Lecture 5 c Introduction 1 HNMR spectroscopy is

- Slides: 10

Lecture 5 c

Introduction • 1 H-NMR spectroscopy is used to determine the structure of the epoxide based on characteristic splitting patterns in the aromatic range and the epoxide range • When analyzing the spectrum, it will become much more difficult if the submitted sample is a mixture of many compounds i. e. , epoxide, aldehyde, water (d=1. 56 ppm), ethyl acetate (d=1. 26 ppm, 2. 05 ppm and 4. 12 ppm), hexane (d=0. 88 ppm, 1. 26 ppm), etc. (see SKR, p. 284) • The proton spectrum will exhibit a singlet at d=7. 26 ppm due to the presence of CDCl 3 if the concentration of the epoxide is very low • The carbon spectrum will show a “triplet” at d=77 ppm due to the presence of CDCl 3

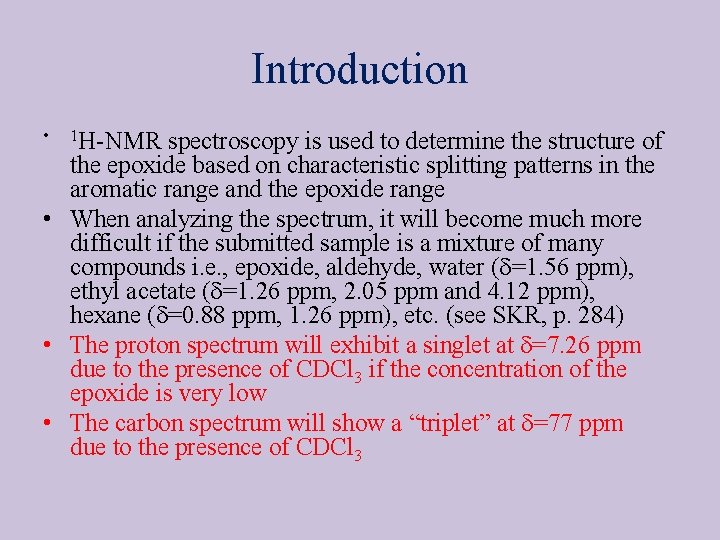
4 -Methylstyrene oxide • 1 H-NMR Spectrum (integration in blue) 4 J 3 H 1 -H 2 H 1 -H 3 H 2 -H 3 3. 31 Hz 3. 30 Hz 5. 68 Hz 3 1 11 H 1, dd H 2, dd H 3, dd CH 3

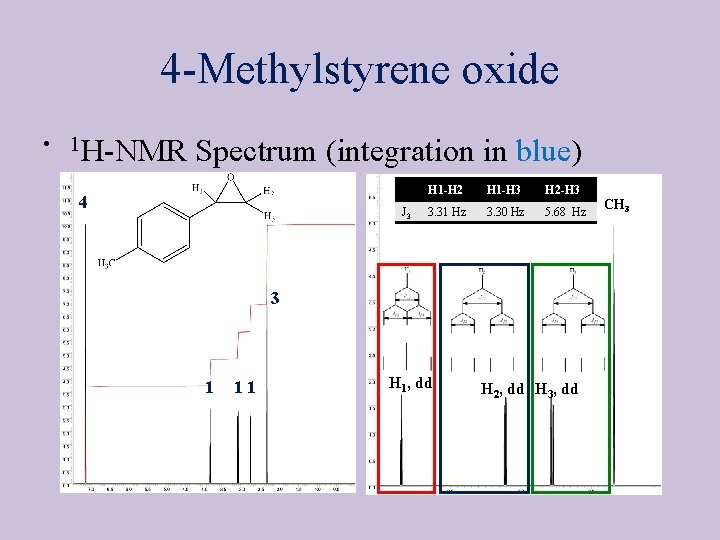
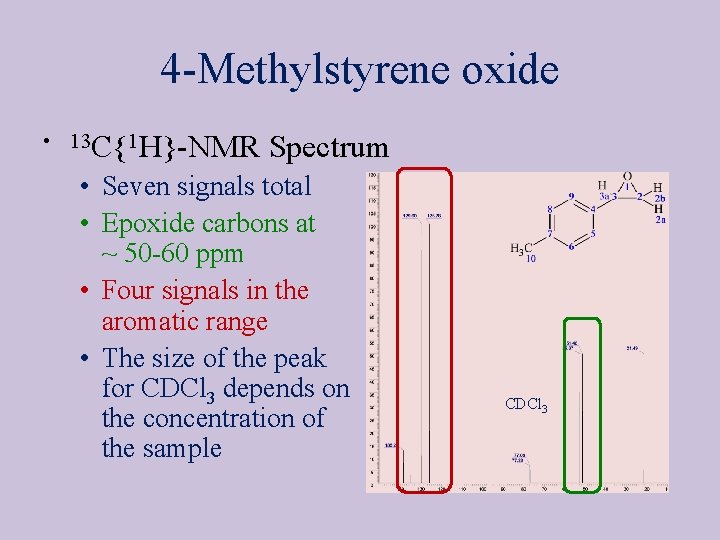
4 -Methylstyrene oxide • 13 C{1 H}-NMR Spectrum • Seven signals total • Epoxide carbons at ~ 50 -60 ppm • Four signals in the aromatic range • The size of the peak for CDCl 3 depends on the concentration of the sample CDCl 3

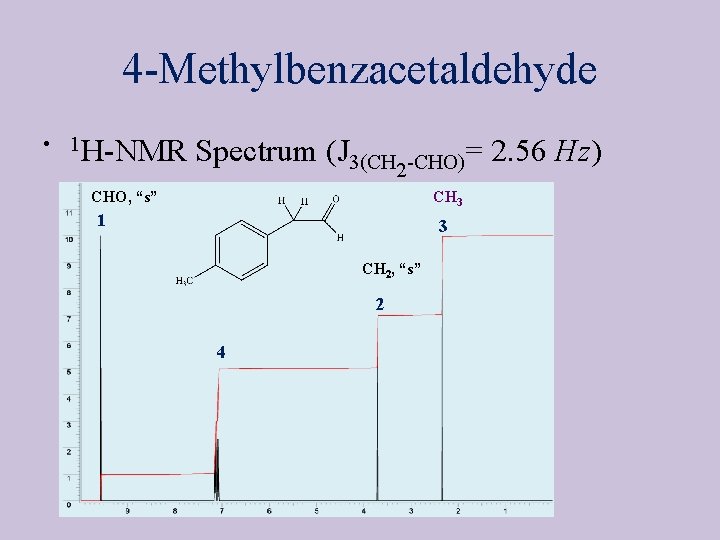
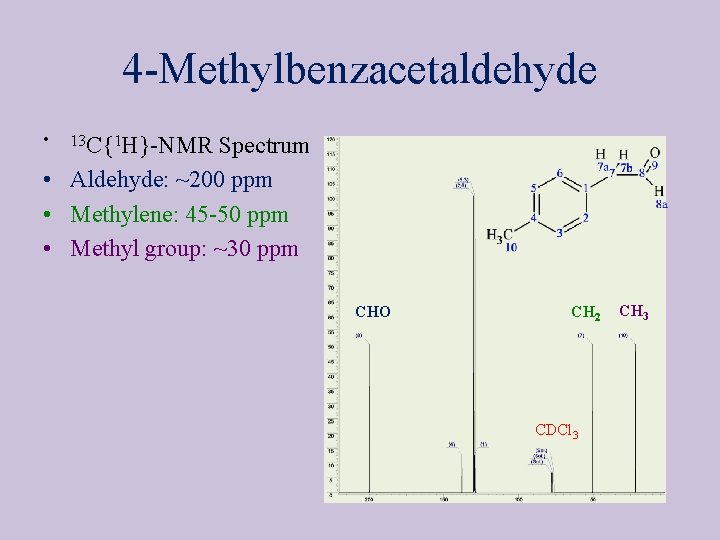
4 -Methylbenzacetaldehyde • 1 H-NMR Spectrum (J 3(CH 2 -CHO)= 2. 56 Hz) CHO, “s” CH 3 1 3 CH 2, “s” 2 4

4 -Methylbenzacetaldehyde • 13 C{1 H}-NMR Spectrum • Aldehyde: ~200 ppm • Methylene: 45 -50 ppm • Methyl group: ~30 ppm CHO CH 2 CDCl 3 CH 3

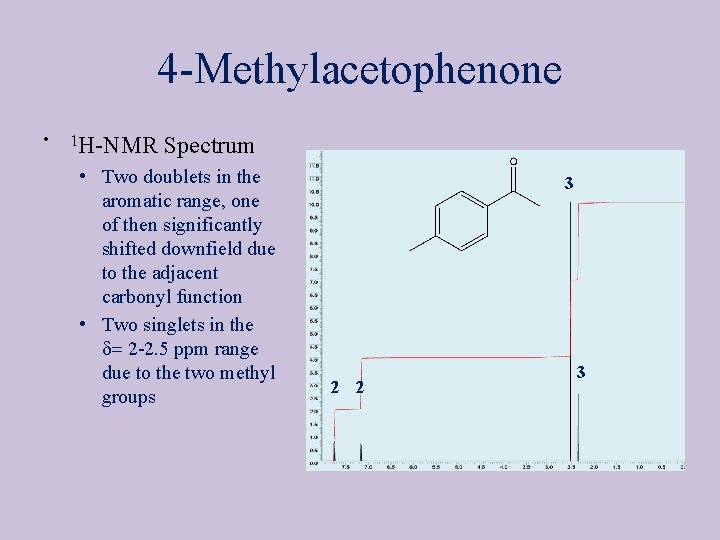
4 -Methylacetophenone • 1 H-NMR Spectrum • Two doublets in the aromatic range, one of then significantly shifted downfield due to the adjacent carbonyl function • Two singlets in the d= 2 -2. 5 ppm range due to the two methyl groups 3 2 2 3

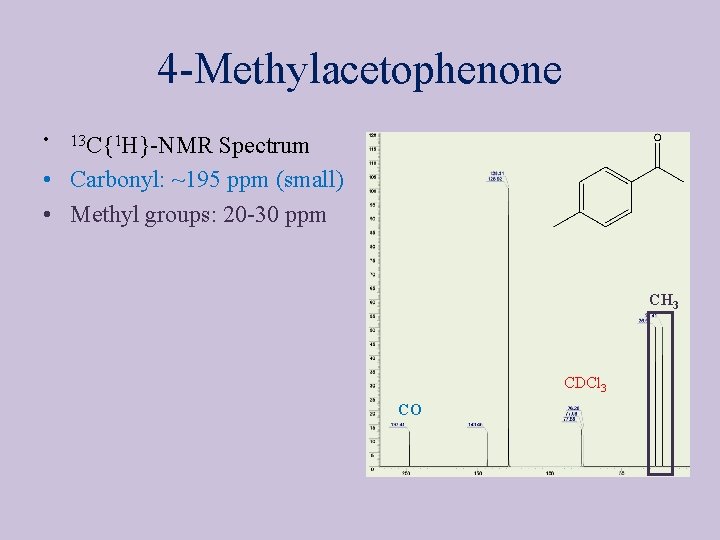
4 -Methylacetophenone • 13 C{1 H}-NMR Spectrum • Carbonyl: ~195 ppm (small) • Methyl groups: 20 -30 ppm CH 3 CDCl 3 CO

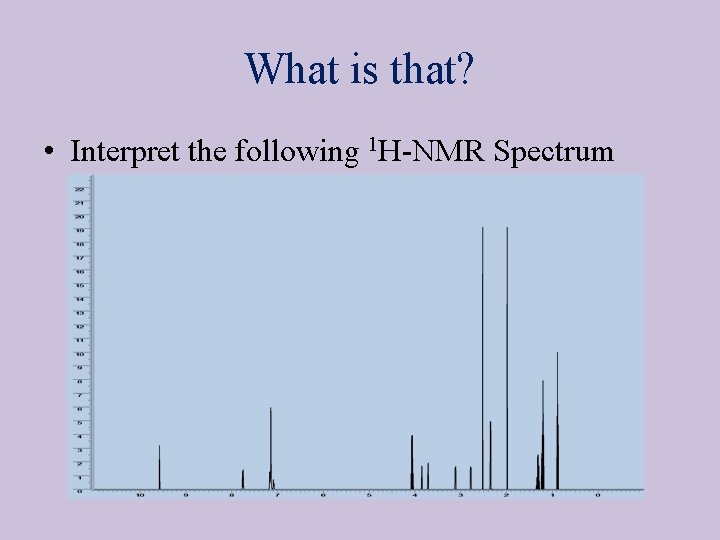
What is that? • Interpret the following 1 H-NMR Spectrum

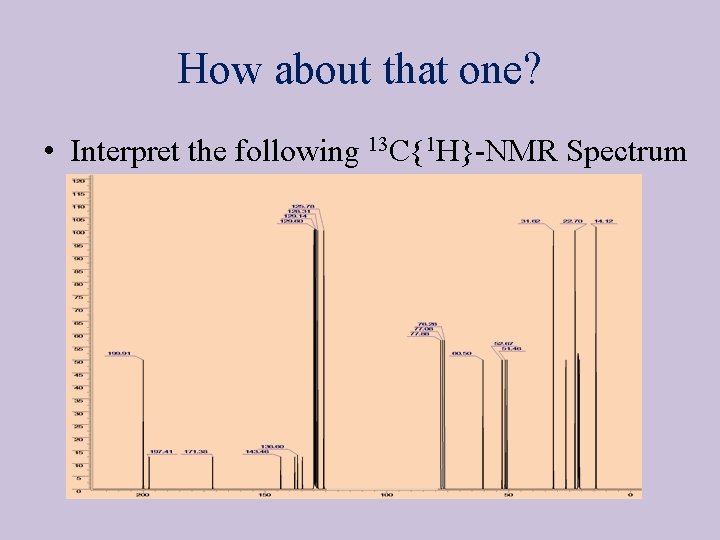
How about that one? • Interpret the following 13 C{1 H}-NMR Spectrum spectrum