Chemistry 125 Lecture 45 January 29 2010 Nucleophilic

![Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1] Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1]](https://slidetodoc.com/presentation_image/eb9c9dea1a77963e9f954b800637c3e6/image-4.jpg)




- Slides: 28

Chemistry 125: Lecture 45 January 29, 2010 Nucleophilic Substitution and Mechanistic Tools: Rate Law, Rate Constant, Structure This For copyright notice see final page of this file

Tools for Testing (i. e. Excluding) Mechanisms: Stereochemistry Rate Law Rate Constant Structure X-Ray and Quantum Mechanics

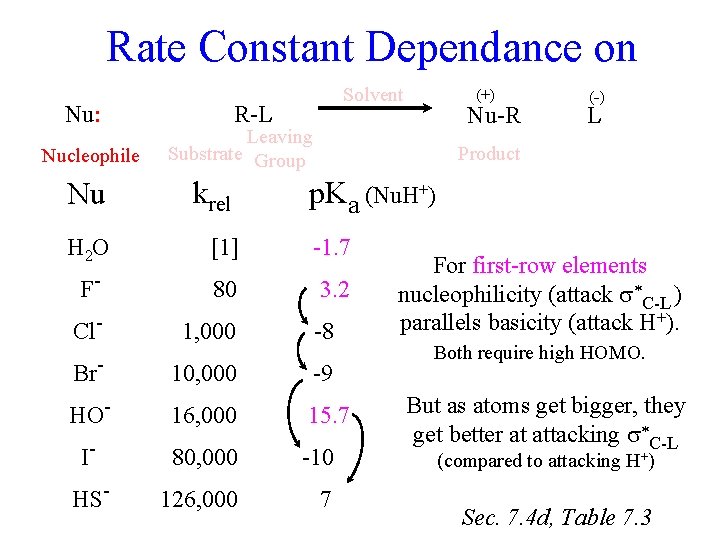
Rate Constant Dependance on Nu: Nucleophile Nu Solvent R-L (+) Nu-R Leaving Substrate Group krel (-) L Product p. Ka (Nu. H+) H 2 O [1] -1. 7 F- 80 3. 2 Cl- 1, 000 -8 Br- 10, 000 -9 HO- 16, 000 15. 7 I- 80, 000 -10 HS- 126, 000 7 For first-row elements nucleophilicity (attack C-L ) parallels basicity (attack H+). Both require high HOMO. But as atoms get bigger, they get better at attacking C-L (compared to attacking H+) Sec. 7. 4 d, Table 7. 3
![Rate Constant Dependance on Nucleophile Nu RL Leaving Substrate Group krel p Ka 1 Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1]](https://slidetodoc.com/presentation_image/eb9c9dea1a77963e9f954b800637c3e6/image-4.jpg)
Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1] -1. 7 F- 80 3. 2 Cl- 1, 000 -8 Br- 10, 000 -9 HO- 16, 000 15. 7 I- 80, 000 -10 HS- 126, 000 7 Nu-R L Polar solvents accelerate reactions that generate (or concentrate) charge, and vice versa. (Nu. H+) krel CH 3 I in H 2 O harder [1] to break 14 H-bonds to smaller ions 160 CH 3 Br in Acetone Backwards H 2 O (-) (+) Sec. 7. 4 dg 11 5 [1] Sensible Nu: Solvent

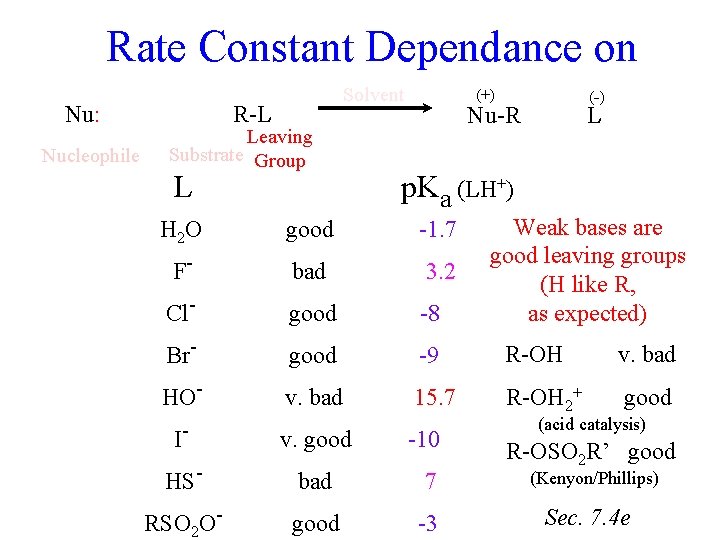
Rate Constant Dependance on Nu: Nucleophile Solvent R-L Leaving Substrate Group L (-) (+) Nu-R L p. Ka (LH+) Weak bases are good leaving groups (H like R, as expected) H 2 O good -1. 7 F- bad 3. 2 Cl- good -8 Br- good -9 R-OH v. bad HO- v. bad 15. 7 R-OH 2+ good I- v. good -10 HS- bad 7 RSO 2 O- good -3 (acid catalysis) R-OSO 2 R’ good (Kenyon/Phillips) Sec. 7. 4 e

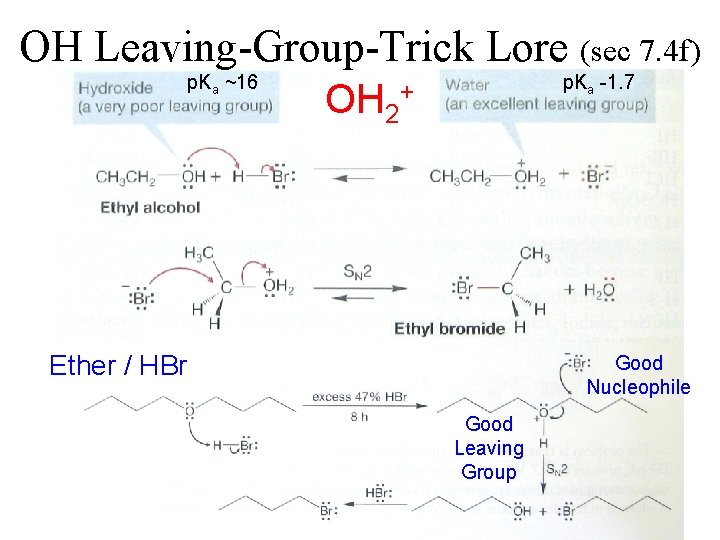
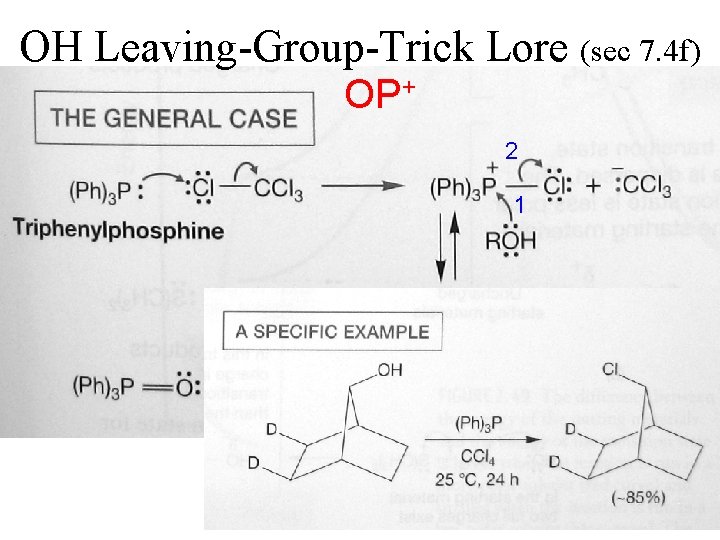
OH Leaving-Group-Trick Lore (sec 7. 4 f) p. Ka ~16 OH 2 p. Ka -1. 7 + Ether / HBr Good Nucleophile Good Leaving Group

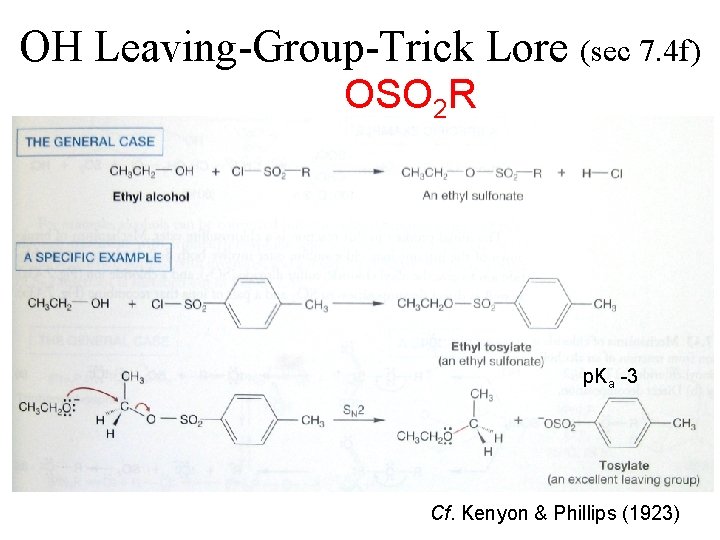
OH Leaving-Group-Trick Lore (sec 7. 4 f) OSO 2 R p. Ka -3 Cf. Kenyon & Phillips (1923)

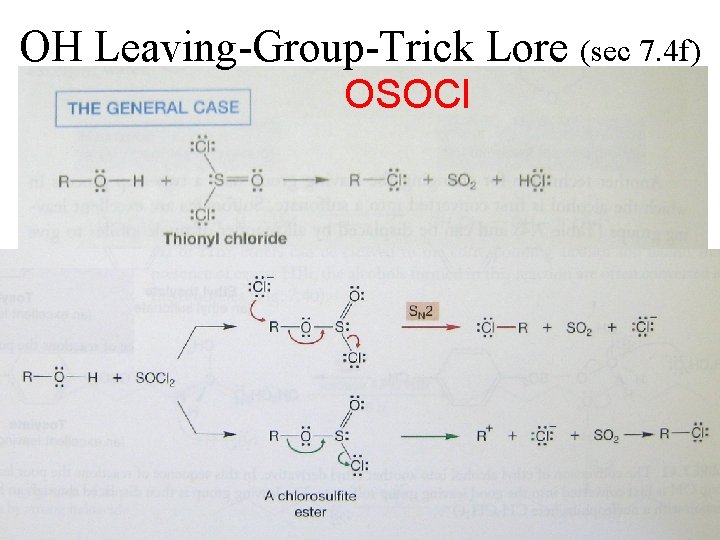
OH Leaving-Group-Trick Lore (sec 7. 4 f) OSOCl gases

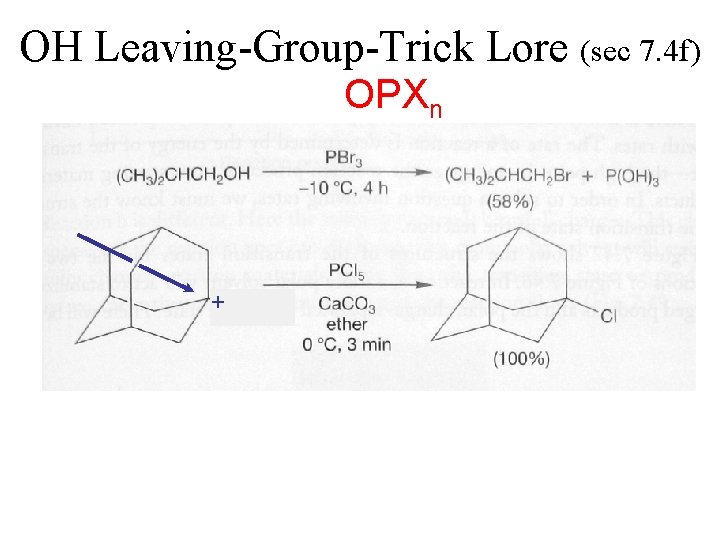
OH Leaving-Group-Trick Lore (sec 7. 4 f) OPXn + PClx

OH Leaving-Group-Trick Lore (sec 7. 4 f) OP+ 2 1

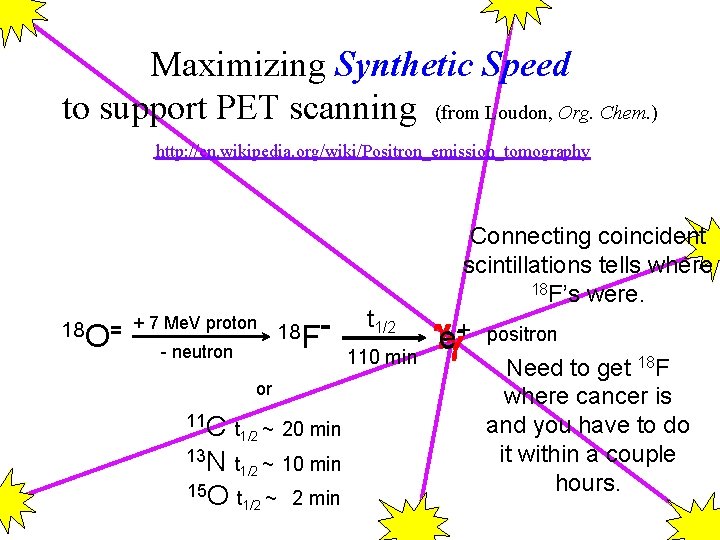
Maximizing Synthetic Speed to support PET scanning (from Loudon, Org. Chem. ) http: //en. wikipedia. org/wiki/Positron_emission_tomography 18 O= + 7 Me. V proton 18 F - neutron or 11 C t ~ 20 min 1/2 13 N t ~ 10 min 1/2 15 O t ~ 2 min 1/2 t 1/2 110 min Connecting coincident scintillations tells where 18 F’s were. e+- positron Need to get 18 F where cancer is and you have to do it within a couple hours.

Yale PET What to synthesize?

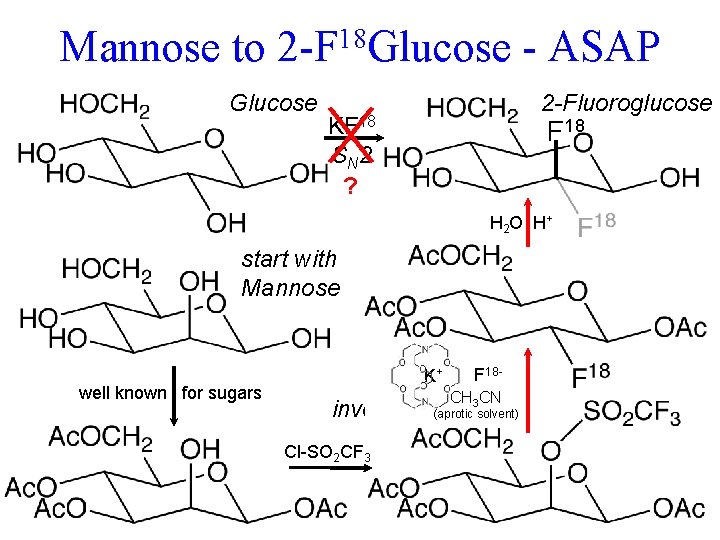
Mannose to 18 2 -F Glucose KF 18 S N 2 ? - ASAP 2 -Fluoroglucose F 18 H 2 O H+ and by K+cation Maybe it would suck up F tied up by H-bonding 2 -Fluoroglucose as well. HO a horrid leaving group trifluormethanesulfonate wrong C-OH could be attacked K+ K+ F 18(Triflate) CH 3 CN inversion (aprotic solvent) gives wrong configuration O Cl-SO 2 CF 3 Ac. O = CH 3 C-O- start with Rapid metabolism of. Mannose tumor sucks up glucose. Problems : well known for sugars (acetate protecting group)

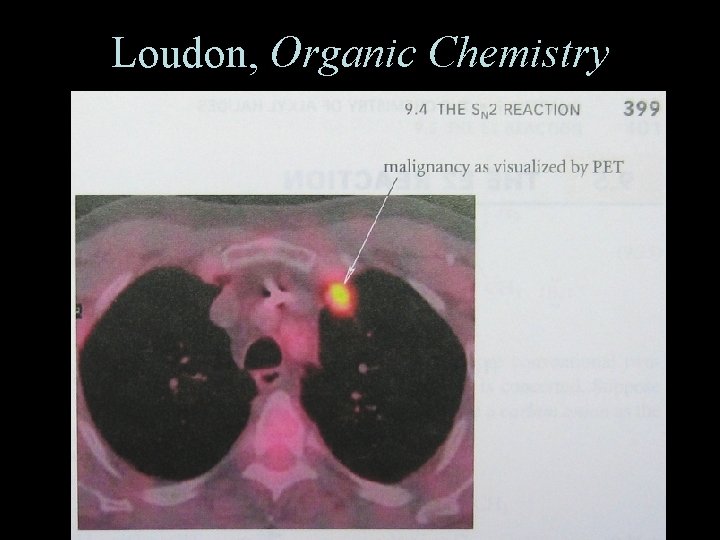
Loudon, Organic Chemistry Office Hours 3 -4: 30 this afternoon 181 SCL

Tools for Testing (i. e. Excluding) Mechanisms: Stereochemistry Rate Law Rate Constant Structure X-Ray and Quantum Mechanics

So far we’ve just been beating up on the D/A mechanism (trivalent C intermediate) though there are cases (SN 1) where it in fact applies. The tougher problem is to distinguish between concerted and A/D with a very weakly stabilized intermediate. (see supplementary reading on Course website)

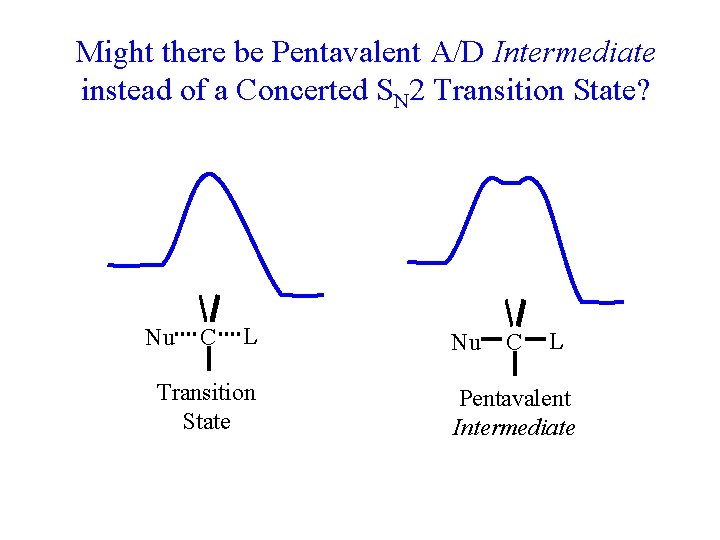
Might there be Pentavalent A/D Intermediate instead of a Concerted SN 2 Transition State? Nu C L Transition State Nu C L Pentavalent Intermediate

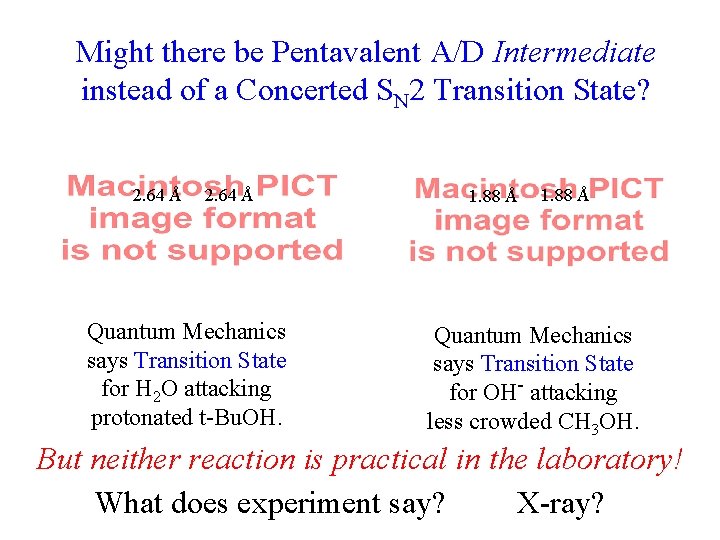
Might there be Pentavalent A/D Intermediate instead of a Concerted SN 2 Transition State? 2. 64 Å Quantum Mechanics says Transition State for H 2 O attacking protonated t-Bu. OH. 1. 88 Å Quantum Mechanics says Transition State for OH- attacking less crowded CH 3 OH. But neither reaction is practical in the laboratory! What does experiment say? X-ray?

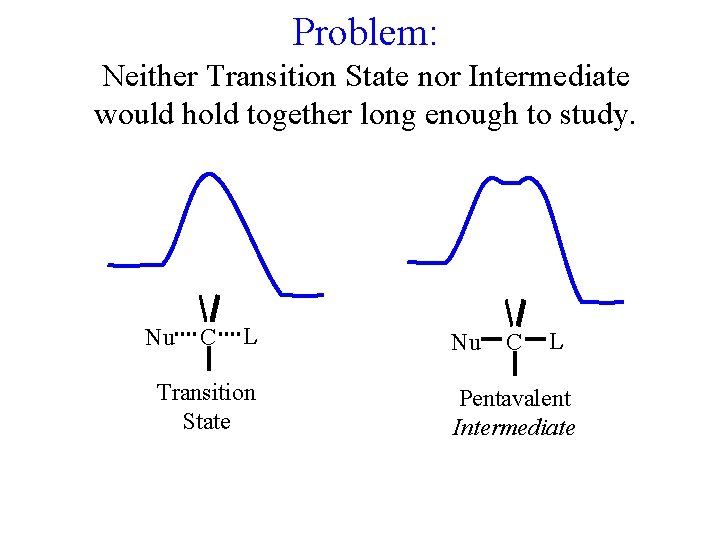
Problem: Neither Transition State nor Intermediate would hold together long enough to study. Nu C L Transition State Nu C L Pentavalent Intermediate

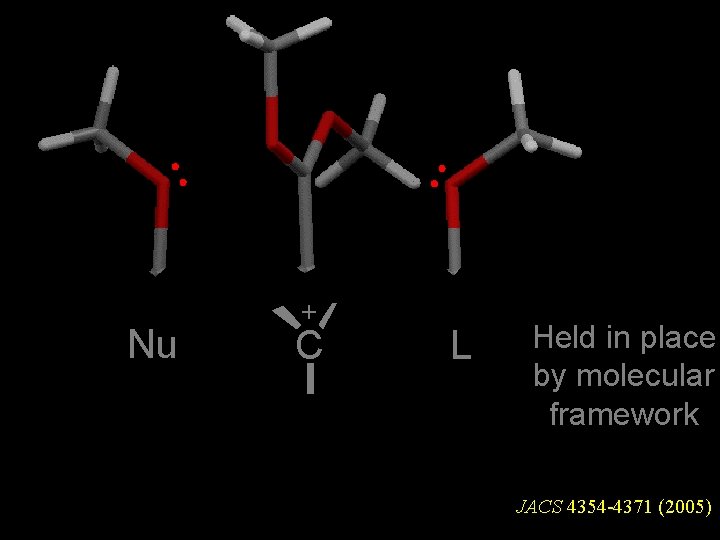
HOYWAT Nu + C L Held in place by molecular framework JACS 4354 -4371 (2005)

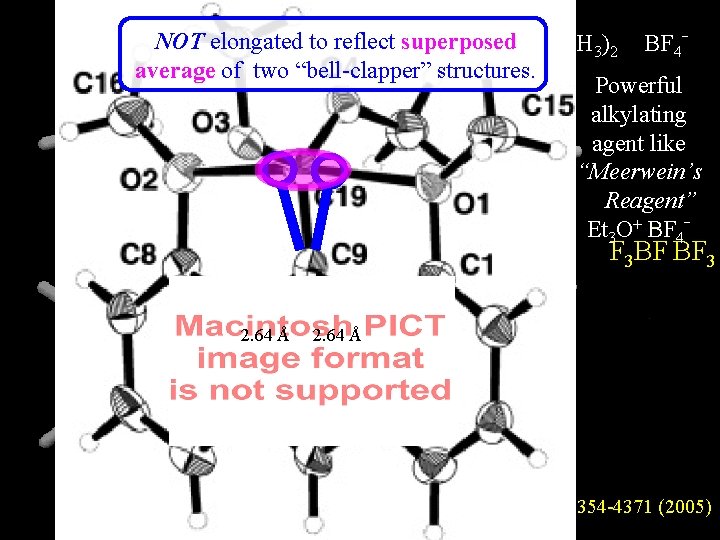
CH 3 + NOT elongated to reflect superposed CH -O(CH ) BF 3 2 4 OCH 3 3 O + O average of two “bell-clapper” C structures. Powerful alkylating + agent like “Meerwein’s : : . Reagent” ARE THERE BONDS HERE? - 4 Et 3 O+ BF HOYWAT F 3 BF BF 3 2. 64 Å JACS 4354 -4371 (2005)

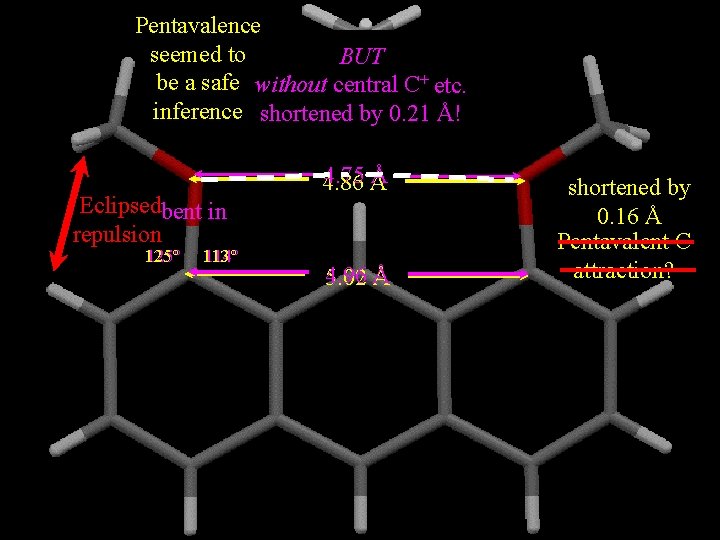
Pentavalence seemed to BUT be a safe without central C+ etc. inference shortened by 0. 21 Å! Eclipsedbent in repulsion 125° 113° 114° 4. 75 Å Å 4. 86 4. 96 5. 02 Å shortened by 0. 16 Å Pentavalent C attraction?

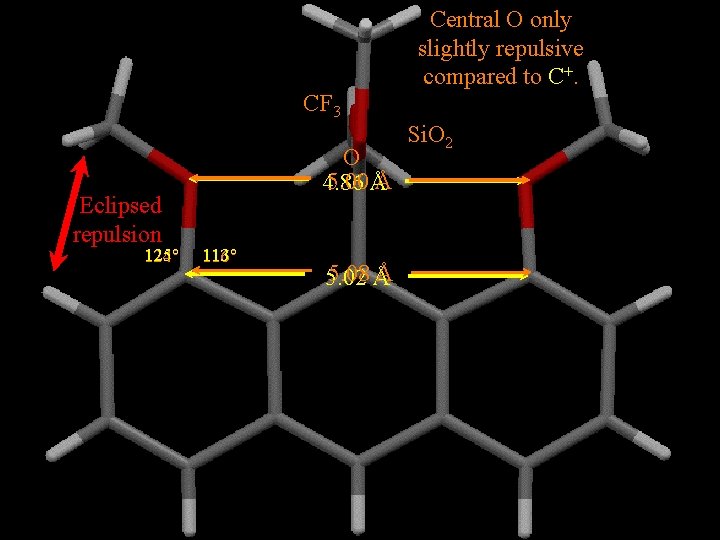
CF 3 Eclipsed repulsion 125° 124° O 5. 00 Å Å 4. 86 113° 116° 5. 08 Å Å 5. 02 Central O only slightly repulsive compared to C+. Si. O 2

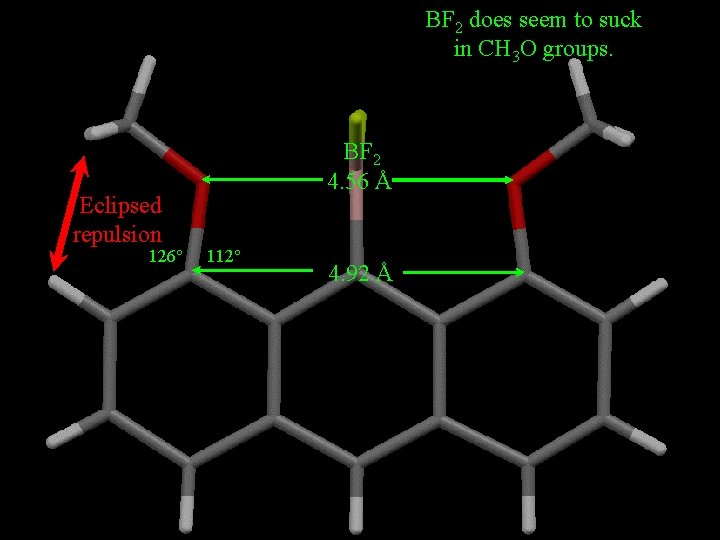
BF 2 does seem to suck in CH 3 O groups. Eclipsed repulsion 126° BF 2 4. 56 Å 112° 4. 92 Å

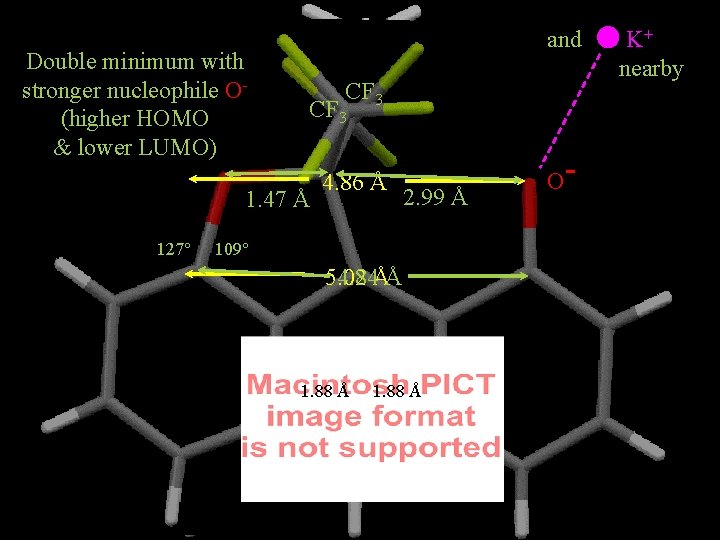
Double minimum with stronger nucleophile O(higher HOMO & lower LUMO) CF 3 1. 47 Å 127° 113° 109° 125° and. 4. 86 Å 2. 99 Å 5. 02 4. 84ÅÅ 1. 88 Å O- K+ nearby

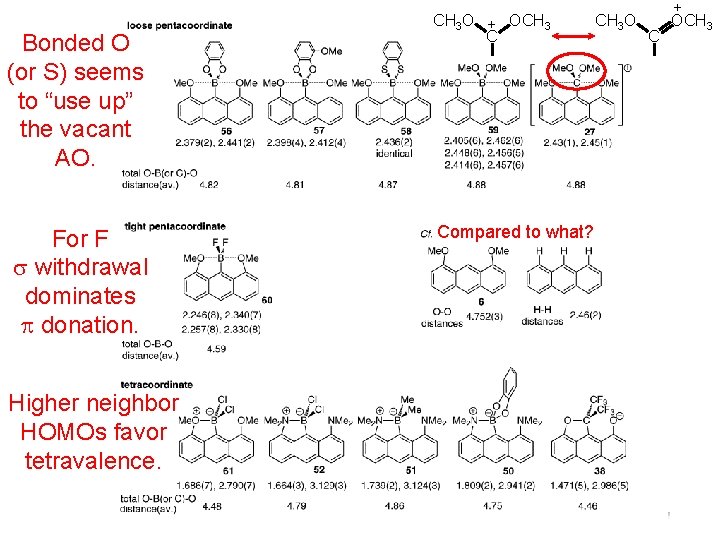
CH 3 O + OCH 3 C Bonded O (or S) seems to “use up” the vacant AO. For F withdrawal dominates donation. Higher neighbor HOMOs favor tetravalence. Range Compared to what? CH 3 O C + OCH 3

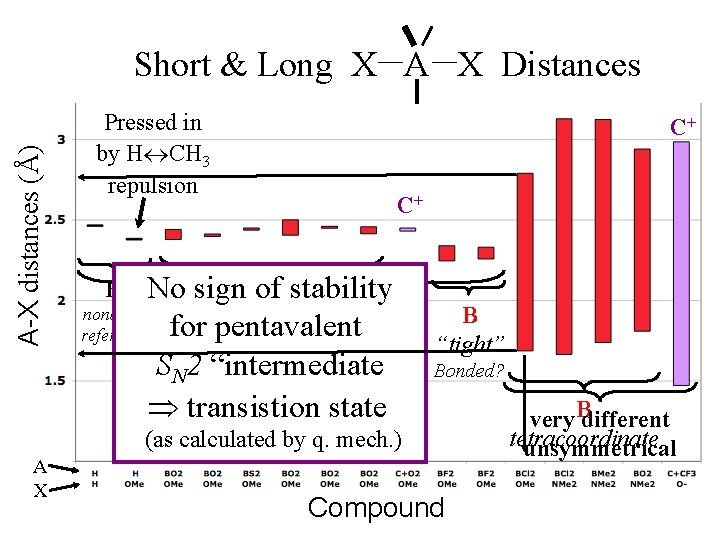
A-X distances (Å) Short & Long X A X Distances A X Pressed in by H CH 3 repulsion C+ C+ B stability No sign of nonbond “loose” ~ equal for pentavalent reference like H symmetrical SN 2 “intermediate ” transistion state (as by q. state mech. ) calculated transistion H B “tight” Bonded? Compound very Bdifferent tetracoordinate unsymmetrical

End of Lecture 45 Jan. 29, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 January 2012 chemistry regents answers
January 2012 chemistry regents answers May 2019 ib grade boundaries
May 2019 ib grade boundaries January 2017 chemistry regents answers
January 2017 chemistry regents answers Nysedregents chemistry
Nysedregents chemistry 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Identify nucleophile and electrophile
Identify nucleophile and electrophile Nucleophilic acyl substitution reaction
Nucleophilic acyl substitution reaction Nucleophilic addition
Nucleophilic addition Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Nucleophilicity trend
Nucleophilicity trend Unimolecular nucleophilic substitution reaction
Unimolecular nucleophilic substitution reaction Nucleophilic substitution of carboxylic acids
Nucleophilic substitution of carboxylic acids Bruice
Bruice Alkyl halide examples
Alkyl halide examples Nucleophilic substitution of alkyl halides
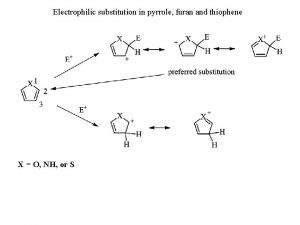
Nucleophilic substitution of alkyl halides Thiophene nucleophilic substitution
Thiophene nucleophilic substitution Isoquinoline nucleophilic substitution
Isoquinoline nucleophilic substitution Least reactive carboxylic acid derivatives
Least reactive carboxylic acid derivatives Bischler-napieralski synthesis
Bischler-napieralski synthesis Ag(nh3)2 reaction with aldehyde
Ag(nh3)2 reaction with aldehyde Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Alkyl halide examples
Alkyl halide examples Order of reactivity of carboxylic acid derivatives
Order of reactivity of carboxylic acid derivatives Hindered vs unhindered base
Hindered vs unhindered base Antiperiplaner
Antiperiplaner Danswer
Danswer An introduction to atmospheric physics
An introduction to atmospheric physics Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes