Chemistry 125 Lecture 45 January 28 2011 This

![Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1] Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1]](https://slidetodoc.com/presentation_image_h2/6a50c2e9f03df256afddec57439ec1ed/image-3.jpg)




- Slides: 22

Chemistry 125: Lecture 45 January 28, 2011 This Nucleophilic Substitution and Mechanistic Tools: Solvent, Leaving Group PET Scanning Pentavalent Carbon? For copyright notice see final page of this file

SN 2 Nucleophilic Substitution Solvent Nu: R-L Nucleophile Substrate (+) (-) Nu-R L Product Leaving Group the Pragmatic Logic of Proving a Mechanism with Experiment & Theory (mostly by disproving all alternative mechanisms)
![Rate Constant Dependance on Nucleophile Nu RL Leaving Substrate Group krel p Ka 1 Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1]](https://slidetodoc.com/presentation_image_h2/6a50c2e9f03df256afddec57439ec1ed/image-3.jpg)
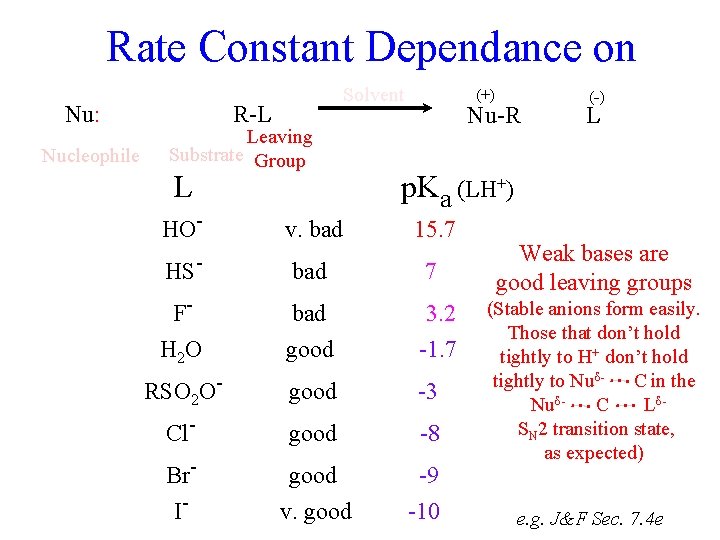
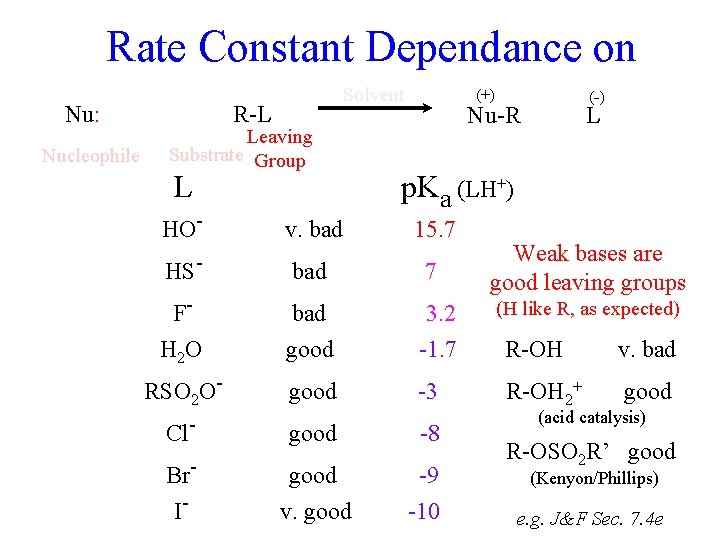
Rate Constant Dependance on Nucleophile Nu R-L Leaving Substrate Group krel p. Ka [1] -1. 7 F- 80 3. 2 Cl- 1, 000 -8 Br- 10, 000 -9 HO- 16, 000 15. 7 I- 80, 000 -10 HS- 126, 000 7 Nu-R L Polar solvents accelerate reactions that generate (or concentrate) charge, and vice versa. (Nu. H+) krel CH 3 I in H 2 O harder [1] to break 14 H-bonds to smaller ions 160 krel CH 3 Br in Acetone Backwards H 2 O (-) (+) 11 5 Sensible Nu: Solvent [1] e. g. J&F Sec. 7. 4 dg

Rate Constant Dependance on Nu: Nucleophile Solvent R-L Leaving Substrate Group L (+) Nu-R (-) L p. Ka (LH+) HO- v. bad 15. 7 HS- bad 7 F- bad 3. 2 H 2 O good -1. 7 RSO 2 O- good -3 Cl- good -8 Br- good -9 I- v. good -10 Weak bases are good leaving groups (Stable anions form easily. Those that don’t hold tightly to H+ don’t hold tightly to Nud- C in the Nud- C Ld. SN 2 transition state, as expected) e. g. J&F Sec. 7. 4 e

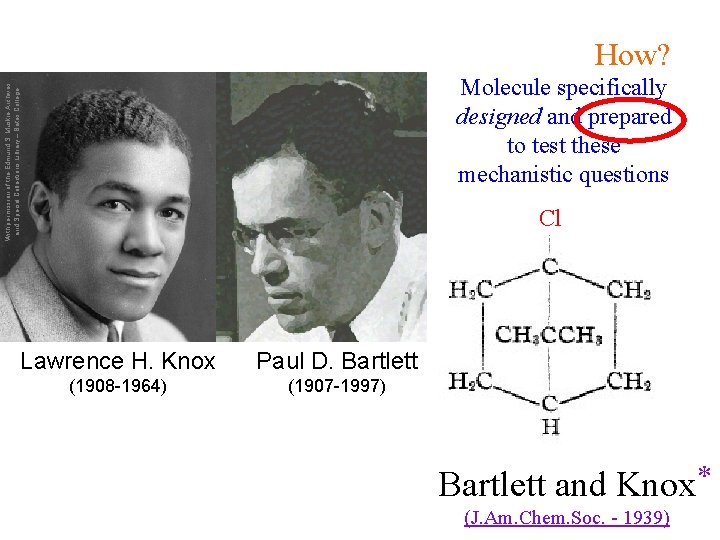
How? With permission of the Edmund S. Muskie Archives and Special Collections Library – Bates College Molecule specifically designed and prepared to test these mechanistic questions Cl Lawrence H. Knox Paul D. Bartlett (1908 -1964) (1907 -1997) Bartlett and Knox * (J. Am. Chem. Soc. - 1939)

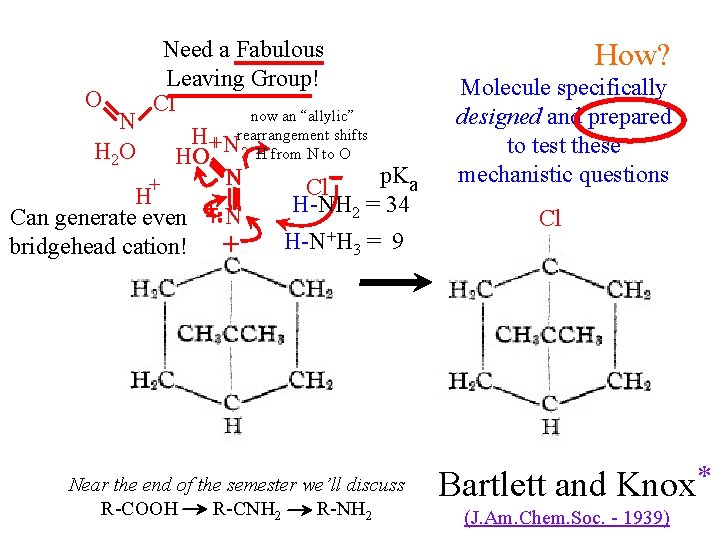
Need a Fabulous Leaving Group! O Cl now an “allylic” N H + Nrearrangement shifts H 2 O HO H O 2 H from N to O p. Ka N + Cl H H-NH 2 = 34 + Can generate even + NH 2 H-N+H 3 = 9 bridgehead cation! + Near the end of the semester we’ll discuss R-COOH R-CNH 2 R-NH 2 How? Molecule specifically designed and prepared to test these mechanistic questions Cl Bartlett and Knox * (J. Am. Chem. Soc. - 1939)

Rate Constant Dependance on Nu: Nucleophile Solvent R-L Leaving Substrate Group L (-) (+) Nu-R L p. Ka (LH+) HO- v. bad 15. 7 HS- bad 7 F- bad 3. 2 H 2 O good -1. 7 R-OH v. bad RSO 2 O- good -3 R-OH 2+ good Cl- good -8 Br- good -9 I- v. good -10 Weak bases are good leaving groups (H like R, as expected) (acid catalysis) R-OSO 2 R’ good (Kenyon/Phillips) e. g. J&F Sec. 7. 4 e

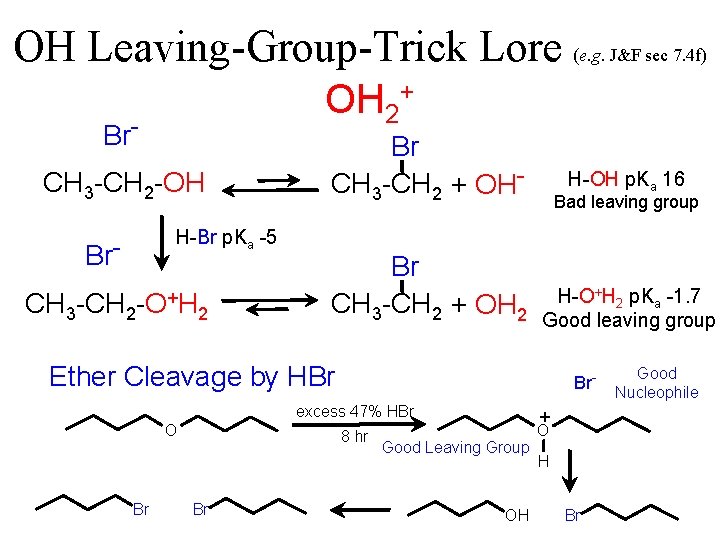
OH Leaving-Group-Trick Lore (e. g. J&F sec 7. 4 f) OH 2+ Br. CH 3 -CH 2 -OH H-Br p. Ka -5 Br. CH 3 -CH 2 -O+H 2 Br CH 3 -CH 2 + OH- Bad leaving group Br CH 3 -CH 2 + OH 2 H-O+H 2 p. Ka -1. 7 Good leaving group H-OH p. Ka 16 Ether Cleavage by HBr Br- excess 47% HBr O Br 8 hr Br + Good Leaving Group OH O H Br Good Nucleophile

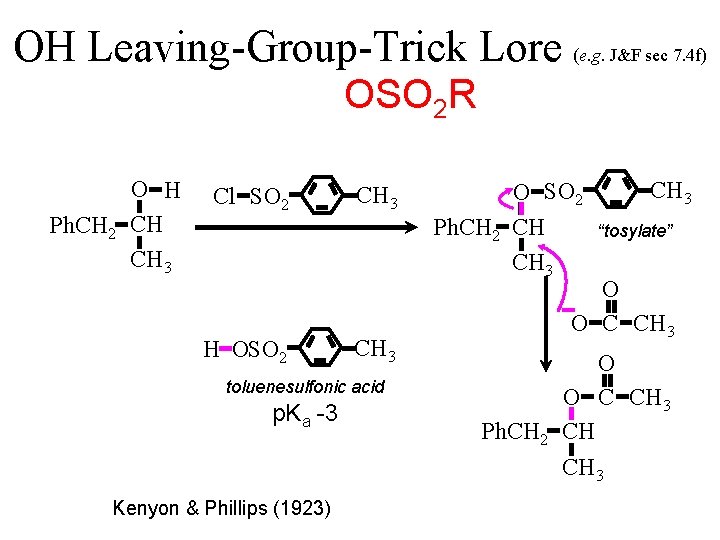
OH Leaving-Group-Trick Lore (e. g. J&F sec 7. 4 f) OSO 2 R O H Ph. CH 2 CH CH 3 Cl SO 2 H OSO 2 CH 3 toluenesulfonic acid p. Ka -3 Kenyon & Phillips (1923) O SO 2 Ph. CH 2 CH CH 3 “tosylate” O O C CH 3 Ph. CH 2 CH CH 3

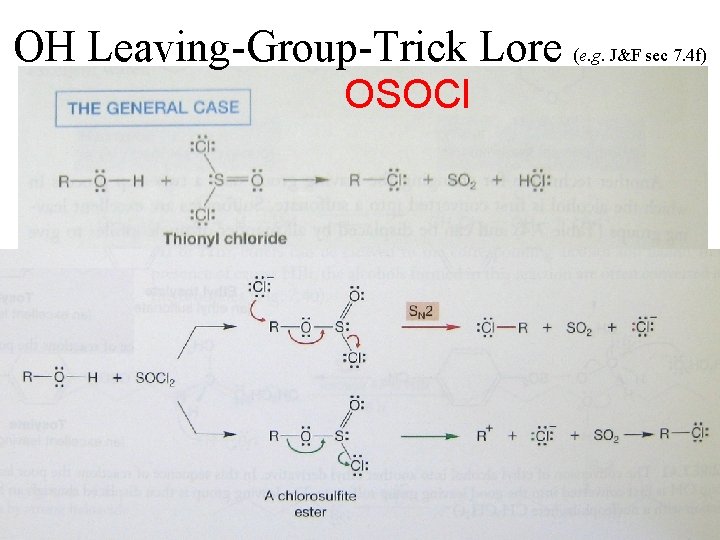
OH Leaving-Group-Trick Lore (e. g. J&F sec 7. 4 f) OSOCl b. p. 75°C 61°C gases

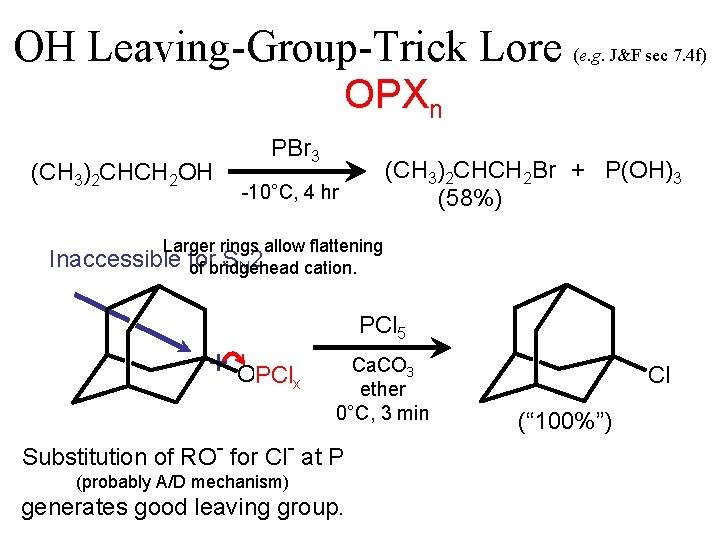
OH Leaving-Group-Trick Lore (e. g. J&F sec 7. 4 f) OPXn (CH 3)2 CHCH 2 OH PBr 3 (CH 3)2 CHCH 2 Br + P(OH)3 (58%) -10°C, 4 hr Larger rings allow flattening Inaccessible for S N 2 of bridgehead cation. PCl 5 + OH PClx Ca. CO 3 ether 0°C, 3 min Substitution of RO- for Cl- at P (probably A/D mechanism) generates good leaving group. Cl (“ 100%”)

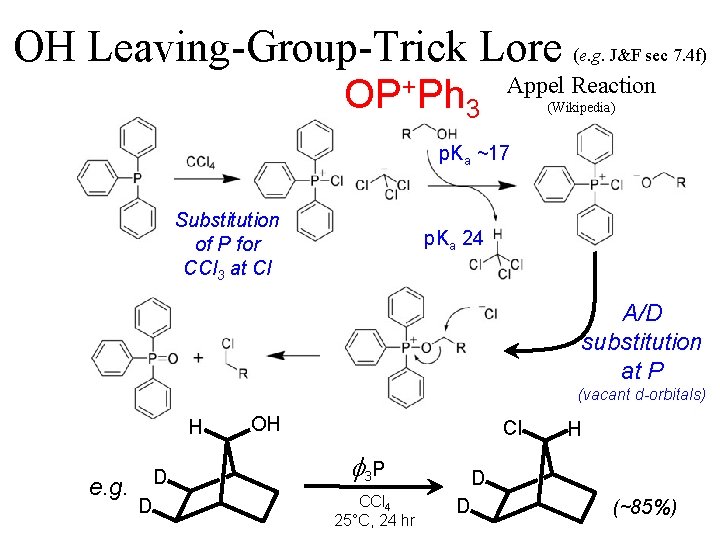
OH Leaving-Group-Trick Lore (e. g. J&F sec 7. 4 f) OP+Ph 3 Appel Reaction (Wikipedia) p. Ka ~17 Substitution of P for CCl 3 at Cl p. Ka 24 A/D substitution at P (vacant d-orbitals) H e. g. D D OH Cl f 3 P CCl 4 25°C, 24 hr H D D (~85%)

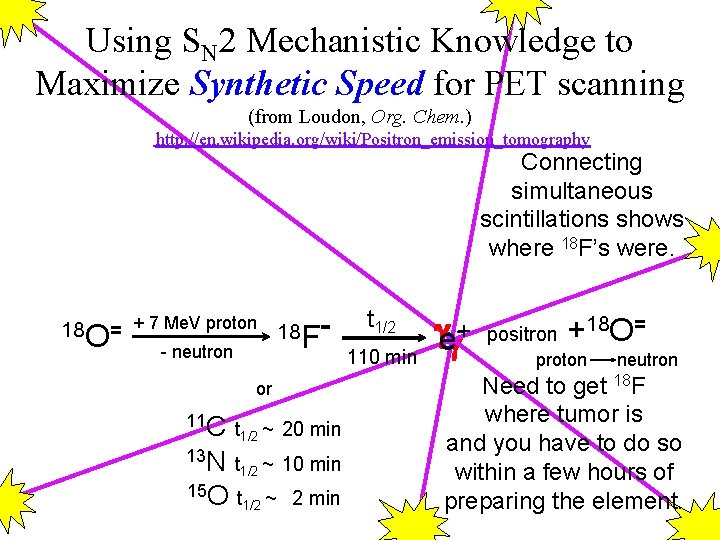
Using SN 2 Mechanistic Knowledge to Maximize Synthetic Speed for PET scanning (from Loudon, Org. Chem. ) http: //en. wikipedia. org/wiki/Positron_emission_tomography Connecting simultaneous scintillations shows where 18 F’s were. 18 O= + 7 Me. V proton 18 F - neutron or 11 C t ~ 20 min 1/2 13 N t ~ 10 min 1/2 15 O t ~ 2 min 1/2 t 1/2 110 min e +- positron +18 O= proton neutron Need to get 18 F where tumor is and you have to do so within a few hours of preparing the element.

Yale PET What to synthesize?

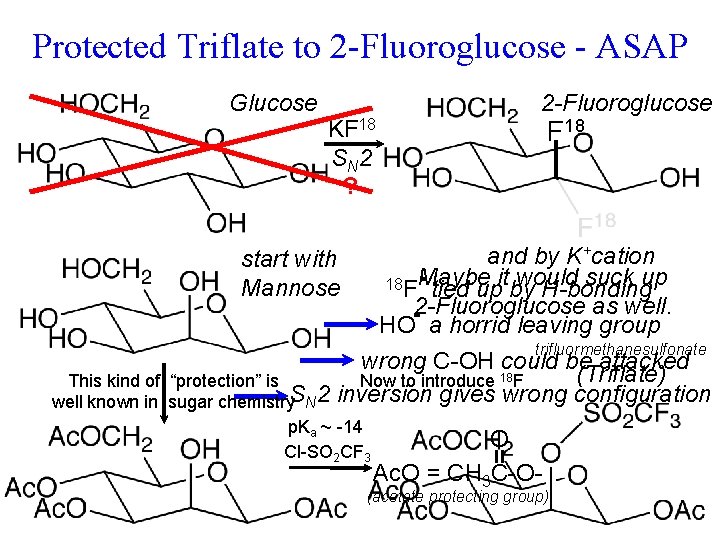
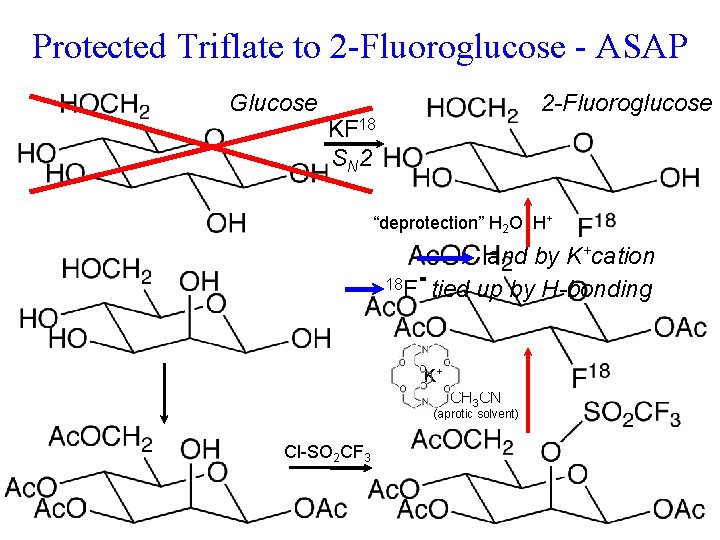
Protected Triflate to 2 -Fluoroglucose - ASAP Glucose KF 18 S N 2 ? 2 -Fluoroglucose F 18 and by K+cation would suck up 18 F Maybe tied upitby H-bonding 2 -Fluoroglucose as well. HO a horrid leaving group trifluormethanesulfonate wrong C-OH could be attacked (Triflate) “protection” is Now to introduce 18 F sugar chemistry. SN 2 inversion gives wrong configuration start with Rapid metabolism of. Mannose tumor sucks up glucose. SN 2 Problems : This kind of well known in p. Ka ~ -14 Cl-SO 2 CF 3 O Ac. O = CH 3 C-O- (acetate protecting group)

Protected Triflate to 2 -Fluoroglucose - ASAP Glucose 2 -Fluoroglucose KF 18 S N 2 “deprotection” H 2 O H+ and by K+cation 18 F tied up by H-bonding K+ K+ 18 FCH 3 CN (aprotic solvent) Cl-SO 2 CF 3

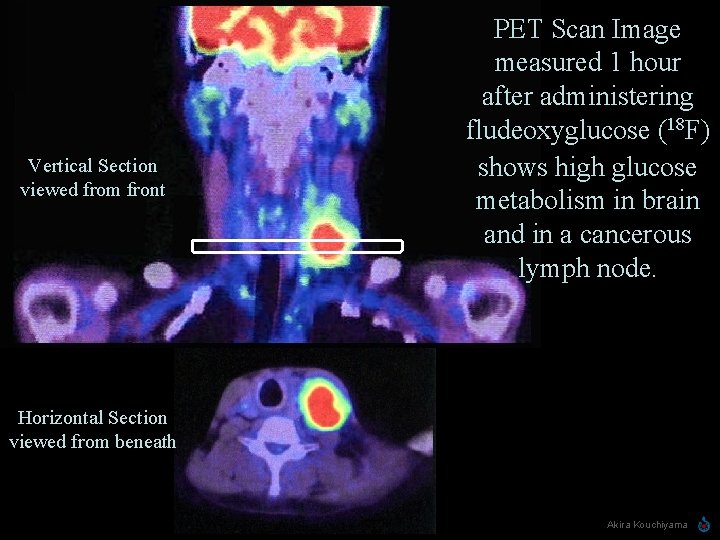
Vertical Section viewed from front PET Scan Image measured 1 hour after administering fludeoxyglucose (18 F) shows high glucose metabolism in brain and in a cancerous lymph node. Horizontal Section viewed from beneath Linus Pauling 1901 -1994 Akira Kouchiyama

Tools for Testing (i. e. Excluding) Mechanisms: Stereochemistry Rate Law Rate Constant Structure X-Ray and Quantum Mechanics

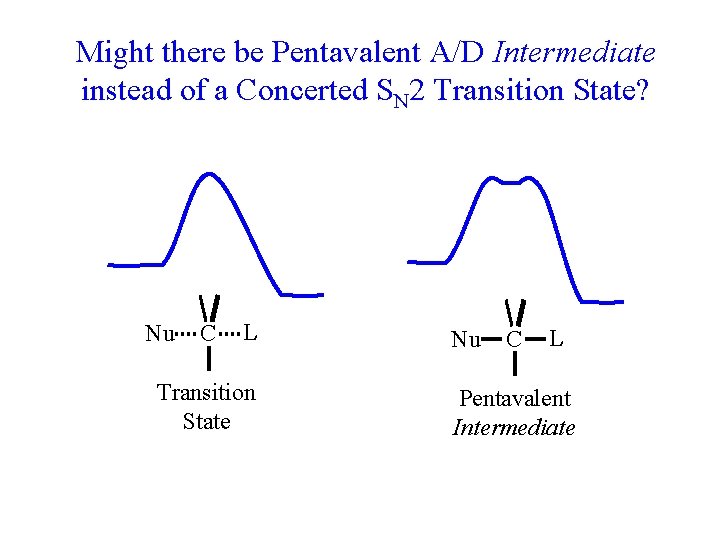
So far we’ve just been beating up on the D/A mechanism (trivalent C intermediate) though there are cases (SN 1) where it in fact applies. The tougher problem is to distinguish between concerted and A/D with a very weakly stabilized intermediate. (see supplementary reading on Course website)

Might there be Pentavalent A/D Intermediate instead of a Concerted SN 2 Transition State? Nu C L Transition State Nu C L Pentavalent Intermediate

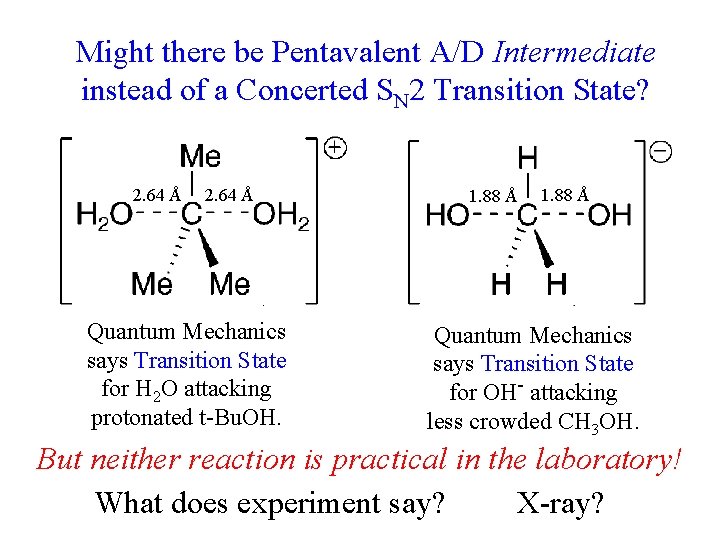
Might there be Pentavalent A/D Intermediate instead of a Concerted SN 2 Transition State? 2. 64 Å Quantum Mechanics says Transition State for H 2 O attacking protonated t-Bu. OH. 1. 88 Å Quantum Mechanics says Transition State for OH- attacking less crowded CH 3 OH. But neither reaction is practical in the laboratory! What does experiment say? X-ray?

End of Lecture 45 Jan. 28, 2011 Copyright © J. M. Mc. Bride 2011. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 Nysedregents chemistry
Nysedregents chemistry Nysedregents chemistry
Nysedregents chemistry 2019 ib grade boundaries
2019 ib grade boundaries January 2018 regents chemistry
January 2018 regents chemistry 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Chemistry regents 2011
Chemistry regents 2011 Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry January 2012
January 2012 Arvod cannot find work as a mall santa in january.
Arvod cannot find work as a mall santa in january. Spatial january
Spatial january An asset was purchased for $120 000 on january 1
An asset was purchased for $120 000 on january 1 How many syllables in flying
How many syllables in flying January february spelling
January february spelling Sunday, tuesday, january, saturday
Sunday, tuesday, january, saturday January 31 2006
January 31 2006 Newton biography
Newton biography January 19, 1809
January 19, 1809 Meridel lesueur, new masses, january 1932
Meridel lesueur, new masses, january 1932 January 2006 calendar
January 2006 calendar 15 janvier 1929
15 janvier 1929