Chemistry 125 Lecture 39 January 12 2011 Fractional
![Rate Laws: Kinetic Order Rate = d [Prod] / d t = k concentration(s)? Rate Laws: Kinetic Order Rate = d [Prod] / d t = k concentration(s)?](https://slidetodoc.com/presentation_image/2fb8593dddb7a65f07ea7d65a0364a7a/image-3.jpg)


- Slides: 29

Chemistry 125: Lecture 39 January 12, 2011 Fractional and Inverse Rate Laws, Bond Dissociation Energies, Radical-Chain Halogenation, Reactivity-Selectivity “Principle” This For copyright notice see final page of this file

Digression on Reaction Order & Complex Reactions The kinetic analogue of the Law of Mass Action (i. e. dependance of rate on concentrations) can provide insight about reaction mechanism.
![Rate Laws Kinetic Order Rate d Prod d t k concentrations Rate Laws: Kinetic Order Rate = d [Prod] / d t = k concentration(s)?](https://slidetodoc.com/presentation_image/2fb8593dddb7a65f07ea7d65a0364a7a/image-3.jpg)
Rate Laws: Kinetic Order Rate = d [Prod] / d t = k concentration(s)? Dependent on Mechanism Discovered by Experiment Complex Reactions The Rate-Limiting Step Fractional Order Importance of e. g. Rate = k [A] [B]1/4 “Dominant” species B 4 dominant / B reactive in B 4 4 B [dominant species] quantity added (how much you think you have) Minor species tag along following the Law of Mass Action.

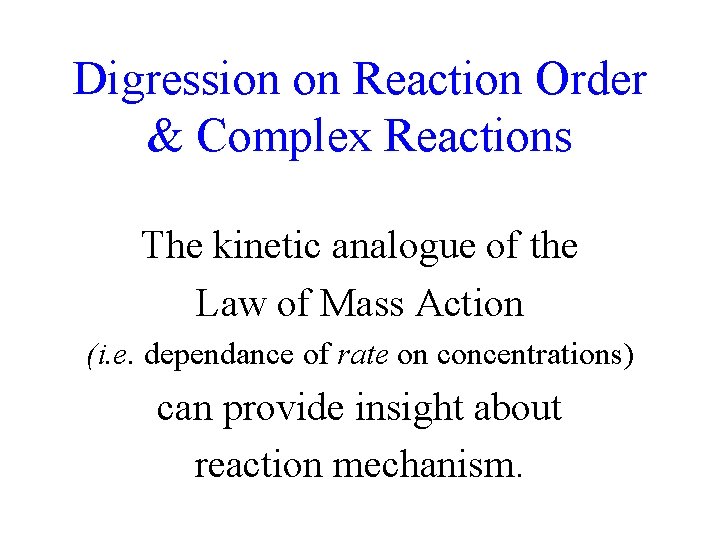
4 CH 3 Li • 3 Me O 2 : Me 2 O: Me 4 O: : O O 2 Excess ether rips aggregates apart CH 3 by competing for vacant Li AOs. Me : H 3 C 2 2 O: (CH 3 Li)4 • 4 Me O 8 Me 2 O 2 Me 2 O: Distorted Cubic Tetramer Me 2 2 O: O Me Me : O M e 2 Me O: : O Me 2

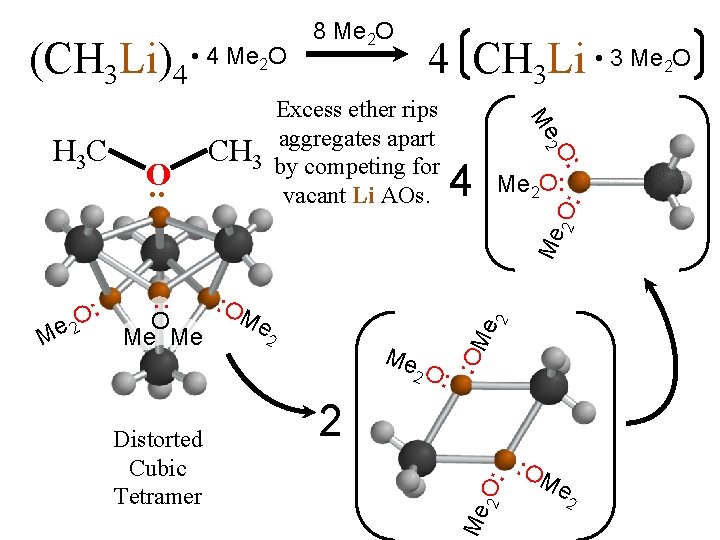
Reaction order proves that monomer is reactive but tetramer is dominant in hydrocarbon. Excess ether rips aggregates apart by bonding with vacant Li AOs to make monomer dominant. Reaction becomes 1 st order. Distorted Cubic Tetramer • 3 Me 2 O 4 CH 3 Li [CH 3 Li]4 K = [CH 3 Li] + 8 Me 2 O (CH 3 Li)4 [(CH 3 Li)4] 1/4 [(CH [CH 33 Li) Li]44] • 4 Me 2 O Reaction of monomer in hydrocarbon solvent is 1/4 iorder in reagent added.

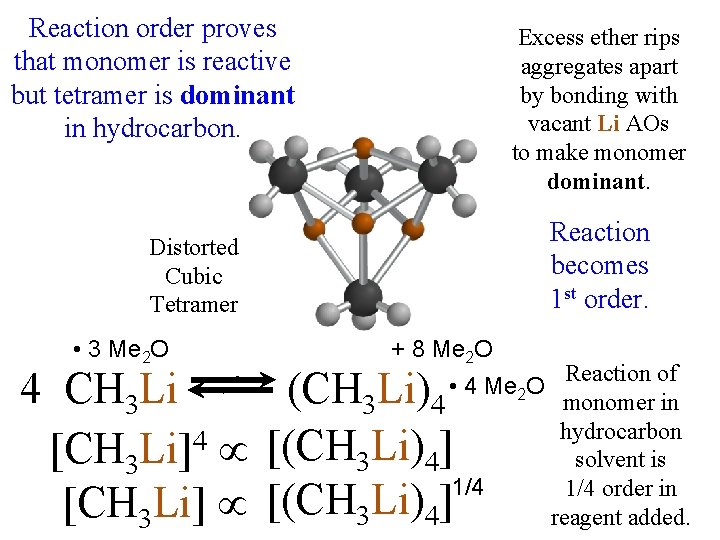
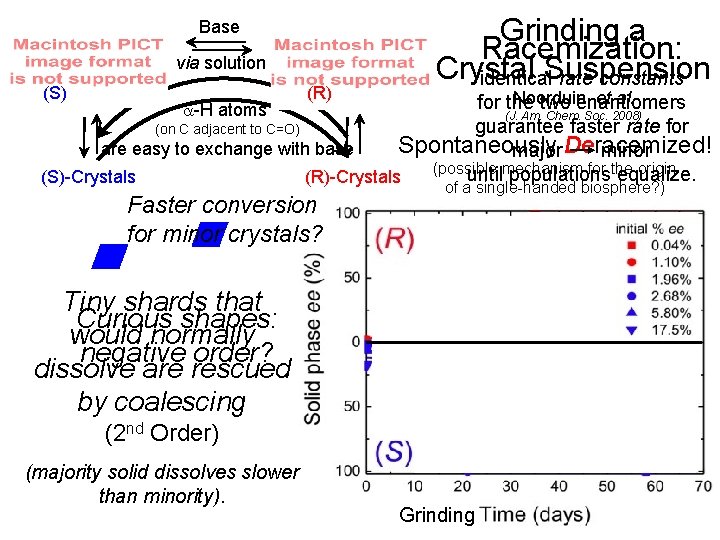
Base via solution (S) (R) Grinding a Racemization: Crystal Suspension identical rate constants Noorduin, et al. for the two enantiomers a-H atoms (J. Am. Chem. Soc. 2008) guarantee faster rate for (on C adjacent to C=O) Spontaneously Deracemized! are easy to exchange with base major minor (possible mechanism for the origin until populations equalize. (S)-Crystals (R)-Crystals Faster conversion for minor crystals? of a single-handed biosphere? ) Tiny shards that Curious shapes: would normally negative order? dissolve are rescued by coalescing (2 nd Order) (majority solid dissolves slower than minority). Grinding

So we’ve seen the guidance rate laws can provide for understanding reaction mechanism. 0 th Order 1 st Order 2 nd Order Fractional Order Negative Order

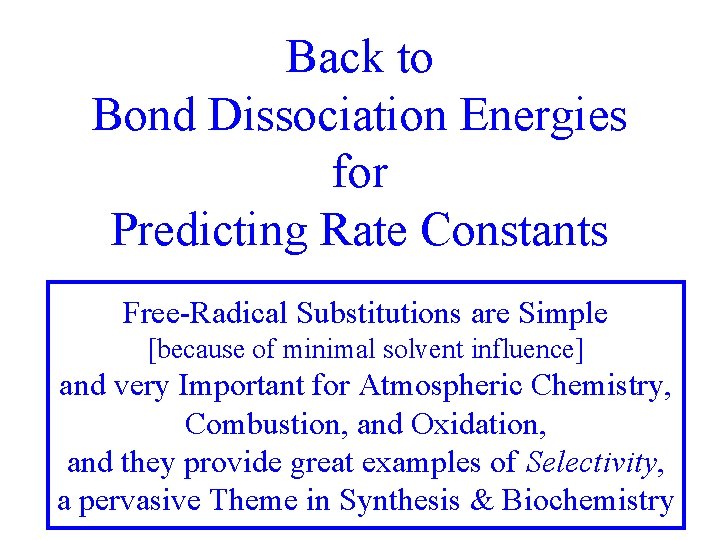
Back to Bond Dissociation Energies for Predicting Rate Constants Free-Radical Substitutions are Simple [because of minimal solvent influence] and very Important for Atmospheric Chemistry, Combustion, and Oxidation, and they provide great examples of Selectivity, a pervasive Theme in Synthesis & Biochemistry

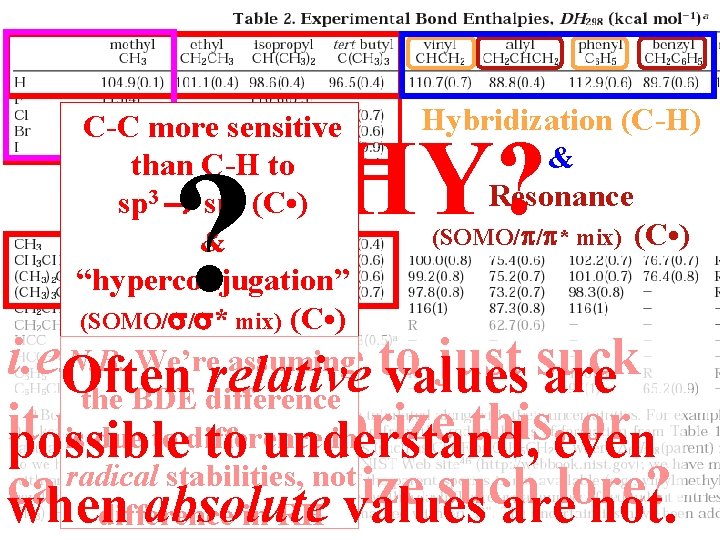
Ellison IIHybridization (C-H) C-C more sensitive than C-H to Overlap (C-X) 2 (C • ) Check with more examples sp 3 sp && E-Match (C-X) “hyperconjugation” (SOMO/ / * mix) (C • ) N. B. We’re assuming the BDE difference is due to difference in radical stabilities, not difference in RH WHY? ? & Resonance (SOMO/ / * mix) (C • ) i. e. Often Do we have to just suck relative values are it up and memorize this, or possible to understand, even can we rationalize such lore? when absolute values are not.

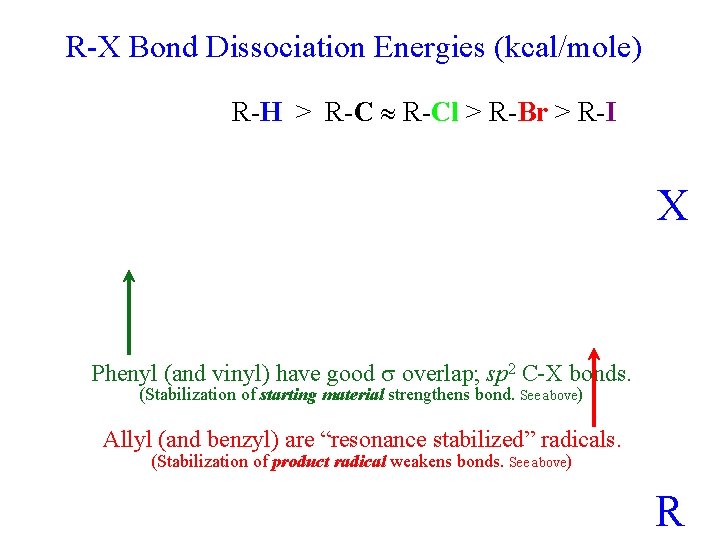
R-X Bond Dissociation Energies (kcal/mole) R-H > R-Cl > R-Br > R-I X Phenyl (and vinyl) have good overlap; sp 2 C-X bonds. (Stabilization of starting material strengthens bond. See above) Allyl (and benzyl) are “resonance stabilized” radicals. (Stabilization of product radical weakens bonds. See above) R

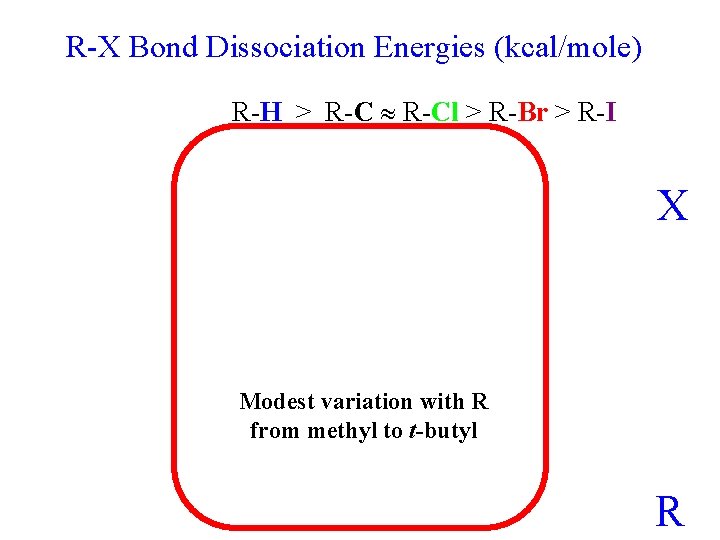
R-X Bond Dissociation Energies (kcal/mole) R-H > R-Cl > R-Br > R-I X Modest variation with R from methyl to t-butyl R

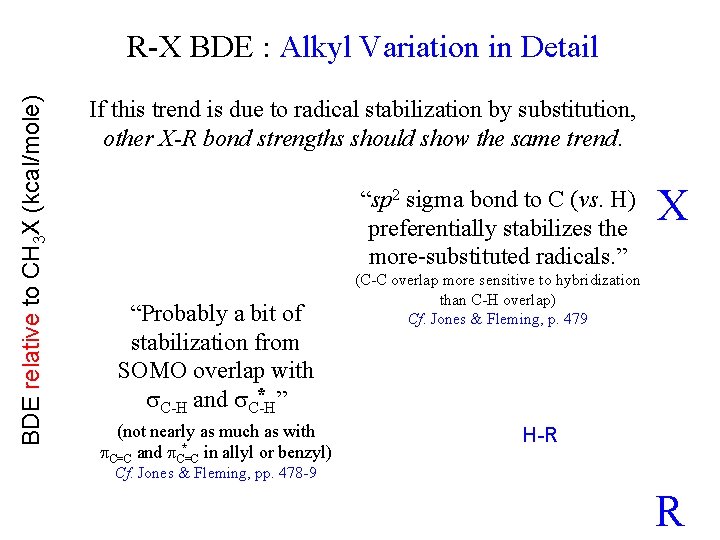
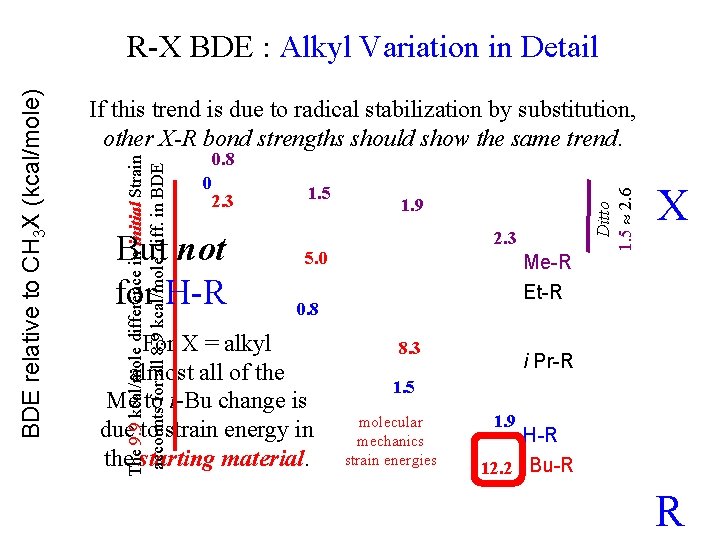
BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. “sp 2 sigma bond to C (vs. H) preferentially stabilizes the more-substituted radicals. ” “Probably a bit of stabilization from SOMO overlap with * ” C-H and C-H (not nearly as much as with * in allyl or benzyl) C=C and C=C X (C-C overlap more sensitive to hybridization than C-H overlap) Cf. Jones & Fleming, p. 479 H-R Cf. Jones & Fleming, pp. 478 -9 R

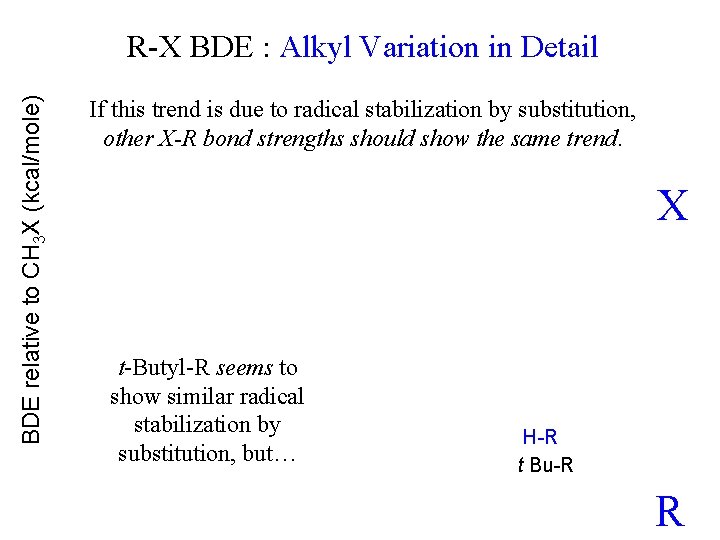
BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. X t-Butyl-R seems to show similar radical stabilization by substitution, but… H-R t Bu-R R

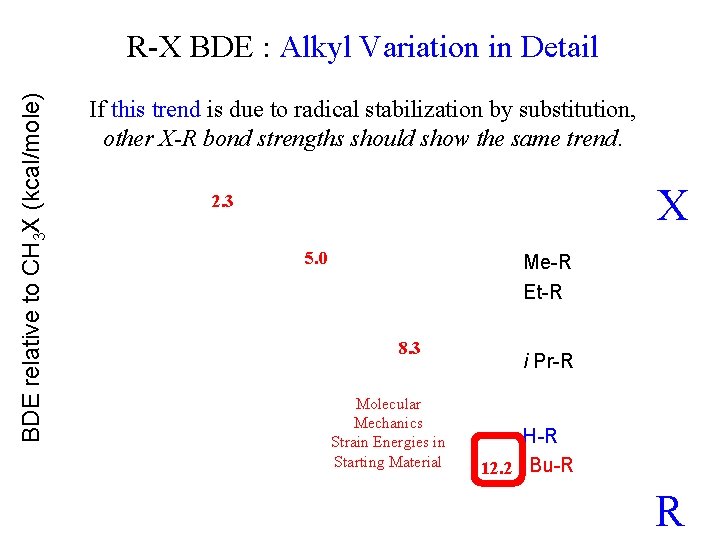
BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. X 2. 3 5. 0 Me-R Et-R 8. 3 Molecular Mechanics Strain Energies in Starting Material i Pr-R H-R 12. 2 t Bu-R R

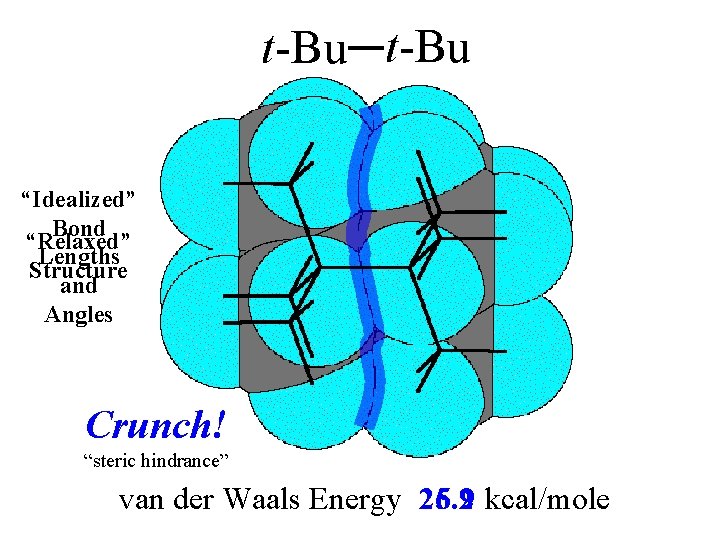
t-Bu “Idealized” Bond “Relaxed” Lengths Structure and Angles Crunch! “steric hindrance” van der Waals Energy 26. 9 5. 2 kcal/mole

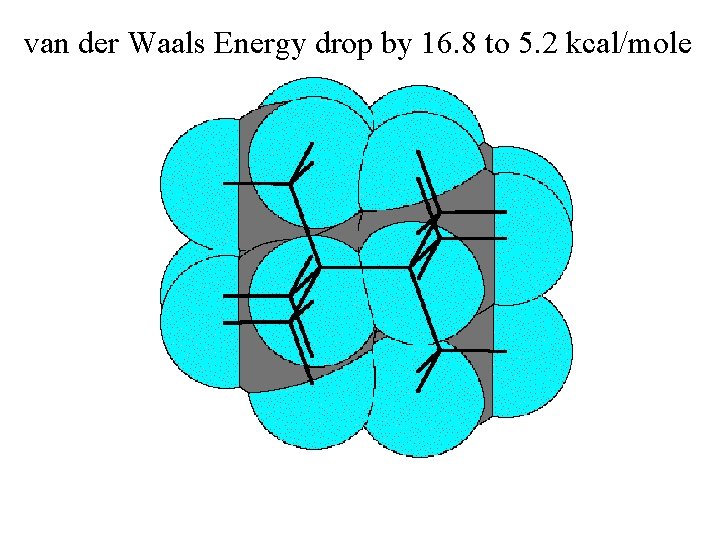
van der Waals Energy drop by 16. 8 to 5. 2 kcal/mole

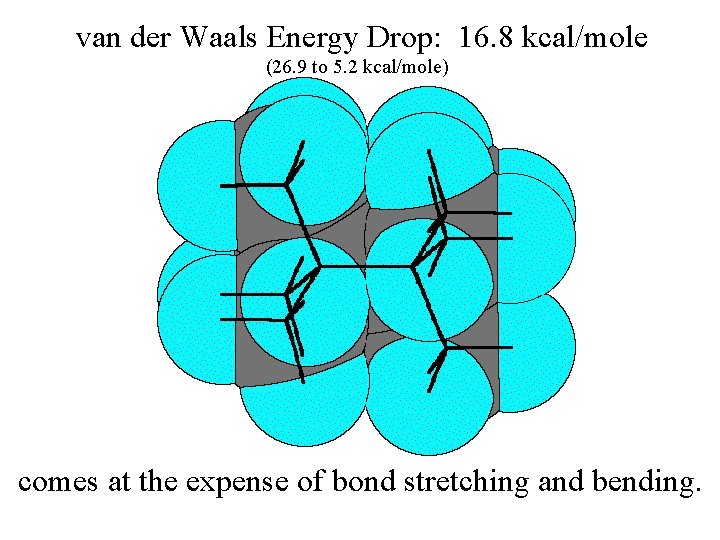
van der Waals Energy Drop: 16. 8 kcal/mole (26. 9 to 5. 2 kcal/mole) comes at the expense of bond stretching and bending.

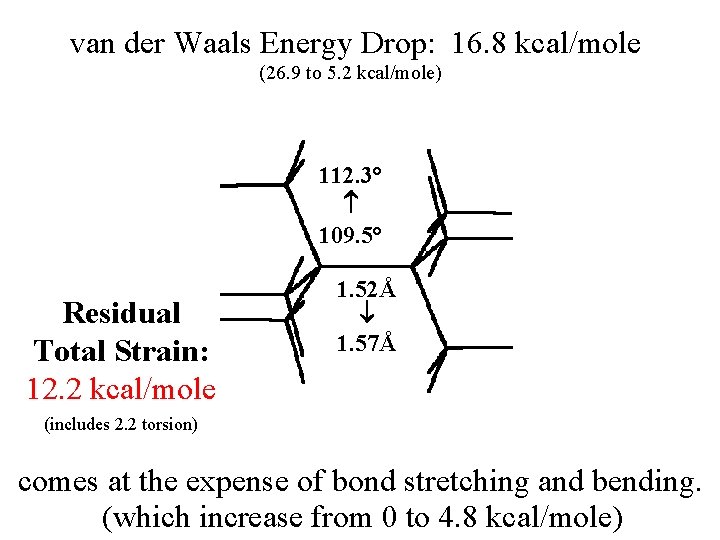
van der Waals Energy Drop: 16. 8 kcal/mole (26. 9 to 5. 2 kcal/mole) 112. 3° 109. 5° Residual Total Strain: 12. 2 kcal/mole 1. 52Å 1. 57Å (includes 2. 2 torsion) comes at the expense of bond stretching and bending. (which increase from 0 to 4. 8 kcal/mole)

If this trend is due to radical stabilization by substitution, other X-R bond strengths should show the same trend. 0. 8 0 2. 3 But not for H-R 1. 5 1. 9 2. 3 5. 0 Me-R Et-R 0. 8 For X = alkyl almost all of the Me to t-Bu change is due to strain energy in the starting material. 8. 3 Ditto 1. 5 2. 6 The 9. 9 kcal/mole difference in initial Strain accounts for all 8. 9 kcal/mole diff. in BDE relative to CH 3 X (kcal/mole) R-X BDE : Alkyl Variation in Detail X i Pr-R 1. 5 molecular mechanics strain energies 1. 9 H-R 12. 2 t Bu-R R

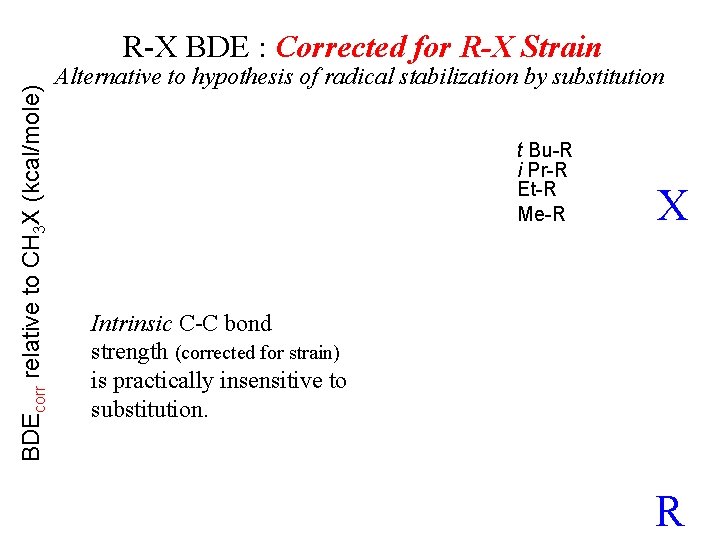
BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution t Bu-R i Pr-R Et-R Me-R X Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. R

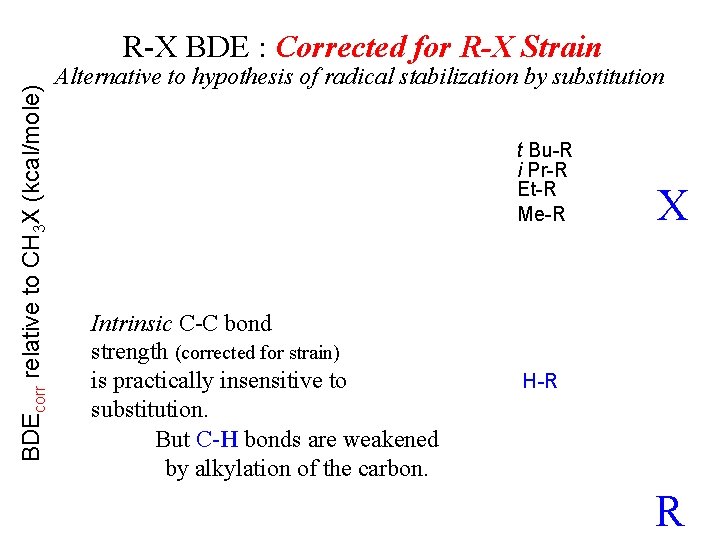
BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution t Bu-R i Pr-R Et-R Me-R Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. But C-H bonds are weakened by alkylation of the carbon. X H-R R

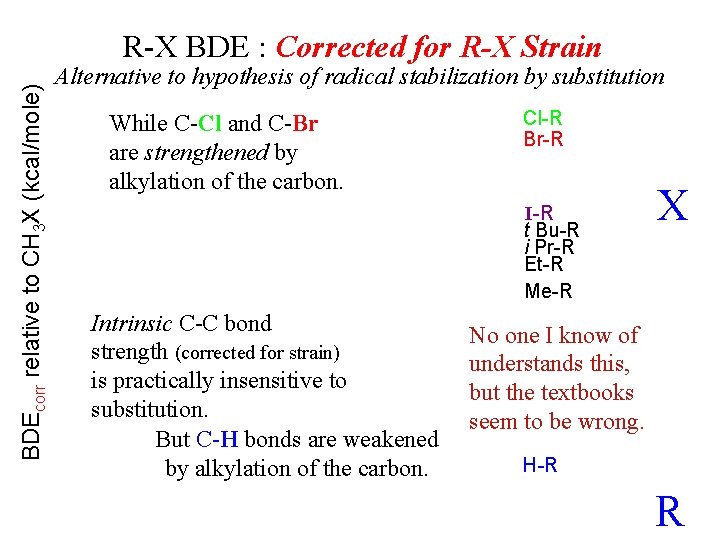
BDEcorr relative to CH 3 X (kcal/mole) R-X BDE : Corrected for R-X Strain Alternative to hypothesis of radical stabilization by substitution While C-Cl and C-Br are strengthened by alkylation of the carbon. Cl-R Br-R I-R t Bu-R i Pr-R Et-R Me-R Intrinsic C-C bond strength (corrected for strain) is practically insensitive to substitution. But C-H bonds are weakened by alkylation of the carbon. X No one I know of understands this, but the textbooks seem to be wrong. H-R R

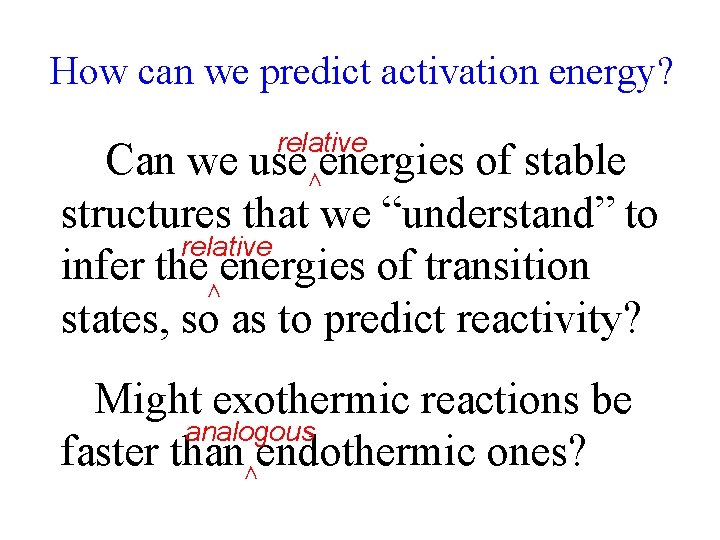
How can we predict activation energy? relative Can we use energies of stable structures that we “understand” to relative infer the energies of transition states, so as to predict reactivity? ∧ ∧ Might exothermic reactions be analogous faster than endothermic ones? ∧

How can we predict activation energy? This is no easy task a priori, especially when interaction with solvent is important. But often one can say something sensible about relative values of Ea (or DG‡). Compared to What?

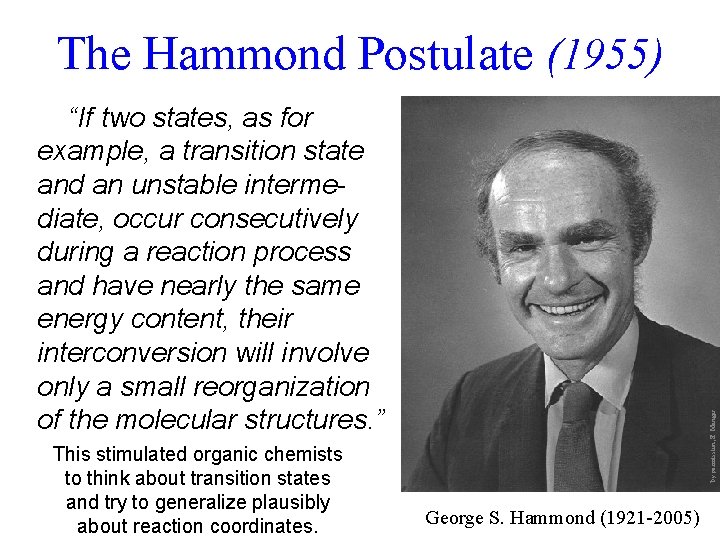
The Hammond Postulate (1955) This stimulated organic chemists to think about transition states and try to generalize plausibly about reaction coordinates. by permission, E. Menger “If two states, as for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures. ” George S. Hammond (1921 -2005)

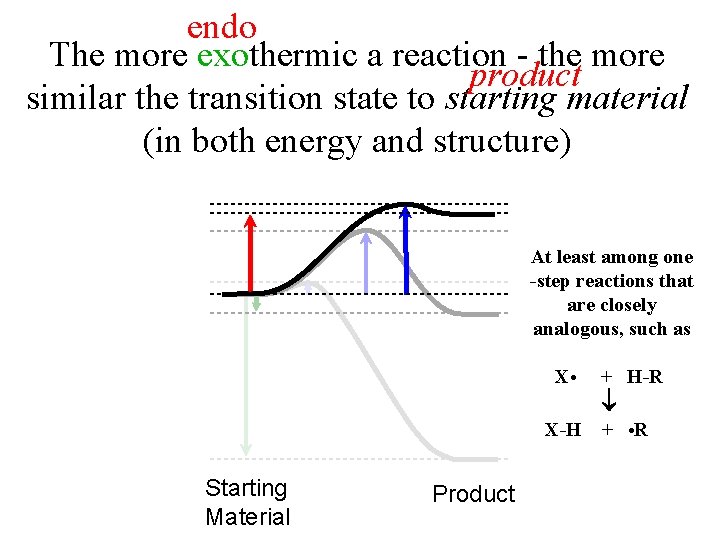
endo The more exothermic a reaction - the more product similar the transition state to starting material (in both energy and structure) At least among one -step reactions that are closely analogous, such as Starting Material Product X • + H-R. X-H + • R…. . .

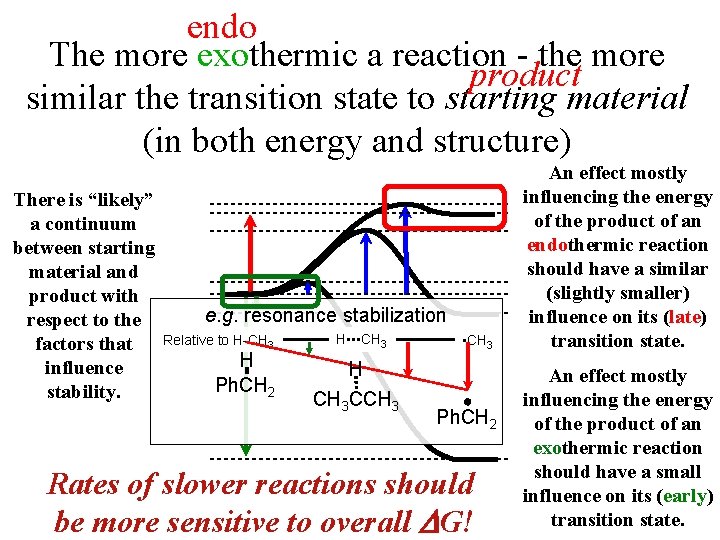
endo The more exothermic a reaction - the more product similar the transition state to starting material (in both energy and structure) There is “likely” a continuum between starting material and product with respect to the factors that influence stability. e. g. resonance stabilization Relative to H-CH 3 H Ph. CH 2 H • • • CH 3 H CH 3 CCH 3 • CH 3 Ph. CH 2 Rates of slower reactions should be more sensitive to overall G! An effect mostly influencing the energy of the product of an endothermic reaction should have a similar (slightly smaller) influence on its (late) transition state. An effect mostly influencing the energy of the product of an exothermic reaction should have a small influence on its (early) transition state.

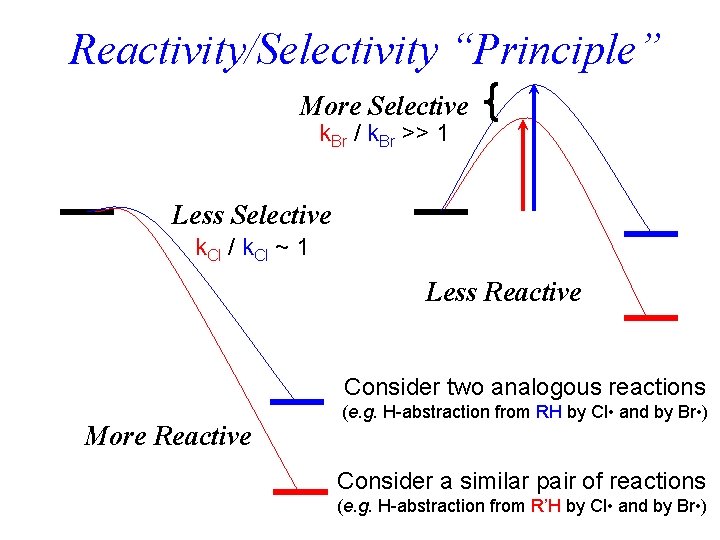
Reactivity/Selectivity “Principle” More Selective k. Br / k. Br >> 1 Less Selective k. Cl / k. Cl ~ 1 Less Reactive Consider two analogous reactions More Reactive (e. g. H-abstraction from RH by Cl • and by Br • ) Consider a similar pair of reactions (e. g. H-abstraction from R’H by Cl • and by Br • )

End of Lecture 39 Jan. 12, 2011 Copyright © J. M. Mc. Bride 2011. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 January 2012 chemistry regents answers
January 2012 chemistry regents answers 2019 ib boundaries
2019 ib boundaries Chemistry january 2018 answers
Chemistry january 2018 answers Nysedregents
Nysedregents 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Chemistry regents 2011
Chemistry regents 2011 Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Fractional reserve theory
Fractional reserve theory Fractional reserve banking example
Fractional reserve banking example Example of medium of exchange
Example of medium of exchange Fractional distillation
Fractional distillation How to find a missing endpoint geometry
How to find a missing endpoint geometry Fractional change in length
Fractional change in length Rational coefficients example
Rational coefficients example How to simplify fraction exponents
How to simplify fraction exponents Negative indices
Negative indices Example of pure substance
Example of pure substance Relative refractive index
Relative refractive index Find the coordinates of the midpoint
Find the coordinates of the midpoint Rational coefficient
Rational coefficient Application of steam distillation
Application of steam distillation Fractional interest discount
Fractional interest discount Fractional reserve banking example
Fractional reserve banking example Application of vacuum distillation in food industry
Application of vacuum distillation in food industry Fractional anisotropy meaning
Fractional anisotropy meaning Binomial series expansion
Binomial series expansion Fractional liberation
Fractional liberation