Chemistry 125 Lecture 40 January 15 2010 Predicting




- Slides: 25

Chemistry 125: Lecture 40 January 15, 2010 Predicting Rate Constants, and Reactivity - Selectivity Relation. Rates of Chain Reactions. This For copyright notice see final page of this file

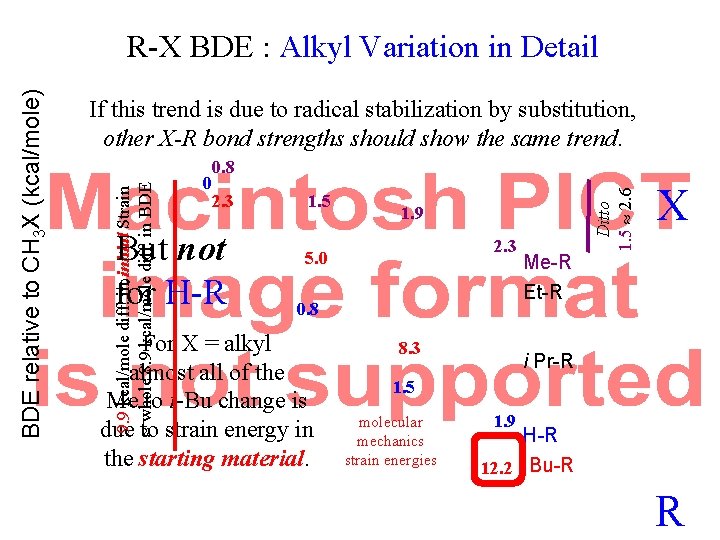
BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. X 2. 3 5. 0 Me-R Et-R 8. 3 Molecular Mechanics Strain Energies in Starting Material i Pr-R H-R 12. 2 t Bu-R R

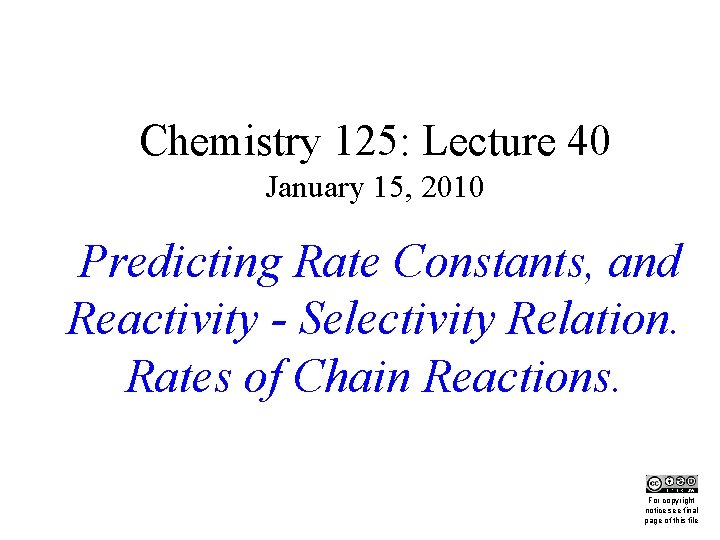
t-Bu “Idealized” Bond “Relaxed” Lengths Structure and Angles Crunch! “steric hindrance” van der Waals Energy 26. 9 5. 2 kcal/mole

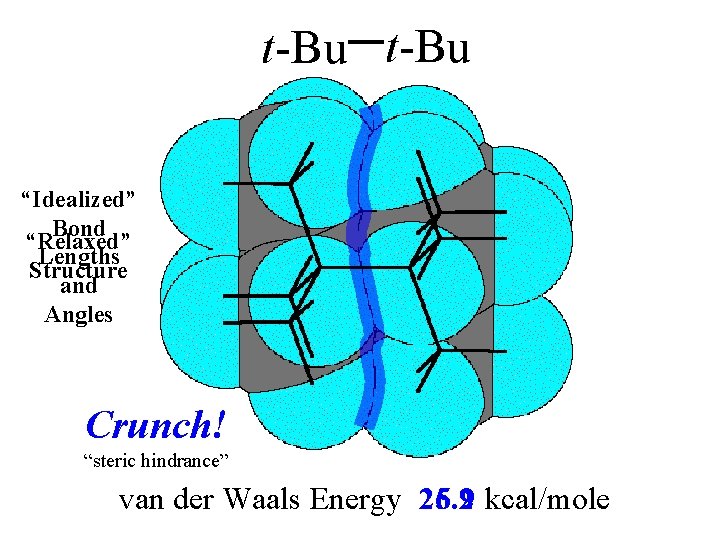
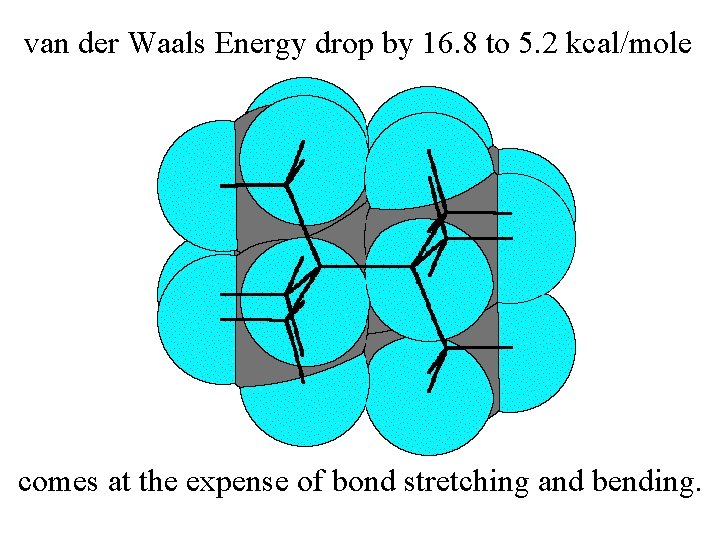
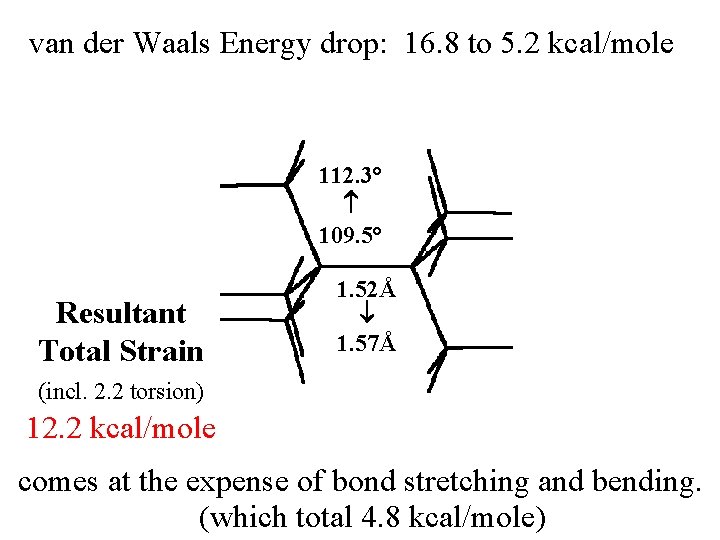
van der Waals Energy drop by 16. 8 to 5. 2 kcal/mole

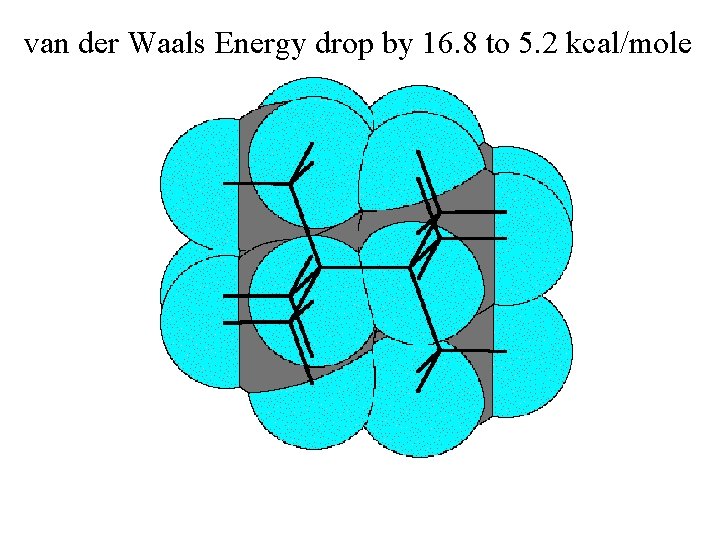
van der Waals Energy drop by 16. 8 to 5. 2 kcal/mole comes at the expense of bond stretching and bending.

van der Waals Energy drop: 16. 8 to 5. 2 kcal/mole 112. 3° 109. 5° Resultant Total Strain 1. 52Å 1. 57Å (incl. 2. 2 torsion) 12. 2 kcal/mole comes at the expense of bond stretching and bending. (which total 4. 8 kcal/mole)

If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. 0 2. 3 But not for H-R 1. 5 1. 9 2. 3 5. 0 0. 8 For X = alkyl almost all of the Me to t-Bu change is due to strain energy in the starting material. 8. 3 Me-R Et-R Ditto 1. 5 2. 6 0. 8 9. 9 kcal/mole diff. in initial Strain whole 8. 9 kcal/mole diff. in BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail X i Pr-R 1. 5 molecular mechanics strain energies 1. 9 H-R 12. 2 t Bu-R R

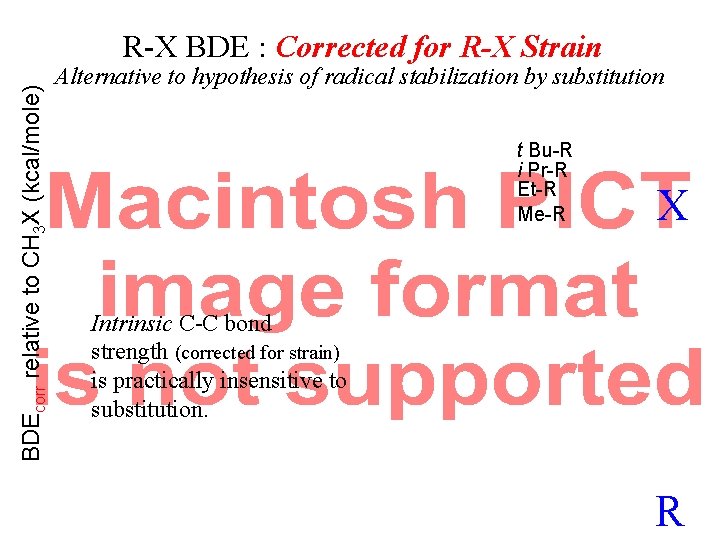
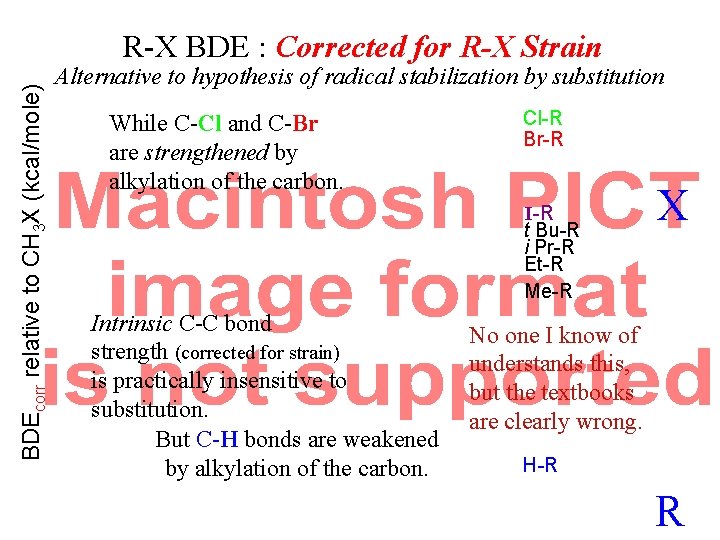
BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution t Bu-R i Pr-R Et-R Me-R X Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. R

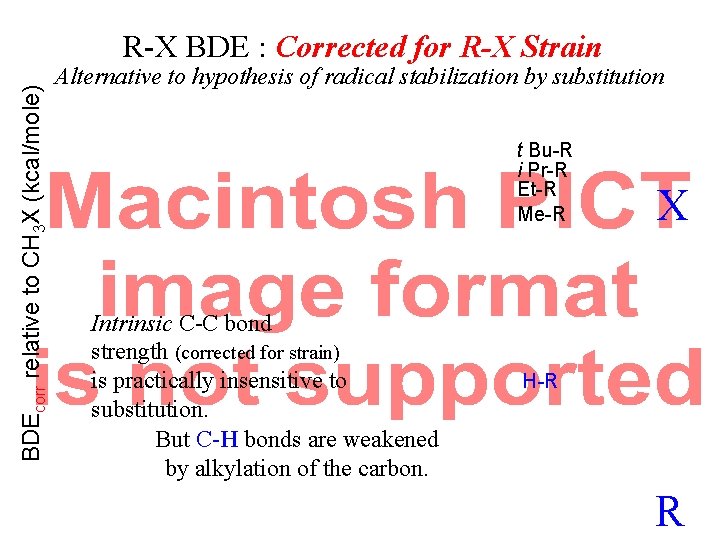
BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution t Bu-R i Pr-R Et-R Me-R Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. But C-H bonds are weakened by alkylation of the carbon. X H-R R

BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution While C-Cl and C-Br are strengthened by alkylation of the carbon. Cl-R Br-R I-R t Bu-R i Pr-R Et-R Me-R Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. But C-H bonds are weakened by alkylation of the carbon. X No one I know of understands this, but the textbooks are clearly wrong. H-R R

How can we predict activation energy? Can we use energies of stable structures that we “understand” to infer the energies of other structures (e. g. transition states), so as to predict reactivity? Might exothermic reactions be faster than endothermic ones?

How can we predict activation energy? This is no easy task a priori, especially when interaction with solvent is important. But often one can say something sensible about relative values of Ea (or G‡). Compared to What?

The Hammond Postulate (1955) This stimulated organic chemists to think about transition states and try to generalize plausibly about reaction coordinates. by permission, E. Menger “If two states, as for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures. ” George S. Hammond (1921 -2005)

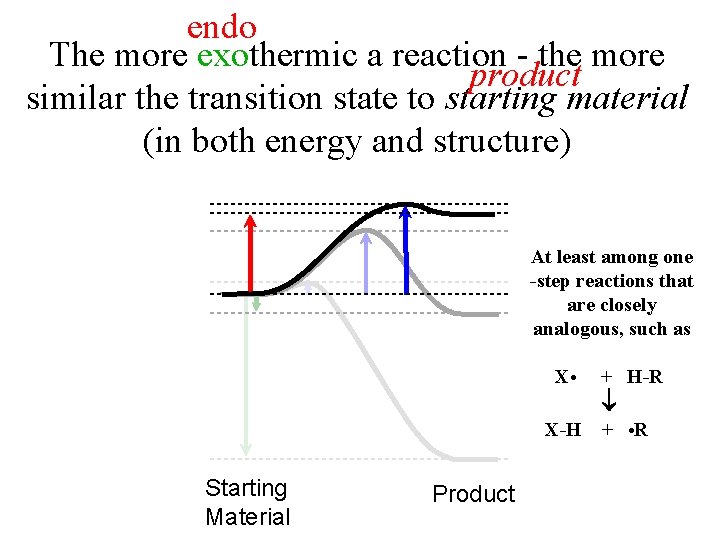
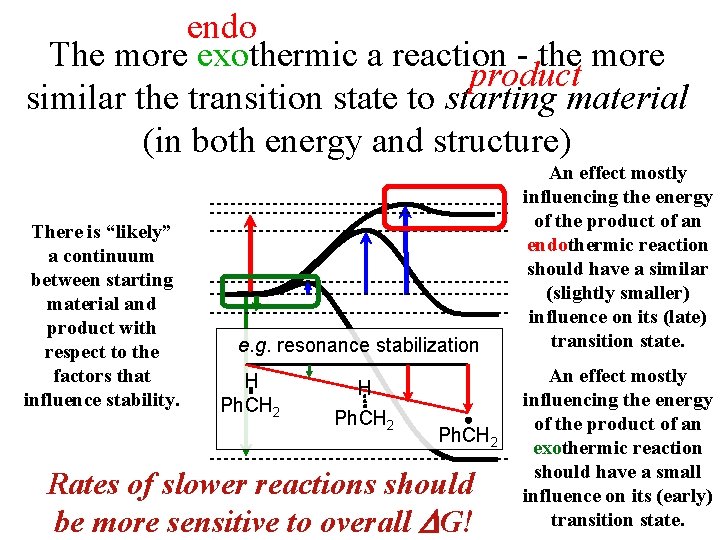
endo The more exothermic a reaction - the more product similar the transition state to starting material (in both energy and structure) At least among one -step reactions that are closely analogous, such as Starting Material Product X • + H-R. X-H + • R…. . .

endo The more exothermic a reaction - the more product similar the transition state to starting material (in both energy and structure) There is “likely” a continuum between starting material and product with respect to the factors that influence stability. e. g. resonance stabilization H Ph. CH 2 Rates of slower reactions should be more sensitive to overall G! An effect mostly influencing the energy of the product of an endothermic reaction should have a similar (slightly smaller) influence on its (late) transition state. An effect mostly influencing the energy of the product of an exothermic reaction should have a small influence on its (early) transition state.

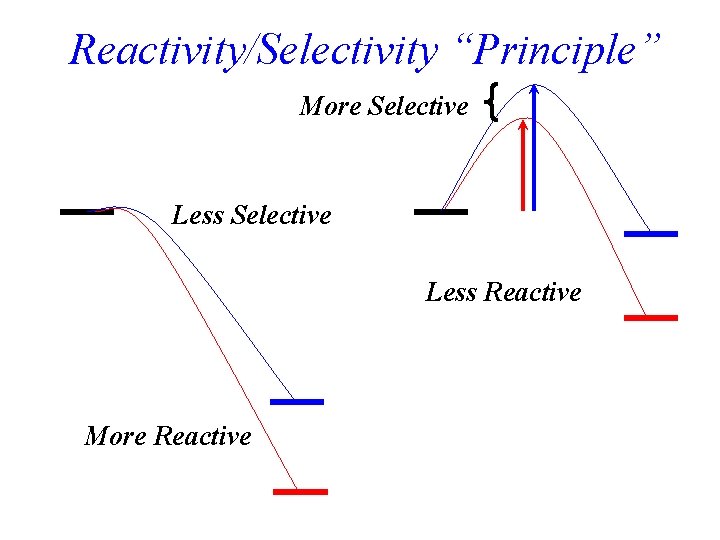
Reactivity/Selectivity “Principle” More Selective Less Reactive More Reactive

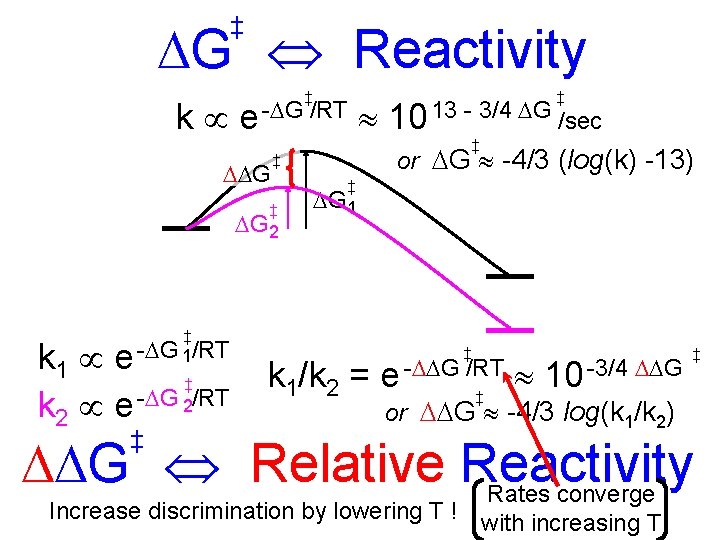
‡ G Reactivity ‡ ‡ k e - G /RT 10 13 - 3/4 G /sec G ‡ or G -4/3 (log(k) -13) ‡ ‡ G 2 ‡ G 1 ‡ k 1 e - G 1/RT ‡ k 2 e - G 2/RT ‡ ‡ -3/4 G k 1/k 2 = e - G /RT 10 ‡ or G -4/3 log(k 1/k 2) ‡ G Relative Reactivity Rates converge Increase discrimination by lowering T ! with increasing T

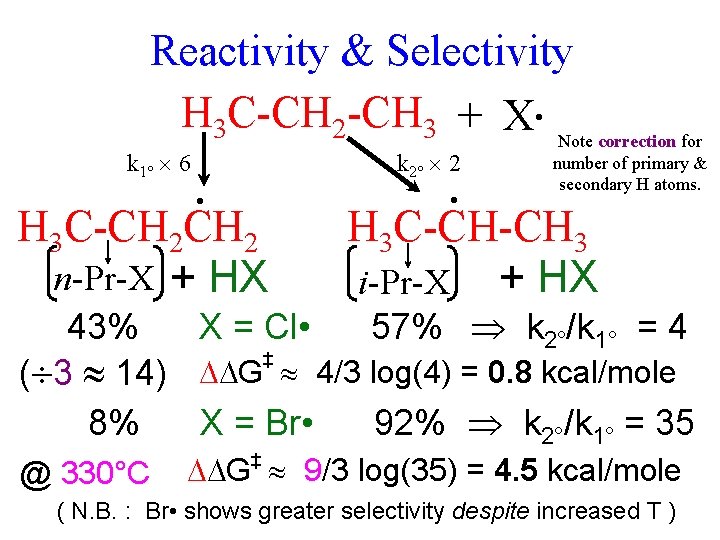
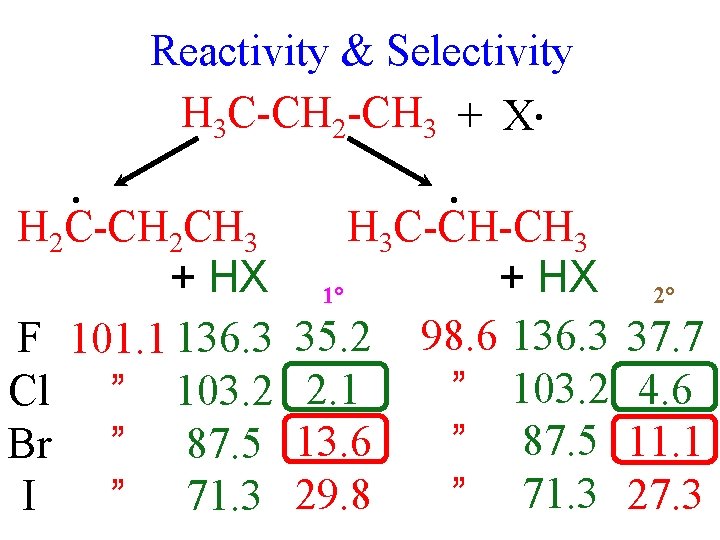
Reactivity & Selectivity H 3 C-CH 2 -CH 3 + X • Note correction for k 1° 6 k 2° 2 • H 3 C-CH 2 n-Pr-X + HX • number of primary & secondary H atoms. H 3 C-CH-CH 3 i-Pr-X + HX 43% X = Cl • 57% k 2°/k 1° = 4 ‡ ( 3 14) G 4/3 log(4) = 0. 8 kcal/mole 8% X = Br • 92% k 2°/k 1° = 35 @ 330°C G 9/3 log(35) = 4. 5 kcal/mole ‡ ( N. B. : Br • shows greater selectivity despite increased T )

Reactivity & Selectivity H 3 C-CH 2 -CH 3 + X • • H 2 C-CH 2 CH 3 + HX F 101. 1 136. 3 Cl ” 103. 2 Br ” 87. 5 I ” 71. 3 • H 3 C-CH-CH 3 + HX 1° 35. 2 98. 6 136. 3 ” 103. 2 2. 1 ” 87. 5 13. 6 ” 71. 3 29. 8 2° 37. 7 4. 6 11. 1 27. 3

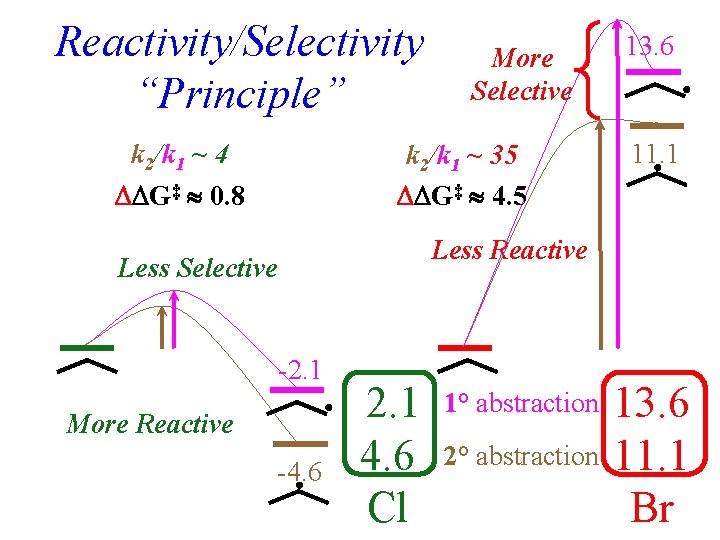
Reactivity/Selectivity “Principle” k 2/k 1 ~ 4 G‡ 0. 8 More Selective k 2/k 1 ~ 35 G‡ 4. 5 13. 6 11. 1 Less Reactive Less Selective -2. 1 More Reactive -4. 6 2 2. 1 4. 6 Cl 1° abstraction 2° abstraction 7 13. 6 11. 1 Br

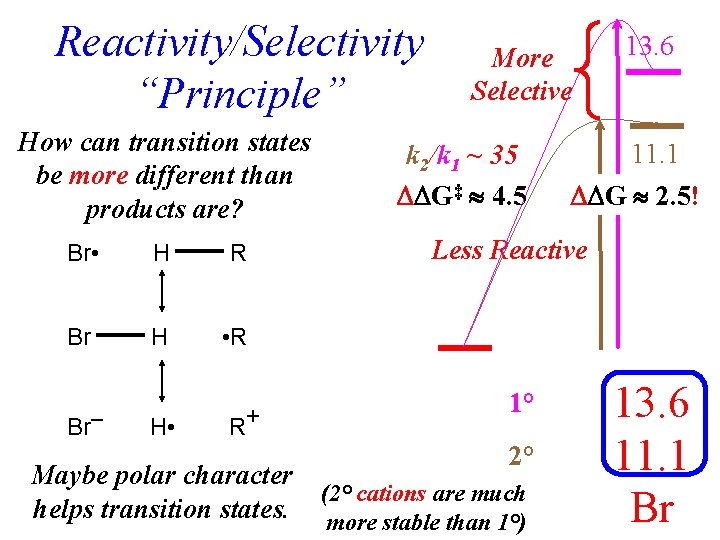
Reactivity/Selectivity “Principle” How can transition states be more different than products are? Br • H R Br H • R H • R+ Br– More Selective k 2/k 1 ~ 35 G‡ 4. 5 13. 6 11. 1 G 2. 5! Less Reactive 1° 2° Maybe polar character (2° cations are much helps transition states. more stable than 1°) 7 13. 6 11. 1 Br

Sometimes factors involved in stabilizing Transition States can be different from those involved in stabilizing either starting materials or products.

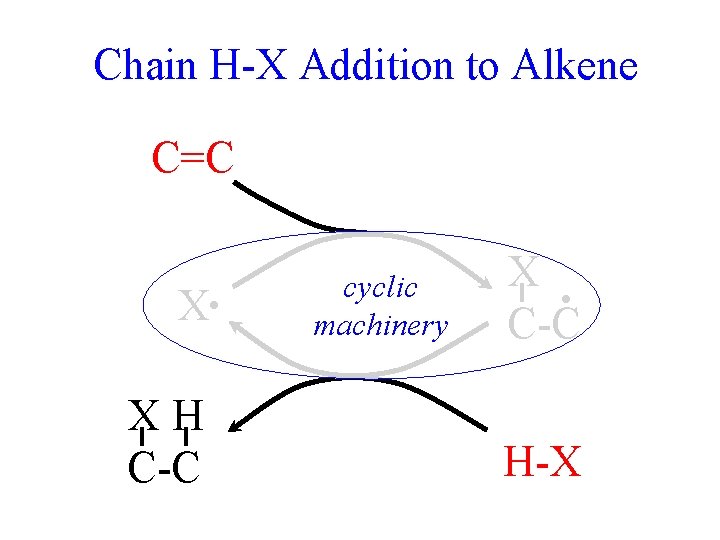
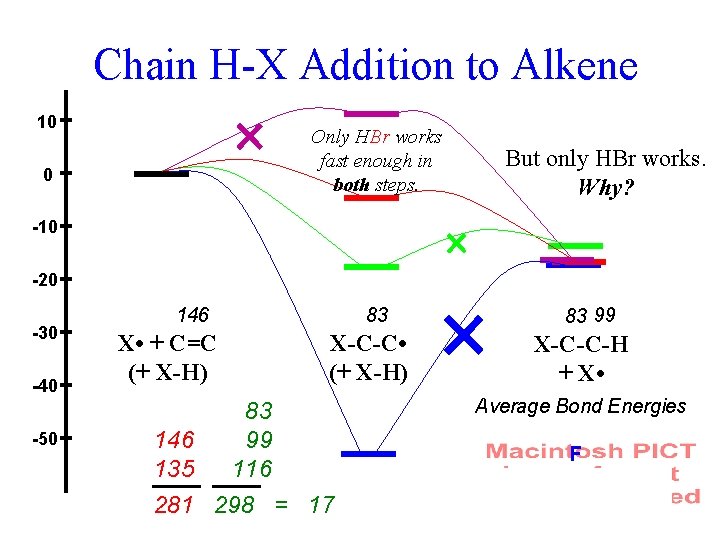
Chain H-X Addition to Alkene C=C X • XH C-C cyclic machinery X • C-C H-X

Chain H-X Addition to Alkene 10 0 Only HBr works fast enough in both steps. -10 -20 -30 -40 -50 But only HBr works. Why? 83 146 X • + C=C (+ X-H) X-C-C • (+ X-H) 83 146 99 135 116 281 298 = 17 83 99 X-C-C-H + X • Average Bond Energies F Cl Br I

End of Lecture 40 Jan. 15, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0