Chemistry 125 Lecture 42 January 22 2010 Solvation






- Slides: 26

Chemistry 125: Lecture 42 January 22, 2010 Solvation, and Ionophores This For copyright notice see final page of this file

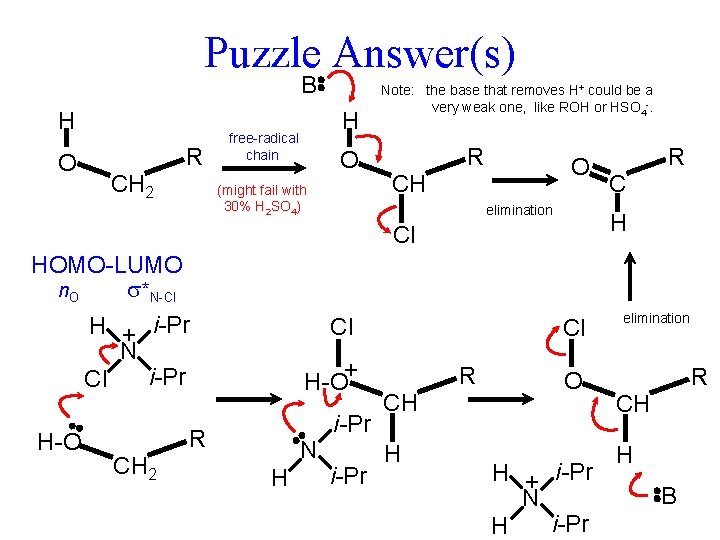
Puzzle Answer(s) B H O R CH 2 H free-radical chain O (might fail with 30% H 2 SO 4) Note: the base that removes H+ could be a very weak one, like ROH or HSO 4 -. R O CH elimination H-O Cl H-O+ R CH 2 N H i-Pr Cl R CH H Cl HOMO-LUMO n. O *N-Cl H + i-Pr N i-Pr Cl R O H + i-Pr N i-Pr H elimination R CH H B

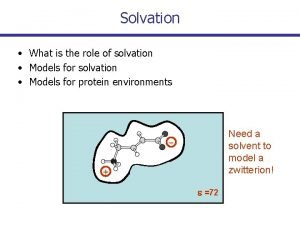
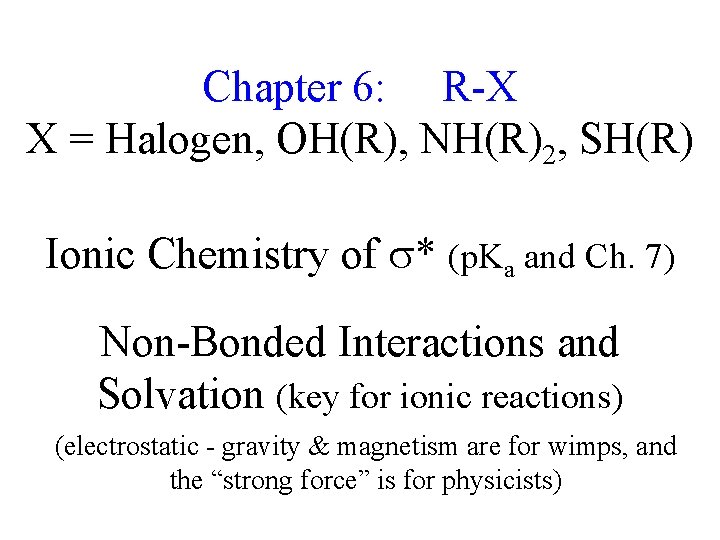
Chapter 6: R-X X = Halogen, OH(R), NH(R)2, SH(R) Ionic Chemistry of * (p. Ka and Ch. 7) Non-Bonded Interactions and Solvation (key for ionic reactions) (electrostatic - gravity & magnetism are for wimps, and the “strong force” is for physicists)

The theory of organic chemistry became manageable because it is often possible to focus on a simple unit with strong interactions (bonds with well defined geometry and energy), neglecting the much weaker (and more numerous and complex) intermolecular interactions. But the weak intermolecular interactions give organic materials many of their most valuable properties.

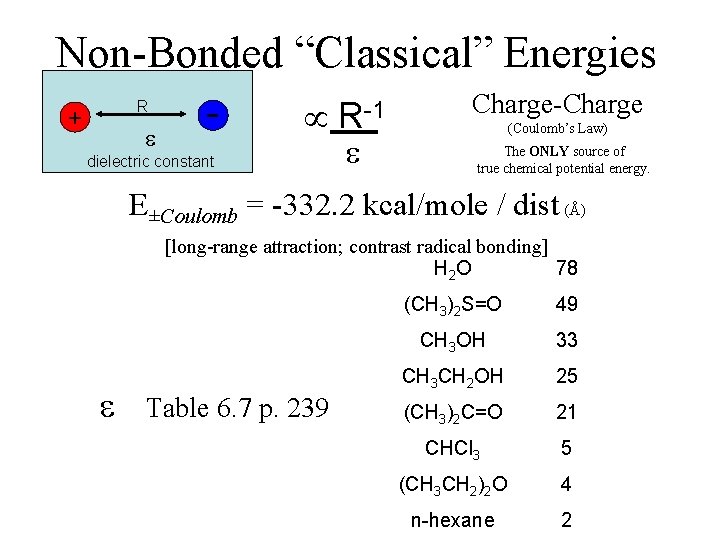
Non-Bonded “Classical” Energies R + - dielectric constant R-1 Charge-Charge (Coulomb’s Law) The ONLY source of true chemical potential energy. E±Coulomb = -332. 2 kcal/mole / dist (Å) [long-range attraction; contrast radical bonding] H 2 O 78 Table 6. 7 p. 239 (CH 3)2 S=O 49 CH 3 OH 33 CH 3 CH 2 OH 25 (CH 3)2 C=O 21 CHCl 3 5 (CH 3 CH 2)2 O 4 n-hexane 2

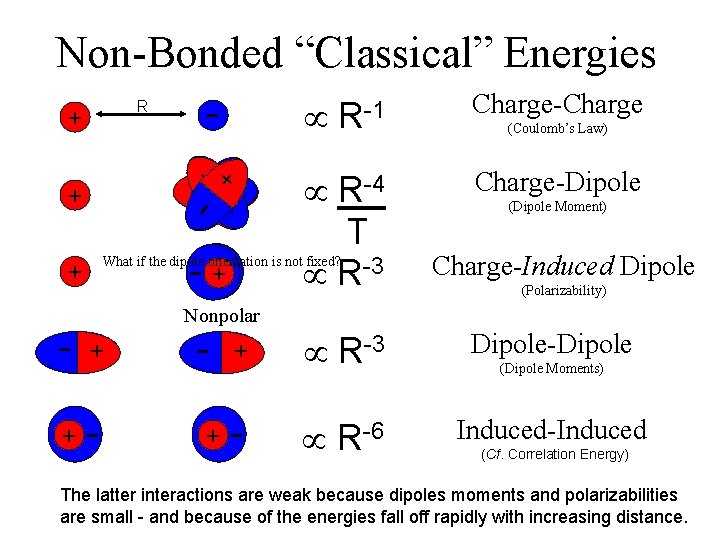
Non-Bonded “Classical” Energies - R - + + -- + + + R-1 Charge-Charge -2 R-4 T R-3 Charge-Dipole - ++ What if the dipole orientation is not fixed? + (Coulomb’s Law) (Dipole Moment) Charge-Induced Dipole (Polarizability) Nonpolar - - + + + R-3 Dipole-Dipole - R-6 Induced-Induced (Dipole Moments) (Cf. Correlation Energy) The latter interactions are weak because dipoles moments and polarizabilities are small - and because of the energies fall off rapidly with increasing distance.

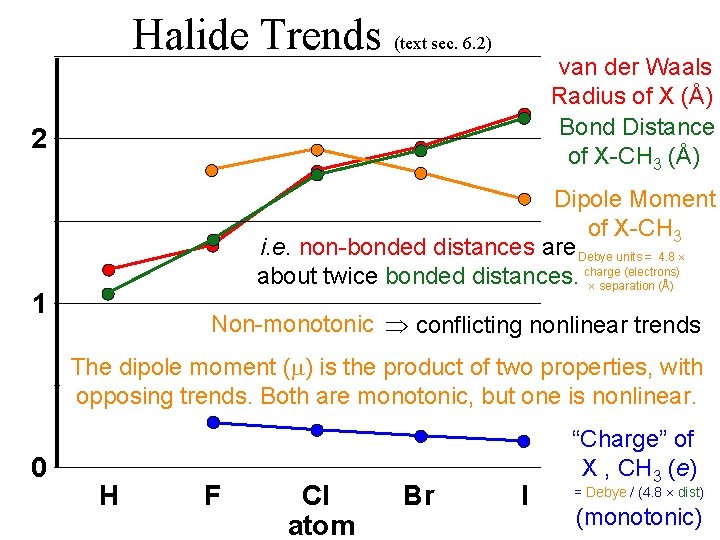
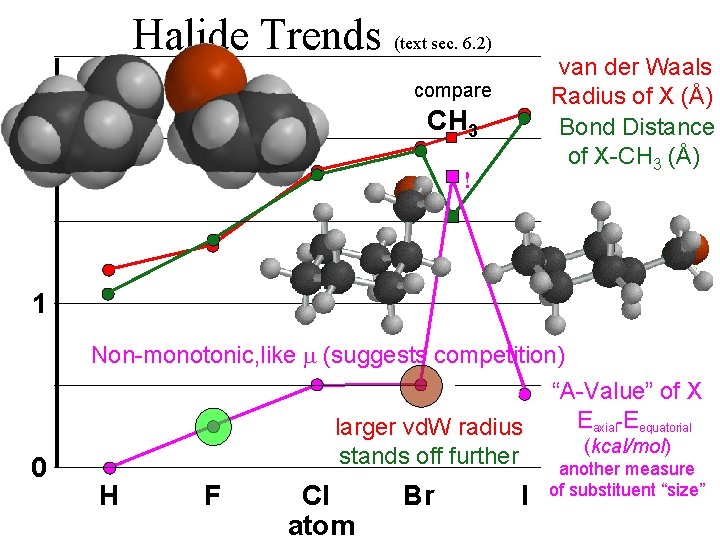
Halide Trends (text sec. 6. 2) van der Waals Radius of X (Å) Bond Distance of X-CH 3 (Å) 2 Dipole Moment of X-CH 3 i. e. non-bonded distances are Debye units = 4. 8 (electrons) about twice bonded distances. charge separation (Å) 1 Non-monotonic conflicting nonlinear trends The dipole moment ( ) is the product of two properties, with opposing trends. Both are monotonic, but one is nonlinear. 0 H F Cl atom Br I “Charge” of X , CH 3 (e) = Debye / (4. 8 dist) (monotonic)

Halide Trends (text sec. 6. 2) van der Waals Radius of X (Å) Bond Distance of X-CH 3 (Å) compare CH 3 2 ! 1 0 Non-monotonic, like (suggests competition) “A-Value” of X Eaxial-Eequatorial larger vd. W radius (kcal/mol) stands off further another measure H F Cl atom Br I of substituent “size”

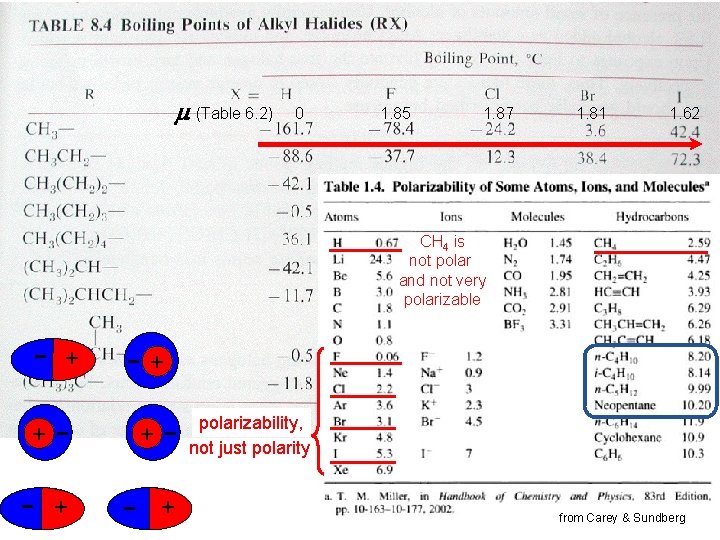
Boiling points (Table 6. 2) 0 1. 85 1. 87 1. 81 1. 62 CH 4 is not polar and not very polarizable - + + + -+ polarizability, not just polarity from Carey & Sundberg -

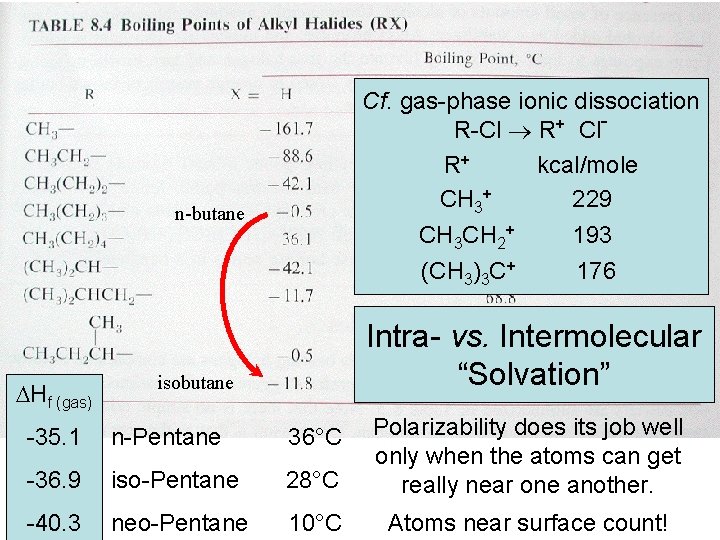
gas-phase ionic dissociation Boiling. Cf. points R-Cl R+ Cl R+ kcal/mole CH 3+ 229 CH 3 CH 2+ 193 (CH 3)3 C+ 176 n-butane Hf (gas) Intra- vs. Intermolecular “Solvation” isobutane -35. 1 n-Pentane 36°C -36. 9 iso-Pentane 28°C Polarizability does its job well only when the atoms can get really near one another. -40. 3 neo-Pentane 10°C Atoms near surface count!

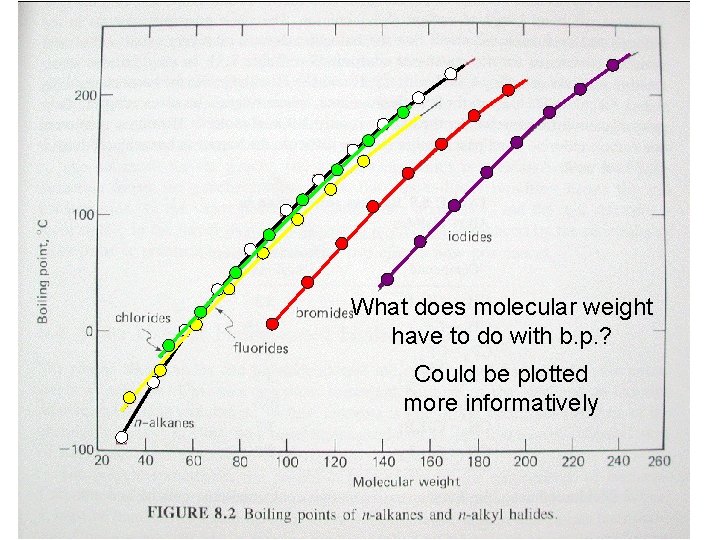
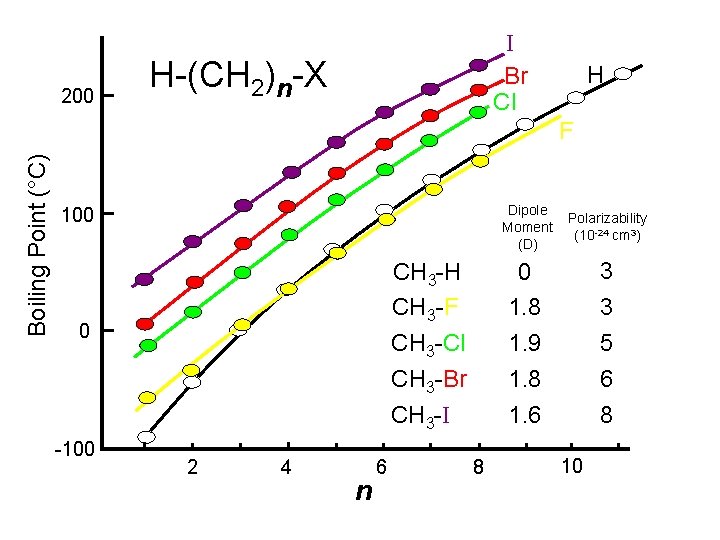
What does molecular weight have to do with b. p. ? Could be plotted more informatively

200 I Br Cl H-(CH 2)n-X H Boiling Point (°C) F Dipole Moment (D) Polarizability (10 -24 cm 3) CH 3 -H CH 3 -F CH 3 -Cl 0 1. 8 1. 9 3 3 5 CH 3 -Br CH 3 -I 1. 8 1. 6 6 8 100 0 -100 2 4 n 6 8 10

“Solvophobic” Forces Like Dissolves Like Hg does not “wet” glass

“Solvophobic” Forces Like Dissolves Like Alkanes and water (or Hg and glass) do not repel one another. Hg does not “wet” hydrocarbon nor does H 2 O but Hg has good reason to be near Hg, and water near water. Hg attracts H 2 O

Water Dipoles

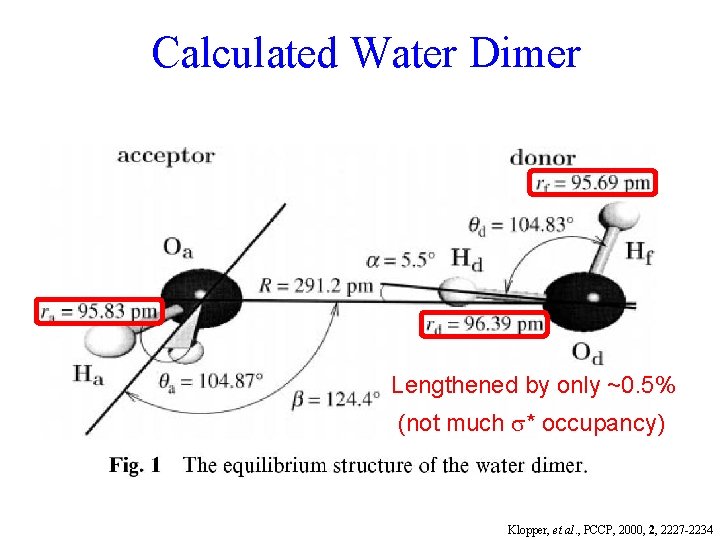
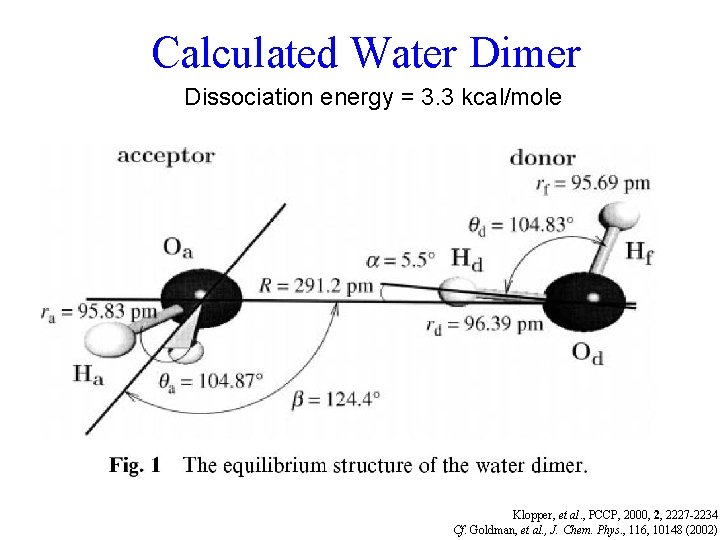
Calculated Water Dimer Lengthened by only ~0. 5% (not much * occupancy) Klopper, et al. , PCCP, 2000, 2, 2227 -2234

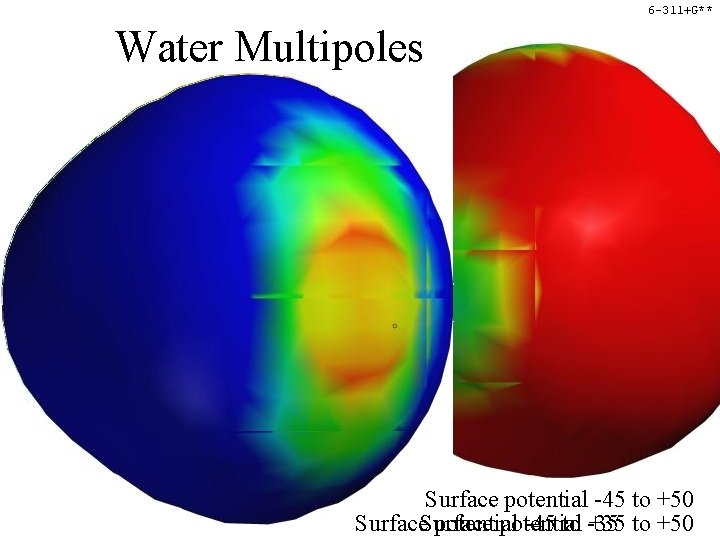
6 -311+G** Water Multipoles Surface potential -45 to +50 Surface potential -45 to -35 +35 to +50

Calculated Water Dimer Dissociation energy = 3. 3 kcal/mole Klopper, et al. , PCCP, 2000, 2, 2227 -2234 Cf. Goldman, et al. , J. Chem. Phys. , 116, 10148 (2002)

The small size of H allows the unusually close approach that makes O-H • • • O-H worth * R R. calling a “hydrogen bond”. * Typically ~ 5% as strong as a covalent bond

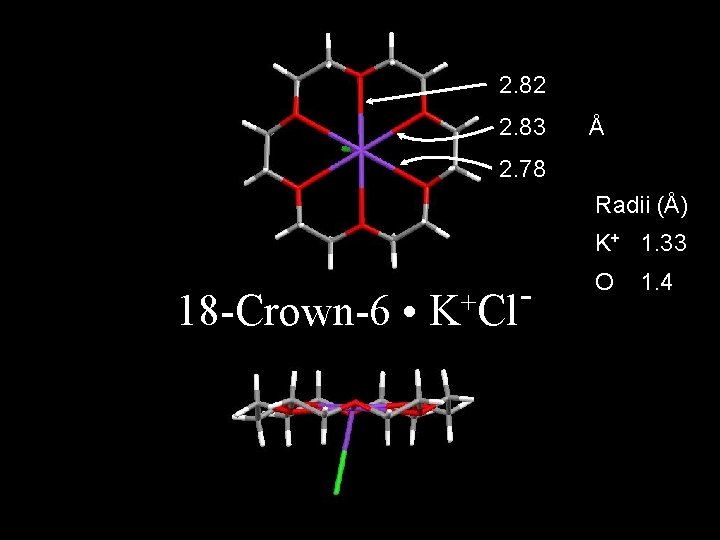
Text Section 6. 10 Crown Ethers and Tailored Ionophores Nobel Prize in Chemistry 1987 “ion carriers” 18 -c-6

2. 82 2. 83 Å 2. 78 Radii (Å) K+ 1. 33 18 -Crown-6 • + K Cl O 1. 4

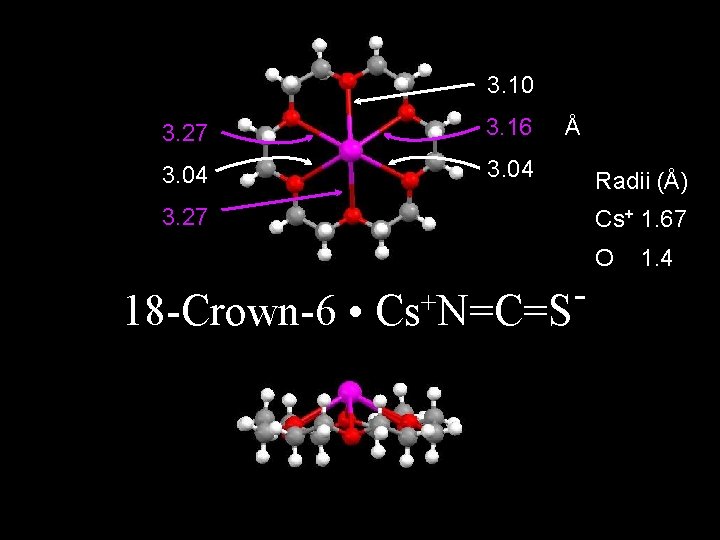
3. 10 3. 27 3. 16 3. 04 Å 3. 27 Radii (Å) Cs+ 1. 67 O 18 -Crown-6 • + Cs N=C=S 1. 4

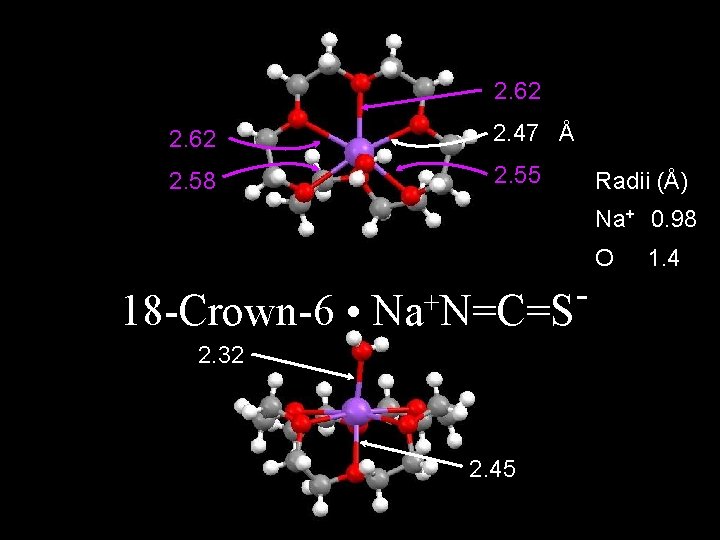
2. 62 2. 47 Å 2. 58 2. 55 Radii (Å) Na+ 0. 98 O 18 -Crown-6 • + Na N=C=S 2. 32 2. 45 1. 4

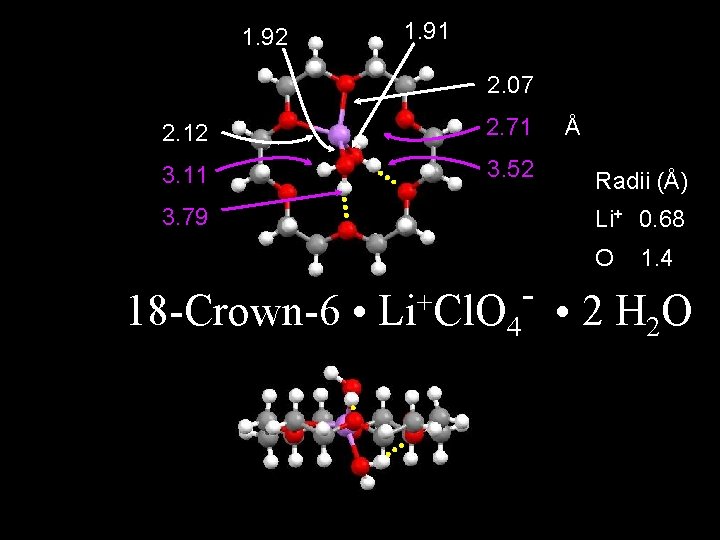
1. 92 1. 91 2. 07 2. 12 2. 71 3. 11 3. 52 3. 79 Å Radii (Å) Li+ 0. 68 O 18 -Crown-6 • + Li Cl. O 1. 4 - • 2 H O 4 2

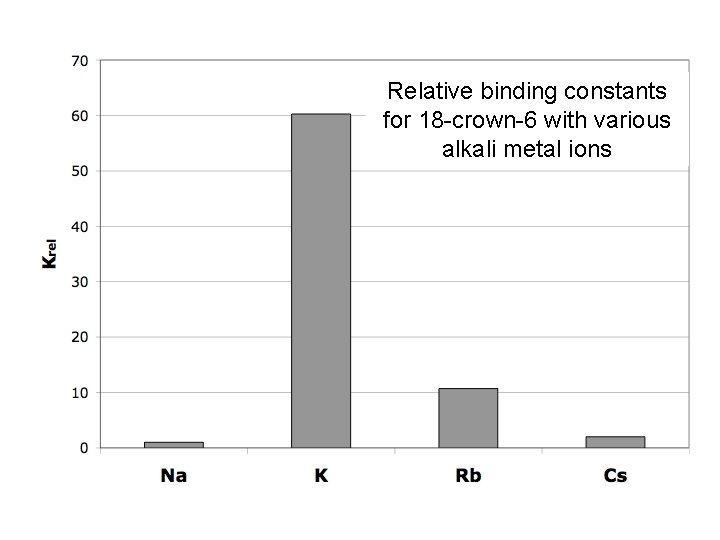
Relative binding constants for 18 -crown-6 with various alkali metal ions

End of Lecture 42 Jan. 22, 2010 Copyright © J. M. Mc. Bride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 Factors affecting solvation
Factors affecting solvation Ib boundaries 2019
Ib boundaries 2019 January 2017 chemistry regents answers
January 2017 chemistry regents answers Nysedregents chemistry
Nysedregents chemistry January 2012 chemistry regents answers
January 2012 chemistry regents answers Solvation process
Solvation process How do intermolecular forces affect solvation
How do intermolecular forces affect solvation 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Danswer
Danswer Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Biography of isaac newton
Biography of isaac newton January 29 2015
January 29 2015 Pro forma journal entries example
Pro forma journal entries example December november october
December november october Zodiac for january 20
Zodiac for january 20 January comes before
January comes before January 24, 1848
January 24, 1848 Character trait respect
Character trait respect 9 months before january 26 2009
9 months before january 26 2009 Arvod cannot find work as a mall santa in january.
Arvod cannot find work as a mall santa in january. Spatial january
Spatial january How many syllables in mother
How many syllables in mother January february spelling
January february spelling January february march april may
January february march april may