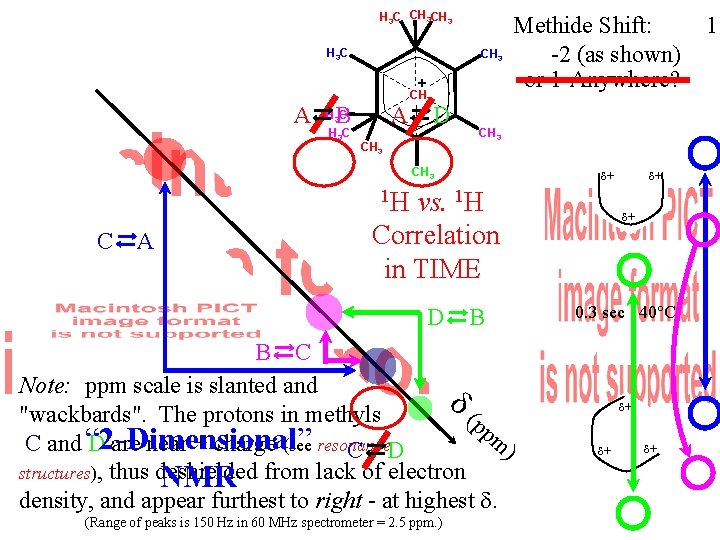
H 3 C CH 3 + A A B H 3 C CH 3 D CH 3 Methide Shift: -2 (as shown) or 1 -Anywhere? CH 3 + + CH 3 1 H C A vs. 1 H Correlation in TIME D B B C Note: ppm scale is slanted and (p "wackbards". The protons in methyls pm C and “ 2 -Dimensional” D are near + charge (see resonance ) C D structures), thus deshielded NMR from lack of electron density, and appear furthest to right - at highest . (Range of peaks is 150 Hz in 60 MHz spectrometer = 2. 5 ppm. ) + + + 0. 3 sec 40°C + + + 1

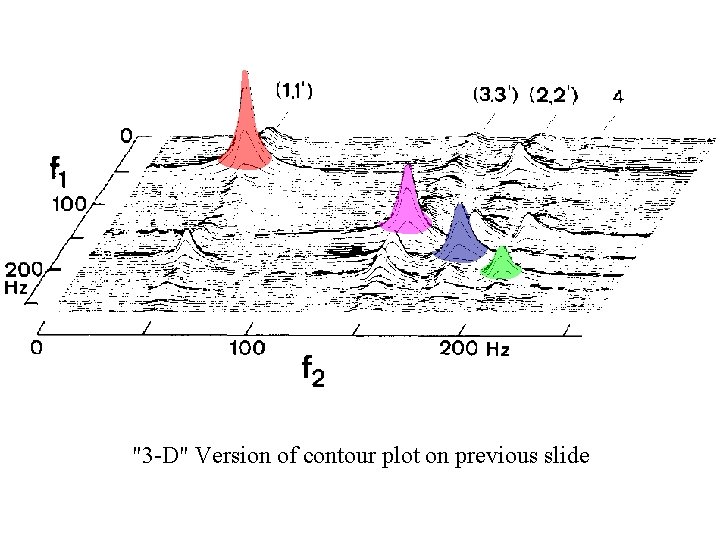
"3 -D" Version of contour plot on previous slide

Power of Correlation: Dilute 13 C Double Labeling 2 -D NMR 2 -D Chromatography

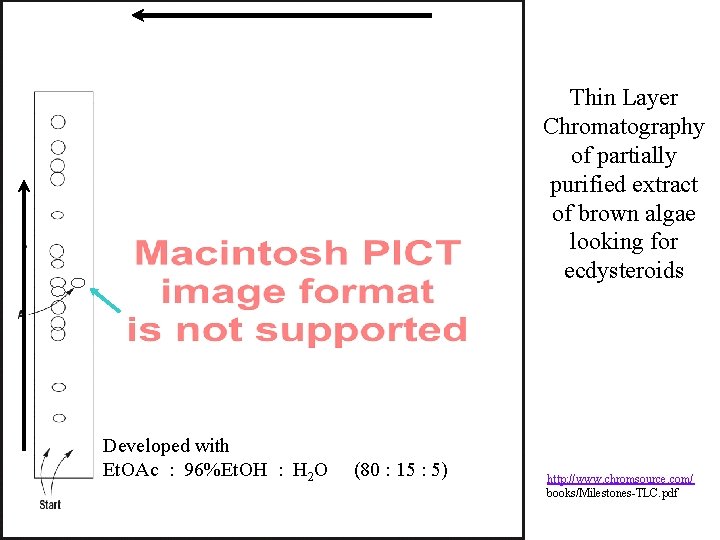
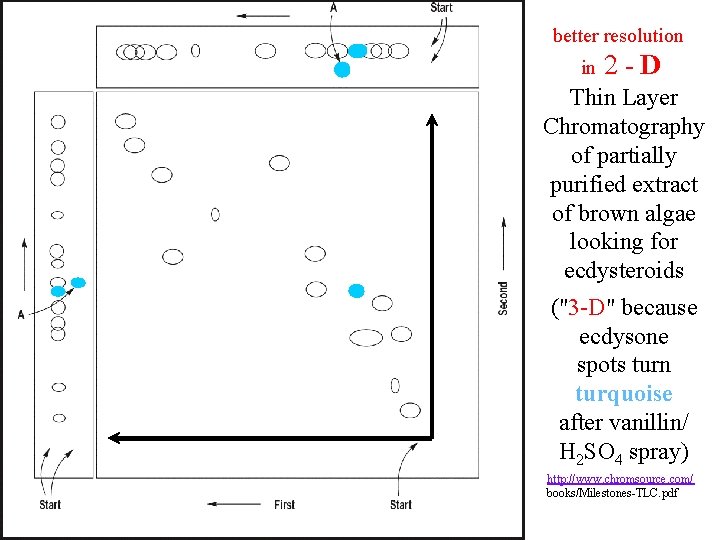
Developed with CHCl 3 : Me. OH : C 6 H 6 (25 : 3) Developed with Et. OAc : 96%Et. OH : H 2 O (80 : 15 : 5) Thin Layer Chromatography of partially purified extract of brown algae looking for ecdysteroids http: //www. chromsource. com/ books/Milestones-TLC. pdf

better resolution in 2 -D Thin Layer Chromatography of partially purified extract of brown algae looking for ecdysteroids ("3 -D" because ecdysone spots turn turquoise after vanillin/ H 2 SO 4 spray) http: //www. chromsource. com/ books/Milestones-TLC. pdf

End of Lecture 61 March 26, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 Poland national anthem lyrics
Poland national anthem lyrics March 22 2010
March 22 2010 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad June 2010 chemistry regents answers
June 2010 chemistry regents answers An introduction to atmospheric physics
An introduction to atmospheric physics Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Przesunięcie chemiczne nmr
Przesunięcie chemiczne nmr Tabel pergeseran kimia h nmr
Tabel pergeseran kimia h nmr 4 methylacetophenone nmr
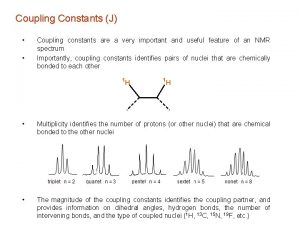
4 methylacetophenone nmr Geminal and vicinal coupling constants
Geminal and vicinal coupling constants Multipletowość nmr
Multipletowość nmr Nmr instrument diagram
Nmr instrument diagram Resonance in nmr
Resonance in nmr Nmr sample requirements
Nmr sample requirements Nmr active and inactive nuclei
Nmr active and inactive nuclei 1 bromopropane nmr
1 bromopropane nmr Benzoic acid hnmr
Benzoic acid hnmr 247remote
247remote Application of nmr
Application of nmr Alkyne carbon nmr
Alkyne carbon nmr Nuclear spin quantum number
Nuclear spin quantum number Nmr polymer
Nmr polymer Nmr
Nmr Nmr spectrum of 1 1 2-tribromoethane
Nmr spectrum of 1 1 2-tribromoethane Tabel pergeseran kimia h nmr
Tabel pergeseran kimia h nmr Nmr esr
Nmr esr Nmr picture
Nmr picture Ketone nmr
Ketone nmr Https www google de
Https www google de