Chemistry 125 Lecture 41 January 20 2010 Rates
![Catalytic Cycle Rate Law R-H X-H k 1 [RH] [X • ] X • Catalytic Cycle Rate Law R-H X-H k 1 [RH] [X • ] X •](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-4.jpg)
![E-ZPass “If you get that [barrier] down, “If there's a slow step, there's 99. E-ZPass “If you get that [barrier] down, “If there's a slow step, there's 99.](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-5.jpg)
![Kinetic Order in Initiator Rate [RO-OR] Initiation : RO-OR ki 1/2 ? 2 RO Kinetic Order in Initiator Rate [RO-OR] Initiation : RO-OR ki 1/2 ? 2 RO](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-9.jpg)




- Slides: 23

Chemistry 125: Lecture 41 January 20, 2010 Rates of Chain Reactions and Electronegativity This � For copyright notice see final page of this file

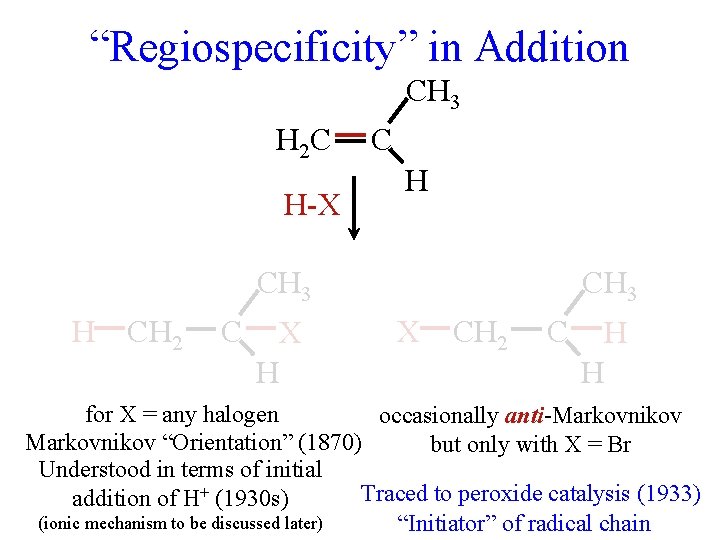
“Regiospecificity” in Addition CH 3 H 2 C H-X C H CH 3 H CH 2 C X H CH 3 X CH 2 C H H for X = any halogen occasionally anti-Markovnikov “Orientation” (1870) but only with X = Br Understood in terms of initial Traced to peroxide catalysis (1933) addition of H+ (1930 s) (ionic mechanism to be discussed later) “Initiator” of radical chain

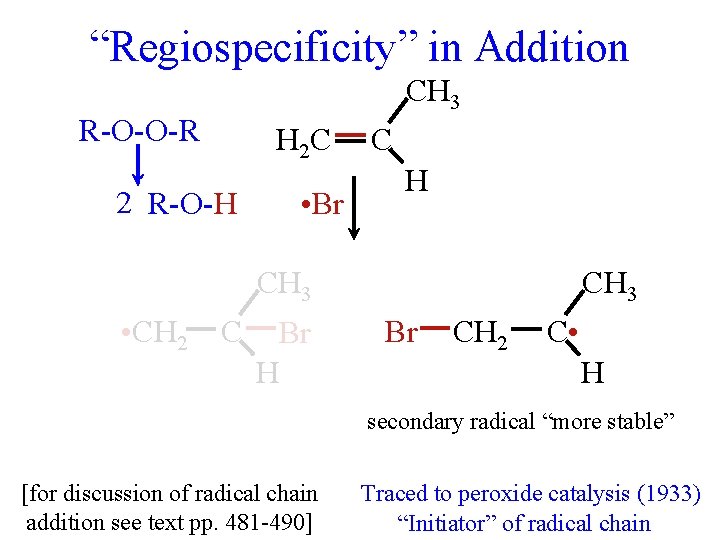
“Regiospecificity” in Addition CH 3 R-O-O-R H 2 C 2 R-O-H R-O • • Br H-Br C H CH 3 • CH 2 C Br H CH 3 Br CH 2 C • H secondary radical “more stable” [for discussion of radical chain addition see text pp. 481 -490] Traced to peroxide catalysis (1933) “Initiator” of radical chain
![Catalytic Cycle Rate Law RH XH k 1 RH X X Catalytic Cycle Rate Law R-H X-H k 1 [RH] [X • ] X •](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-4.jpg)
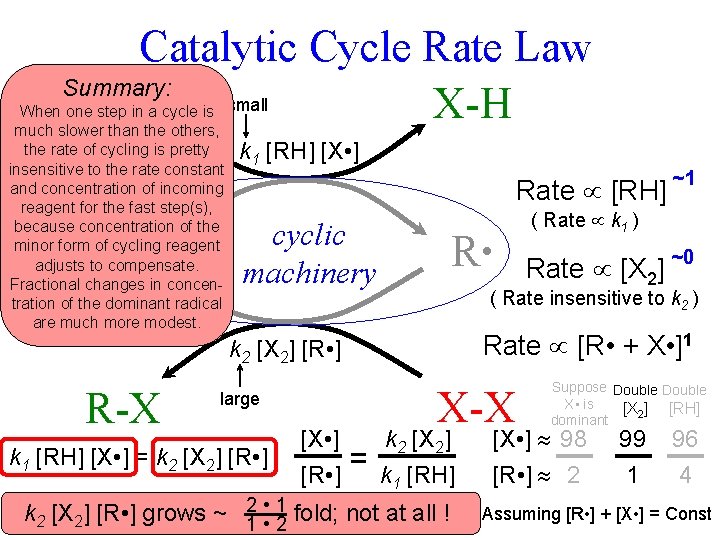
Catalytic Cycle Rate Law R-H X-H k 1 [RH] [X • ] X • cyclic machinery R • k 2 [X 2] [R • ] X-X R-X k 1 [RH] [X • ] = k 2 [X 2] [R • ] “this is a real democracy, catalysis” (K. B. Sharpless, 12/3/08)
![EZPass If you get that barrier down If theres a slow step theres 99 E-ZPass “If you get that [barrier] down, “If there's a slow step, there's 99.](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-5.jpg)
E-ZPass “If you get that [barrier] down, “If there's a slow step, there's 99. 9% of the rate goes way up. And if you the titaniums that you need, stuck before get them all the same height, this one mountain, that goes way up like you're really rolling. ” (Sharpless, 12/3/08) Mount Everest. ” But the two fluxes (cars/min) must be equal at steady state! Low rate constant Cash te a r h ig H t n w a t o s L n n o o c i t tra n e c con High concentration k [ ] = k[ ]

Catalytic Cycle Rate Law Summary: R-H small X-H When one step in a cycle is much slower than the others, the rate of cycling is pretty insensitive to the rate constant and concentration of incoming reagent for the fast step(s), because concentration of the minor form of cycling reagent adjusts to compensate. Fractional changes in concentration of the dominant radical are much more modest. X • k 1 [RH] [X • ] ~1 Rate [RH]? cyclic machinery R • Rate [R • + X • ]1 X-X large k 1 [RH] [X • ] = k 2 [X 2] [R • ] [X • ] [R • ] Rate [X 2]? ~0 ( Rate insensitive to k 2 ) k 2 [X 2] [R • ] R-X ( Rate k 1 ) = k 2 [X 2] k 1 [RH] 2 • 196 fold; not at all ! k 2 k 1[X[RH] ] [R • ] grows ~ [X • ] grows 2 1 • 298 = 1. 96 fold. Suppose Double X • is [X 2] [RH] dominant [X • ] 98 [R • ] 2 99 1 96 4 Assuming [R • ] + [X • ] = Const

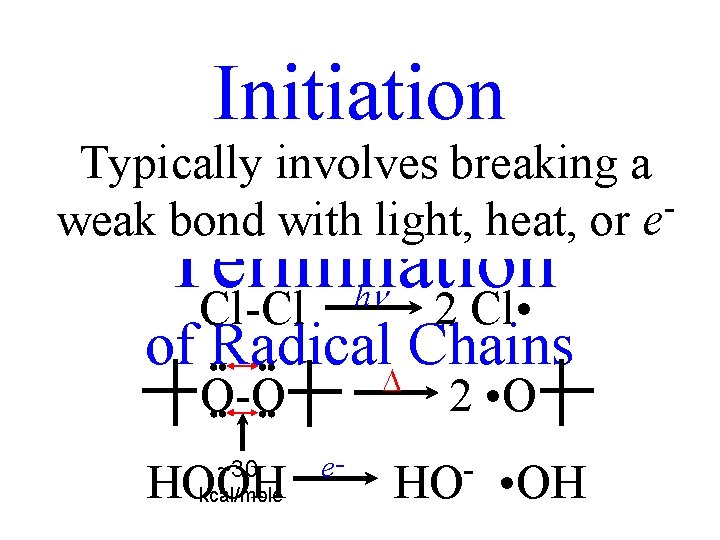
Initiation Typically involves breaking a and weak bond with light, heat, or e Termination Cl-Cl 2 Cl • h of Radical Chains O-O HOOH kcal/mole ~30 2 • O e- HO • OH

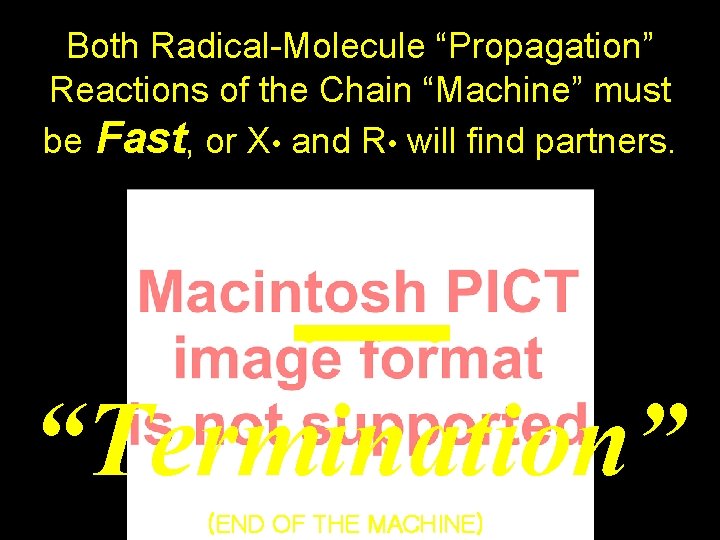
Both Radical-Molecule “Propagation” Reactions of the Chain “Machine” must be Fast, or X • and R • will find partners. • • “Termination” (END OF THE MACHINE)
![Kinetic Order in Initiator Rate ROOR Initiation ROOR ki 12 2 RO Kinetic Order in Initiator Rate [RO-OR] Initiation : RO-OR ki 1/2 ? 2 RO](https://slidetodoc.com/presentation_image_h2/0795c085593233de61d8a96056bd54a4/image-9.jpg)
Kinetic Order in Initiator Rate [RO-OR] Initiation : RO-OR ki 1/2 ? 2 RO • Rate of forming radicals [RO-OR] Termination : 2 R’ • kt R’-R’ Rate of destroying radicals [R’ • ]2 at Steady State : [R’ • ]2 [RO-OR] 1/2

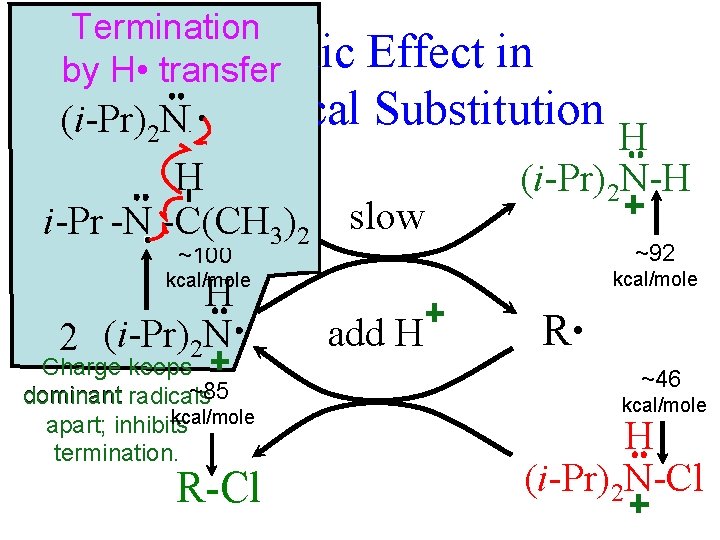
Termination An Ionic by H • transfer Effect in Free-Radical Substitution • (i-Pr) NH 2 H R-H i-Pr -N • =C(CH -C(CH 3)2 slow H (i-Pr)2 N-H ~100 ~92 kcal/mole H 2 (i-Pr)2 N Charge keeps ~85 dominant radicals kcal/mole apart; inhibits termination. R-Cl add H R • ~46 kcal/mole H (i-Pr)2 N-Cl

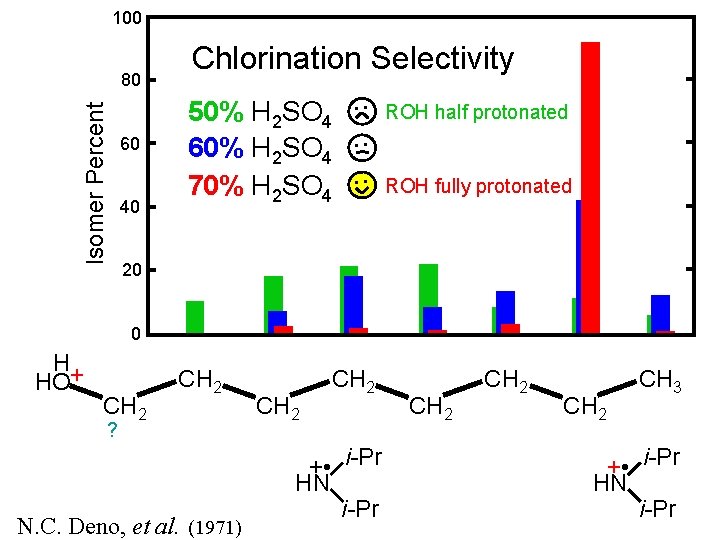
100 Isomer Percent 80 60 40 Chlorination Selectivity 50% H 2 SO 4 60% H 2 SO 4 70% H 2 SO 4 • • ROH half protonated • • ROH fully protonated 20 0 H HO+ CH 2 ? N. C. Deno, et al. (1971) CH 2 + • i-Pr HN i-Pr CH 2 CH 3 + • i-Pr HN i-Pr

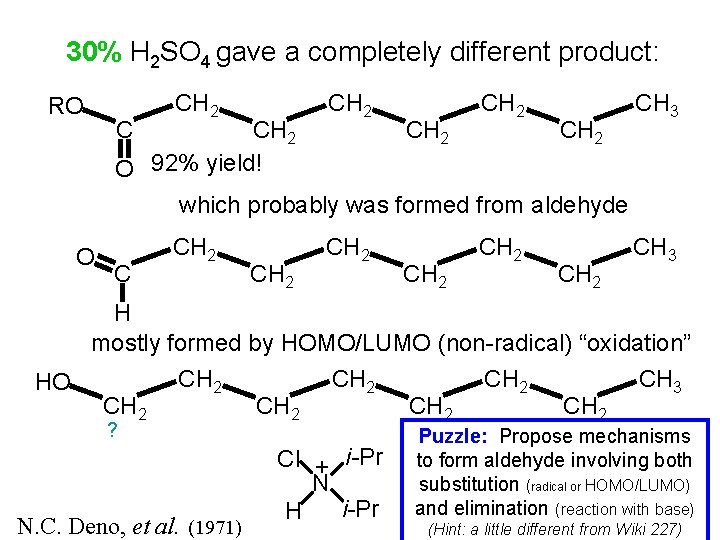
30% H 2 SO 4 gave a completely different product: RO O C CH 2 CH 2 CH 3 CH 2 O 92% yield! which probably was formed from aldehyde C CH 2 H mostly formed by HOMO/LUMO (non-radical) “oxidation” CH 2 CH 3 HO CH 2 ? N. C. Deno, et al. (1971) i-Pr 3 Cl + • + CH HN N CH i-Pr 3 H Puzzle: Propose mechanisms to form aldehyde involving both substitution (radical or HOMO/LUMO) and elimination (reaction with base) (Hint: a little different from Wiki 227)

From 2009 Exam 3. Consider the chlorination reaction : i-Pr 2 NCl + RH i-Pr 2 NH + RCl and these approximate bond dissociation energies (kcal/mole): N-Cl (46), R-Cl (85), N-H (92), R-H (100). G. (3 min) Why should the BDEs of N-Cl and R-Cl be so different, when those for N-H and R-H H. are so similar?

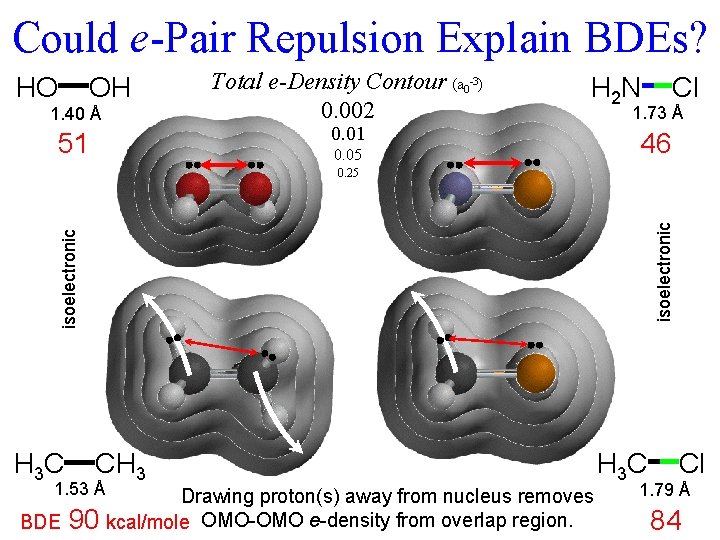
Could e-Pair Repulsion Explain BDEs? 1. 40 Å Total e-Density Contour (a 0 -3) 0. 002 51 0. 05 HO OH H 2 N 0. 01 Cl 1. 73 Å 46 isoelectronic 0. 25 H 3 C CH 3 1. 53 Å Drawing proton(s) away from nucleus removes BDE 90 kcal/mole OMO-OMO e-density from overlap region. H 3 C Cl 1. 79 Å 84

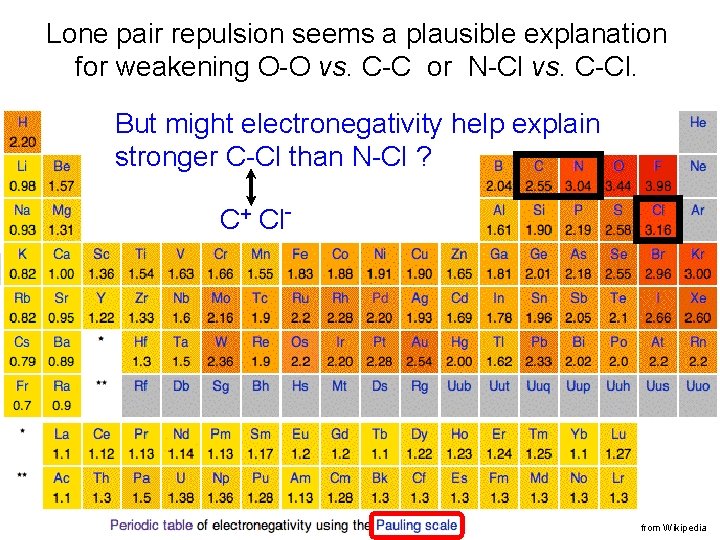
Lone pair repulsion seems a plausible explanation for weakening O-O vs. C-C or N-Cl vs. C-Cl. But might electronegativity help explain stronger C-Cl than N-Cl ? C+ Cl- from Wikipedia

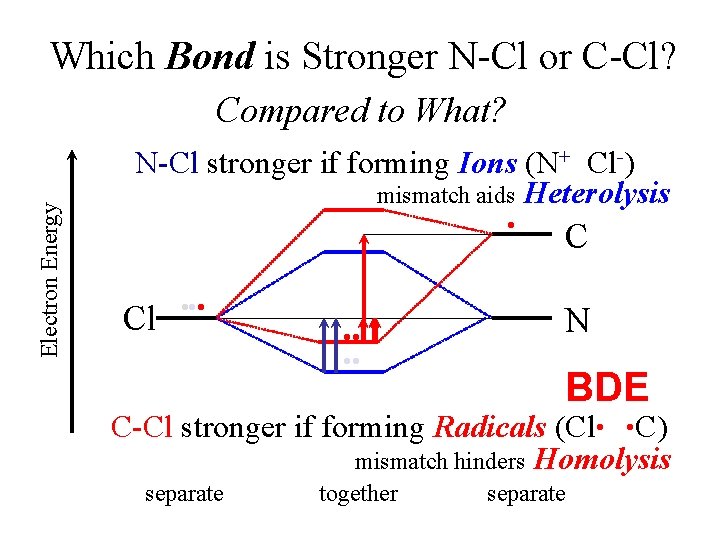
Which Bond is Stronger N-Cl or C-Cl? Electron Energy Compared to What? N-Cl stronger if forming Ions (N+ Cl-) mismatch aids Heterolysis • Cl • • • C N BDE C-Cl stronger if forming Radicals (Cl • • C) mismatch hinders Homolysis separate together separate

“Electronegativity” and Bond Strength First use in English (O. E. D. ) 1837 J. D. Dana Syst. Mineral. 82 When chemistry has so far advanced, that the relative electro-negativity, (if I may so call it, ) or electro-positivity, of the several elements, is fully known, . . we shall probably be able to construct a natural arrangement of minerals on chemical principles. J. D. Dana 1813 -1895 Silliman’s son-in-law Dana House 1849

“Electronegativity” and Bond Strength

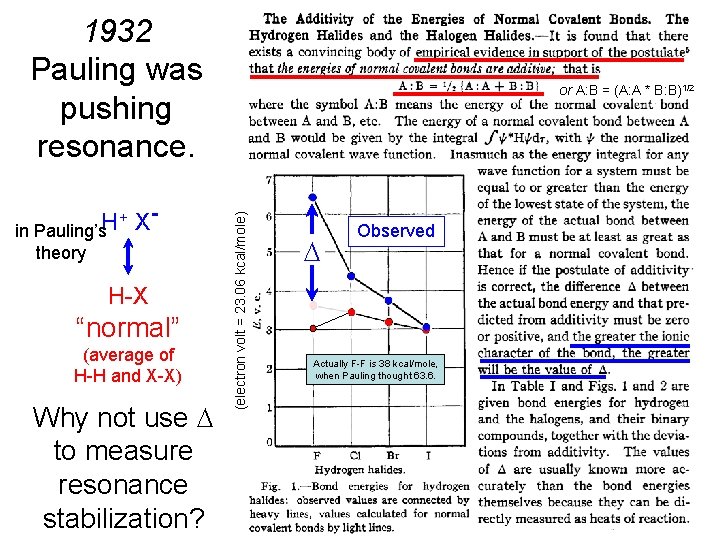
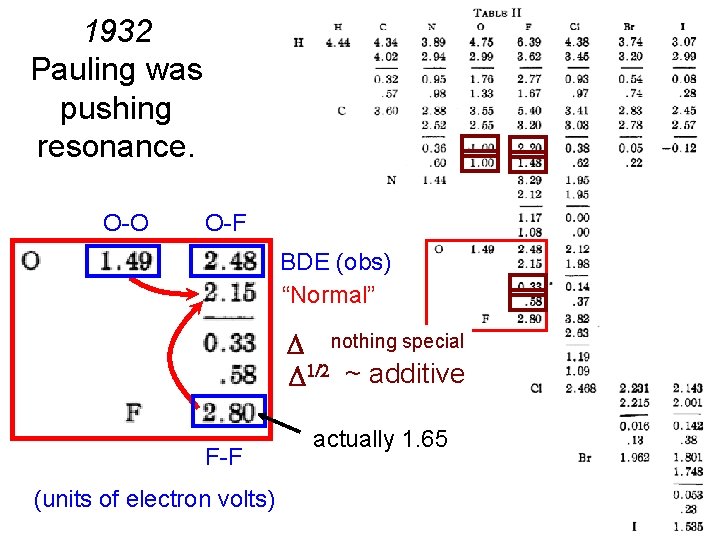
1932 Pauling was pushing resonance. theory H-X “normal” (average of H-H and X-X) Why not use to measure resonance stabilization? (electron volt = 23. 06 kcal/mole) + XH in Pauling’s or A: B = (A: A * B: B)1/2 Observed Actually F-F is 38 kcal/mole, when Pauling thought 63. 6.

1932 Pauling was pushing resonance. O-O O-F BDE (obs) “Normal” nothing special ~ additive F-F (units of electron volts) actually 1. 65

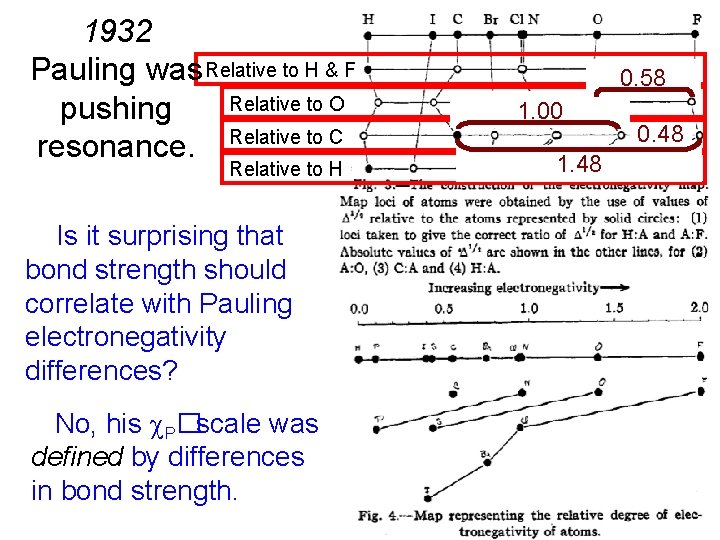
1932 Pauling was Relative to H & F Relative to O pushing resonance. Relative to C Relative to H Is it surprising that bond strength should correlate with Pauling electronegativity differences? No, his P�scale was defined by differences in bond strength. 0. 58 1. 00 1. 48 0. 48

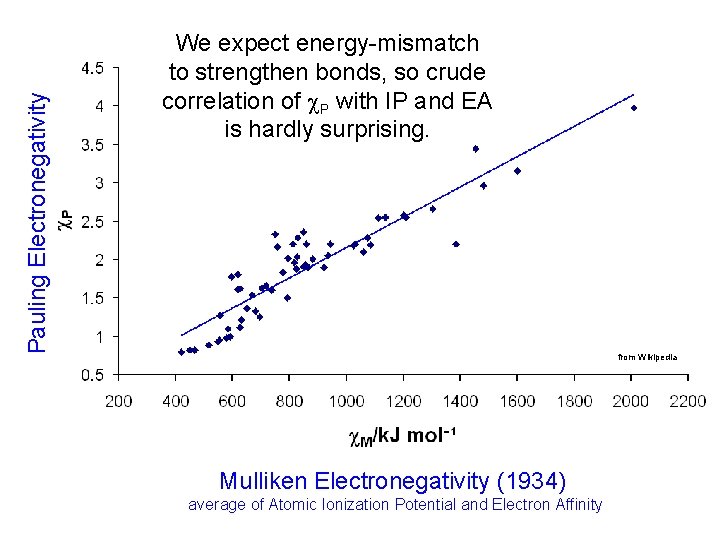
Pauling Electronegativity We expect energy-mismatch to strengthen bonds, so crude correlation of P with IP and EA is hardly surprising. from Wikipedia Mulliken Electronegativity (1934) average of Atomic Ionization Potential and Electron Affinity

End of Lecture 41 Jan. 20, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 Ratio guided notes
Ratio guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates A rate is a ratio
A rate is a ratio Nysedregents chemistry
Nysedregents chemistry Nysedregents chemistry
Nysedregents chemistry 2019 ib boundaries
2019 ib boundaries January 2006 chemistry regents answers
January 2006 chemistry regents answers 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad June 2010 chemistry regents
June 2010 chemistry regents Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry His birth date was on 25 january 1759
His birth date was on 25 january 1759 January 24th 1848
January 24th 1848 9 months before january 26 2009
9 months before january 26 2009 January character trait
January character trait Arvod cannot find work as a mall santa in january.
Arvod cannot find work as a mall santa in january. Spatial january
Spatial january How many syllables does snake have
How many syllables does snake have January february spelling
January february spelling March april may season
March april may season Sunday, tuesday, january, saturday
Sunday, tuesday, january, saturday