Chemistry 125 Lecture 70 April 19 2010 Acyl



- Slides: 18

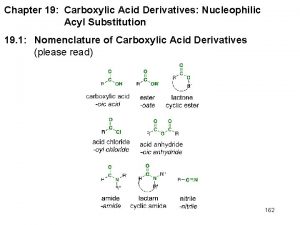
Chemistry 125: Lecture 70 April 19, 2010 Acyl Compounds (Ch. 18) -H Reactivity This (Ch. 19) For copyright notice see final page of this file

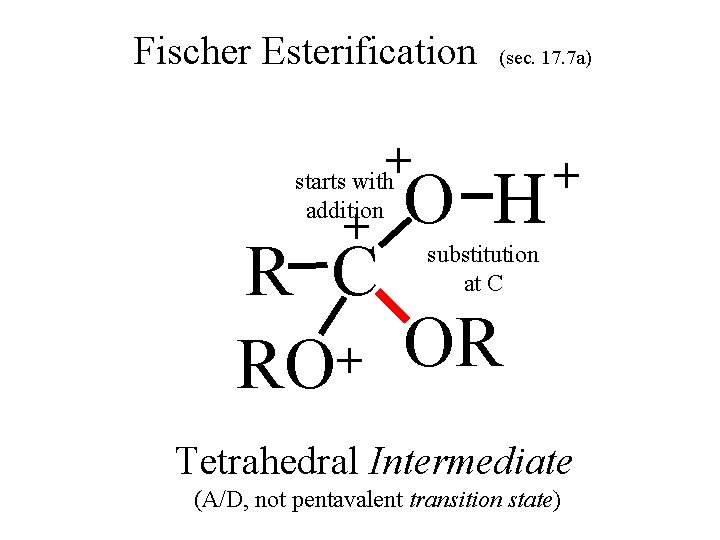
Fischer Esterification (sec. 17. 7 a) + starts with + addition O H + substitution R C OR O H + RO H Tetrahedral Intermediate at C (A/D, not pentavalent transition state)

Victor Meyer (1848 - 1897) Victor Meyer 9/8/48 - 8/8/97 “Geliebte Frau! Geliebte Kinder! Lebt wohl! Meine Nerven sind zerstört; ich kann nicht mehr. ” introduced the idea of Steric Hindrance (1894) “…the source of this behavior is stereochemical…the space filling of the neighboring groups” Configuration van’t Hoff (1874) Conformation Sachse (1891)

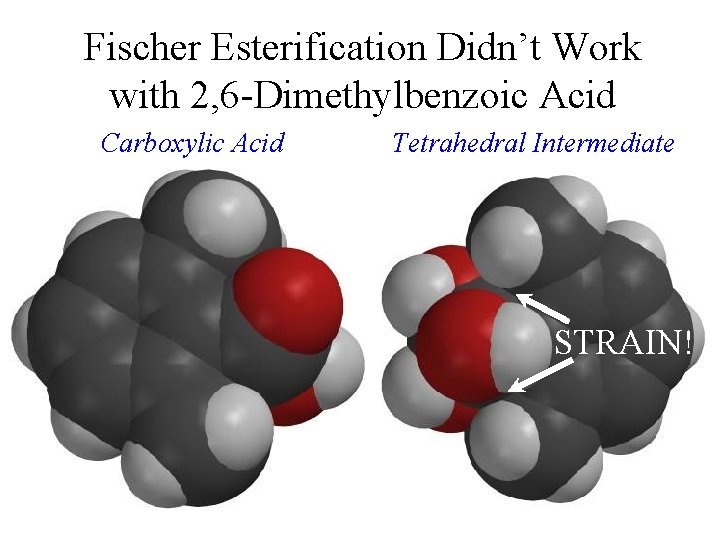
Fischer Esterification Didn’t Work with 2, 6 -Dimethylbenzoic Acid Carboxylic Acid Tetrahedral Intermediate STRAIN!

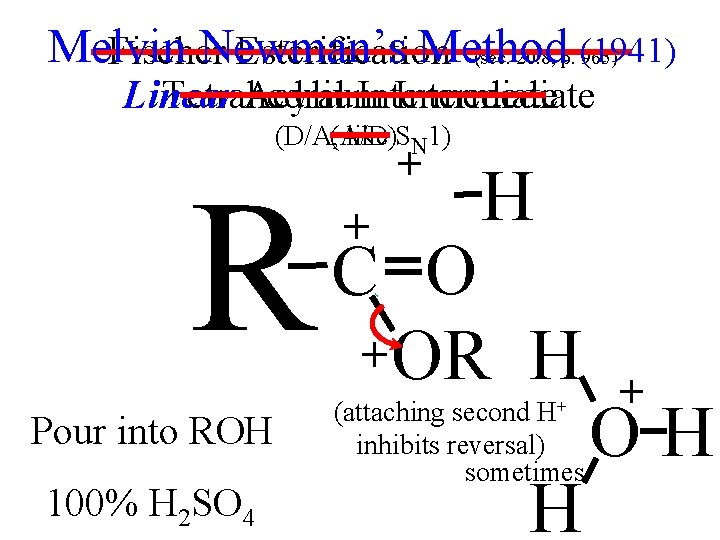
Melvin Newman’s Method Fischer Esterification (sec. 20. 8, p. (1941) 965) Linear Tetrahedral Acylium Intermediate (D/A, (A/D) like SN 1) + + H O + R C +O O HH+ + OR + + Pour into ROH HH OH 100% H SO H R substitution at C (attaching second H+ inhibits reversal) sometimes 2 4

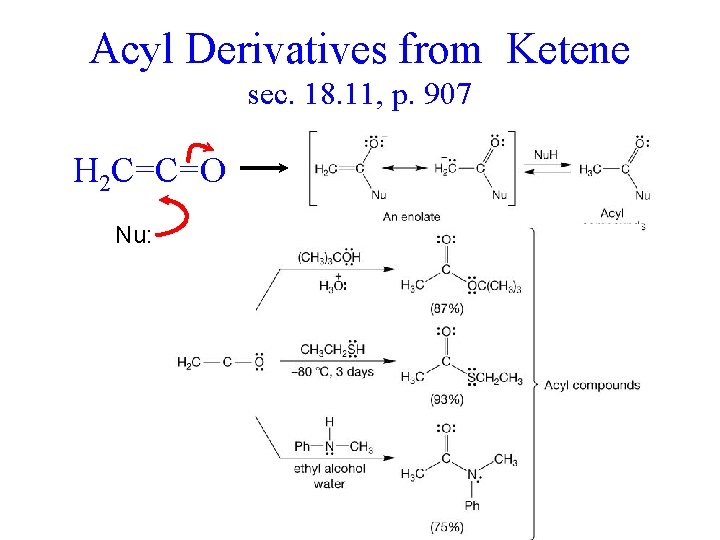
Acyl Derivatives from Ketene sec. 18. 11, p. 907 H 2 C=C=O Nu:

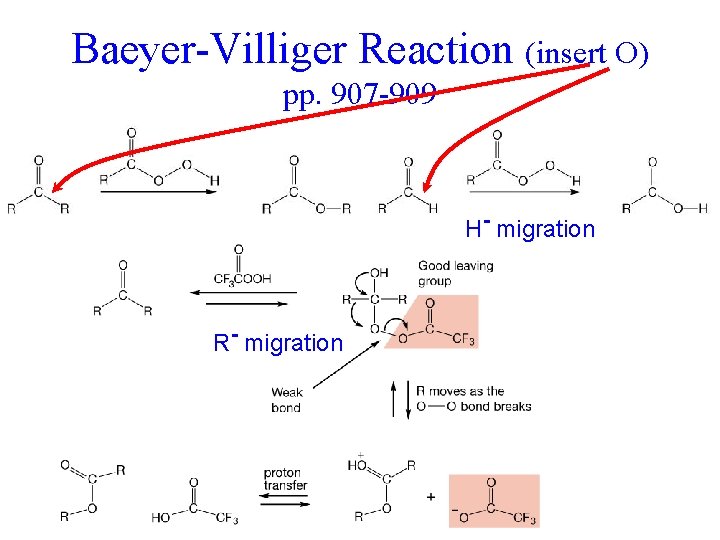
Baeyer-Villiger Reaction (insert O) pp. 907 -909 Remember Ph. CHO + O 2 H- migration R- migration

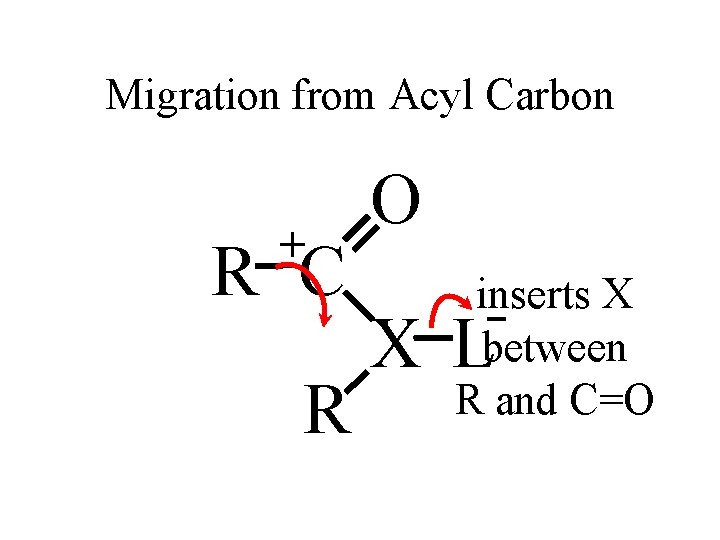
Migration from Acyl Carbon + R C R O inserts X between R and C=O X L

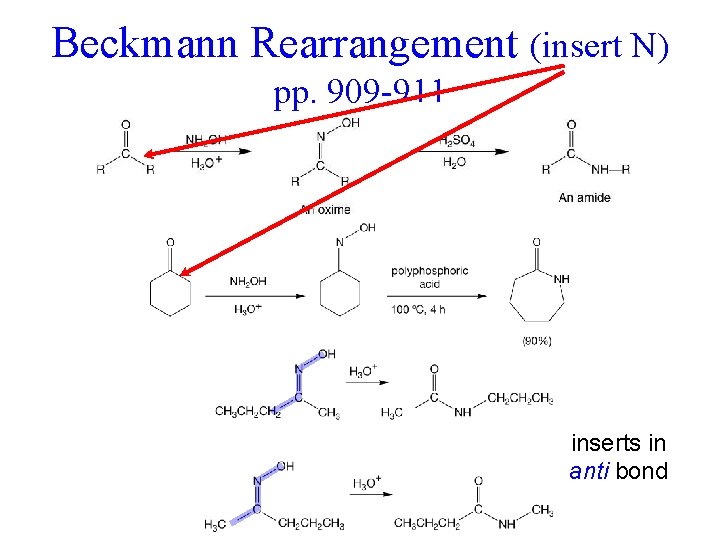
Beckmann Rearrangement (insert N) pp. 909 -911 inserts in anti bond

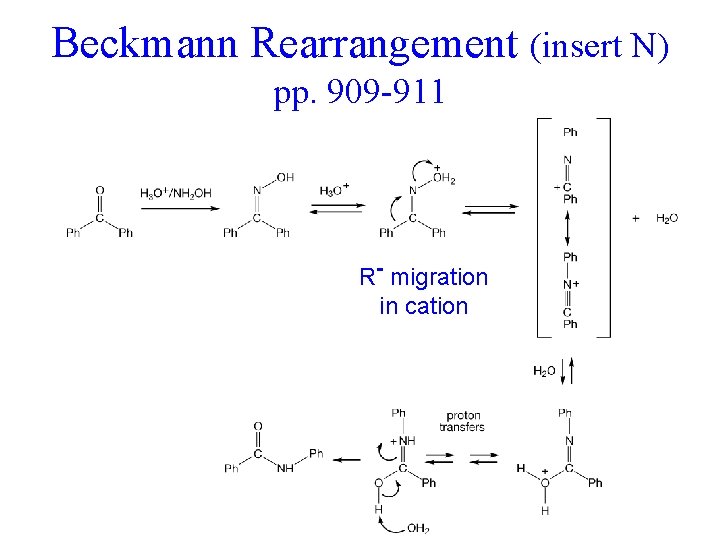
Beckmann Rearrangement (insert N) pp. 909 -911 R- migration in cation

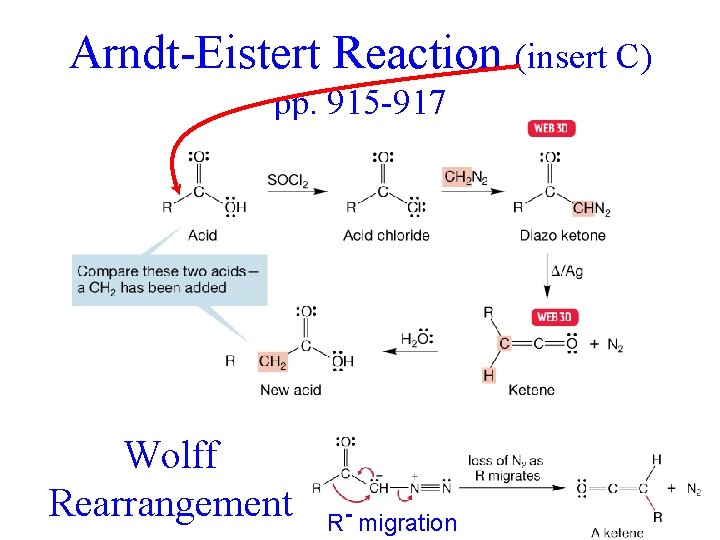
Arndt-Eistert Reaction (insert C) pp. 915 -917 Wolff elongates acid by one carbon Rearrangement R- migration

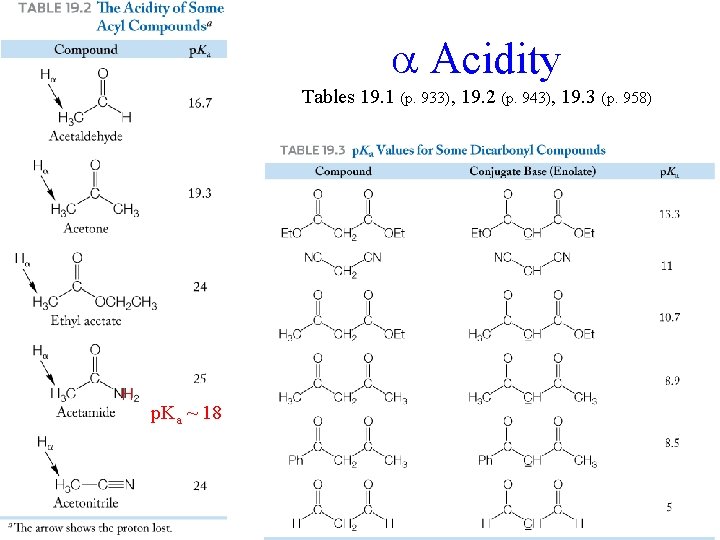
Acidity Tables 19. 1 (p. 933), 19. 2 (p. 943), 19. 3 (p. 958) H p. Ka ~ 18

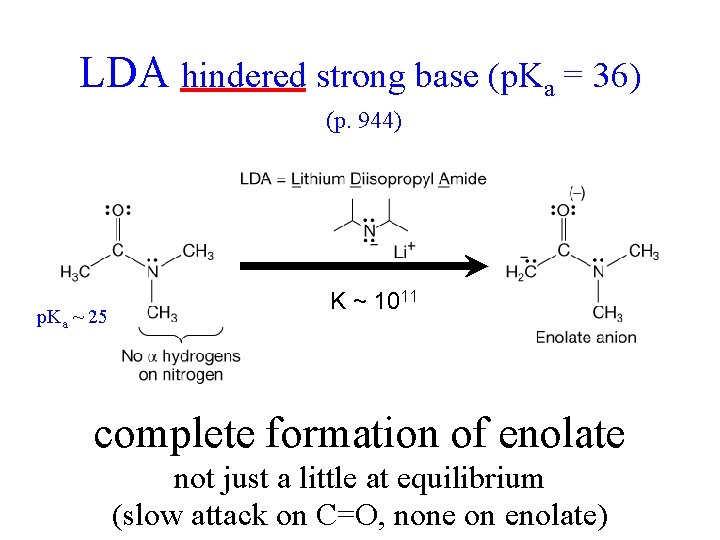
LDA hindered strong base (p. Ka = 36) (p. 944) p. Ka ~ 25 K ~ 1011 complete formation of enolate not just a little at equilibrium (slow attack on C=O, none on enolate)

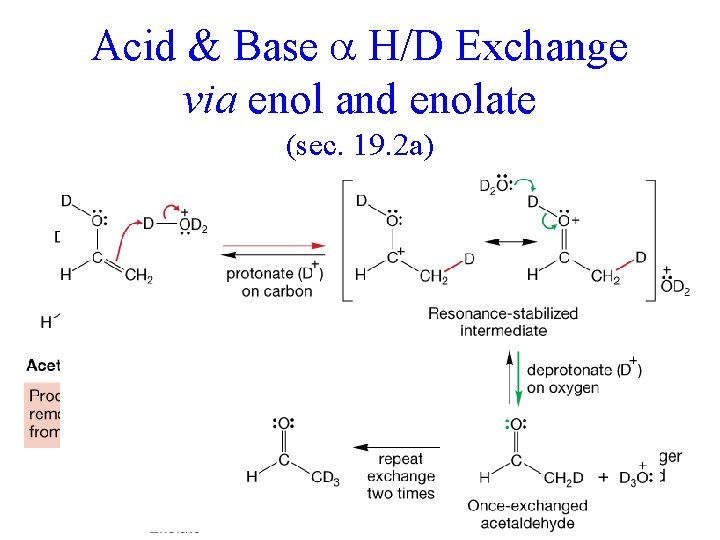
Acid & Base H/D Exchange via enol and enolate (sec. 19. 2 a)

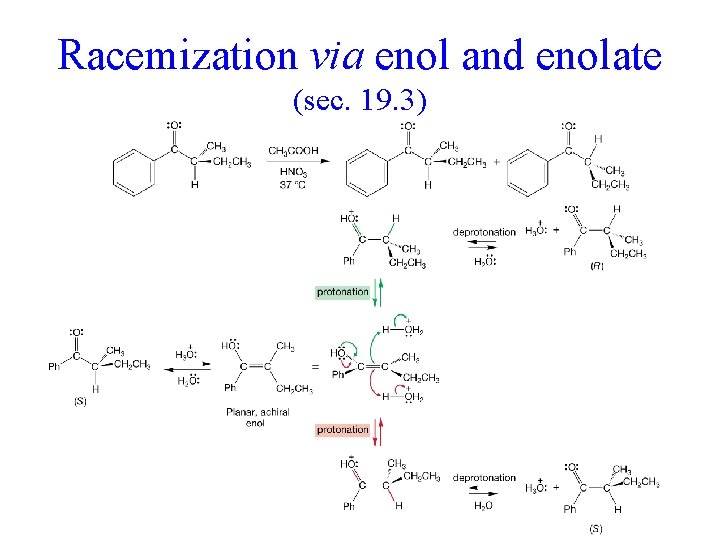
Racemization via enol and enolate (sec. 19. 3)

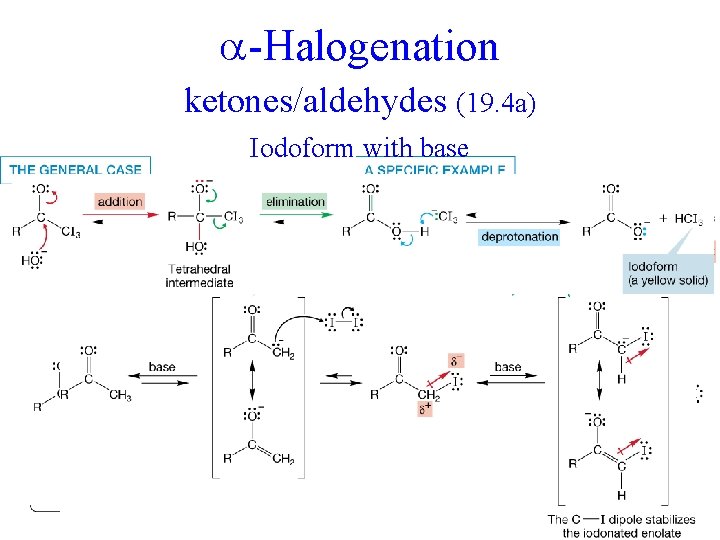
-Halogenation ketones/aldehydes (19. 4 a) Iodoform with base +

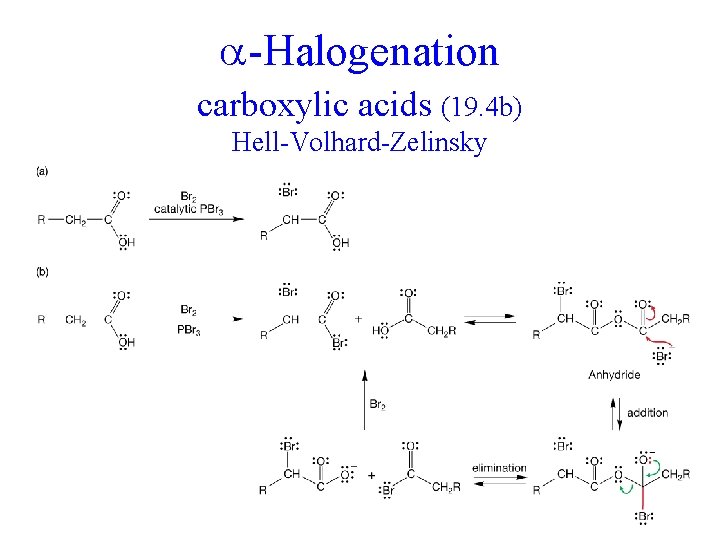
-Halogenation carboxylic acids (19. 4 b) Hell-Volhard-Zelinsky

End of Lecture 70 April 19, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 Alkanal and alkanone
Alkanal and alkanone Acyl group
Acyl group Hydrolysis of ether gives
Hydrolysis of ether gives Acid catalyzed hydrolysis of nitrile
Acid catalyzed hydrolysis of nitrile Nitrile to acyl chloride
Nitrile to acyl chloride In nucleophilic acyl substitution,
In nucleophilic acyl substitution, Ethanoylchlorid
Ethanoylchlorid Nucleophilic acyl substitution reaction
Nucleophilic acyl substitution reaction Order of reactivity of carboxylic acid derivatives
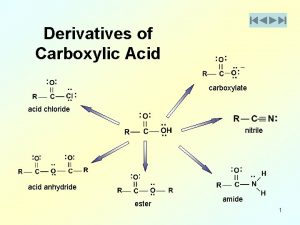
Order of reactivity of carboxylic acid derivatives Acyl derivatives
Acyl derivatives Nucleophilic acyl substitution mechanism
Nucleophilic acyl substitution mechanism 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad June 2010 chemistry regents answers
June 2010 chemistry regents answers An introduction to atmospheric physics
An introduction to atmospheric physics Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Half-life of iodine 125
Half-life of iodine 125 Ca 125 valores normales
Ca 125 valores normales