Solutions Ch 15 16 What is a solution














- Slides: 25

Solutions Ch 15 & 16

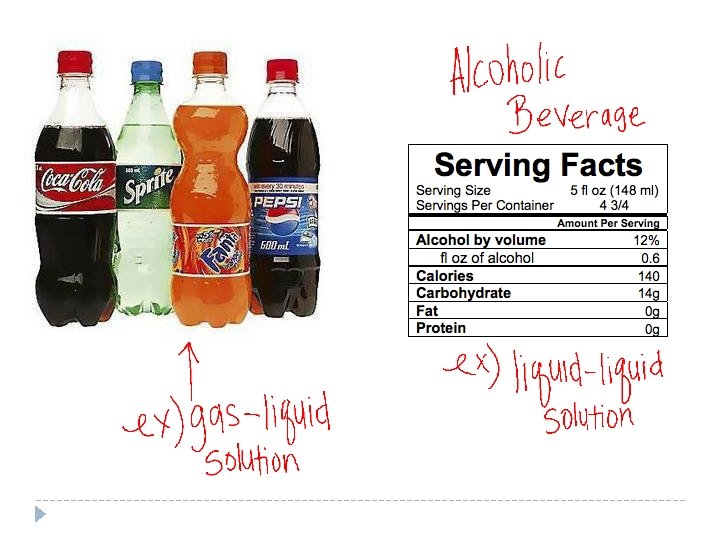
What is a solution? A solution is uniform mixture that may contain solids, liquids, or gases. Known as a homogenous mixture Solution = solute + solvent Solvent – The substance in greater abundance in the solution A solvent dissolves the solute. (dissolving medium) Solute – The substance dissolved in the solvent



Characteristics of Solutions Soluble – The solute’s ability to dissolve in a solvent Insoluble – The solute is not able to dissolve in a solvent Immiscible – Two liquids that can be mixed together, but separate shortly after you stop mixing them Miscible – Two liquids that are soluble in each other


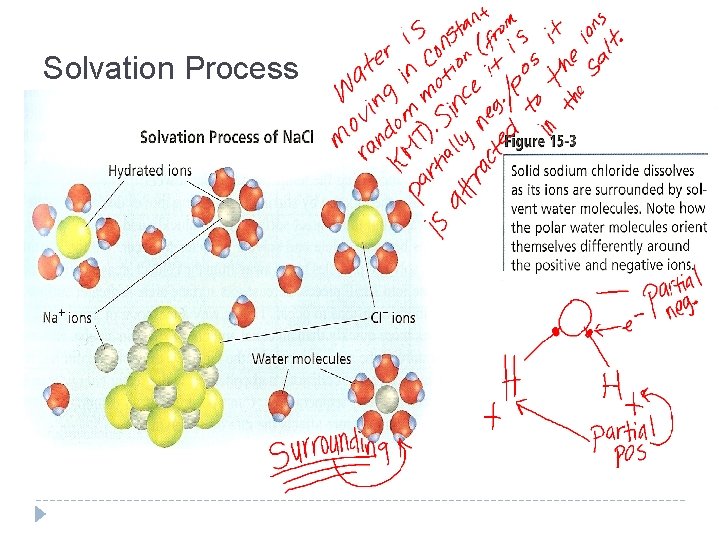
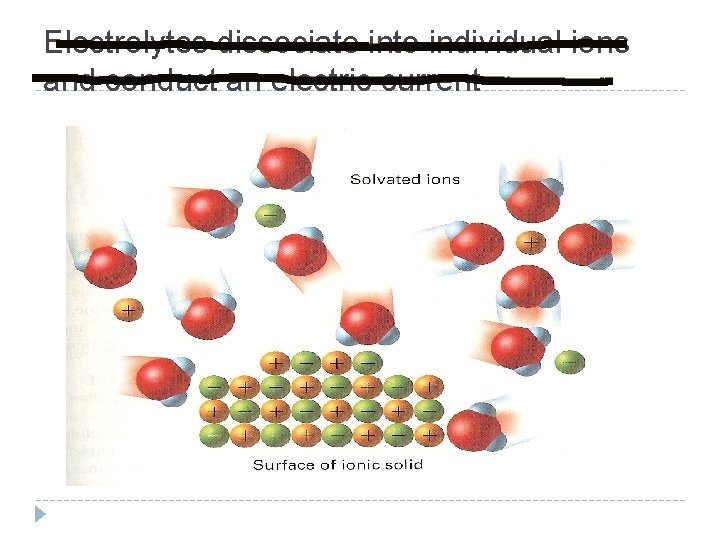
Solvation in Aqueous Solutions Solvation – The process of surrounding solute particles with solvent particles to form a solution Aqueous solution – A solute dissolved in water Caused by constant, random molecular motion of solute and solvent particles. (Remember KMT) In an aqueous solution each ion is surrounded by water molecules Individual solute ions break away from the crystal and the charged ions become surrounded by solvent molecules (H 2 O) and the ionic crystal dissolves

Solvation Process

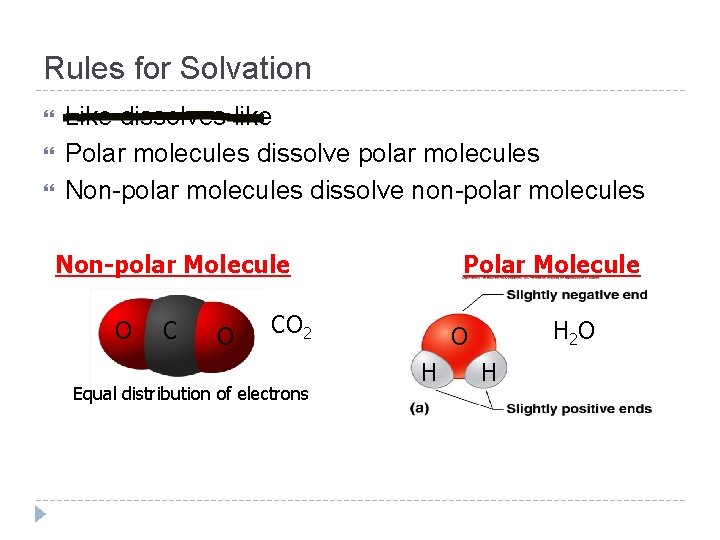
Rules for Solvation Like dissolves like Polar molecules dissolve polar molecules Non-polar molecules dissolve non-polar molecules Non-polar Molecule O C O Polar Molecule CO 2 Equal distribution of electrons H 2 O O H H

Factors that Affect Rate of Solvation (How fast something dissolves) Need to increase collisions between solute and solvent molecules 1. Agitating the mixture (Stirring) New collisions between solute and solvent will occur Increasing the surface area of the solute (Crushing the solute) 2. Greater surface area allows more collisions to occur Increasing the temperature of the solvent Increases kinetic energy of molecules and more frequent collisions occur 4. Increasing the pressure (For gases) 3. Increases the rate of collision between particles.

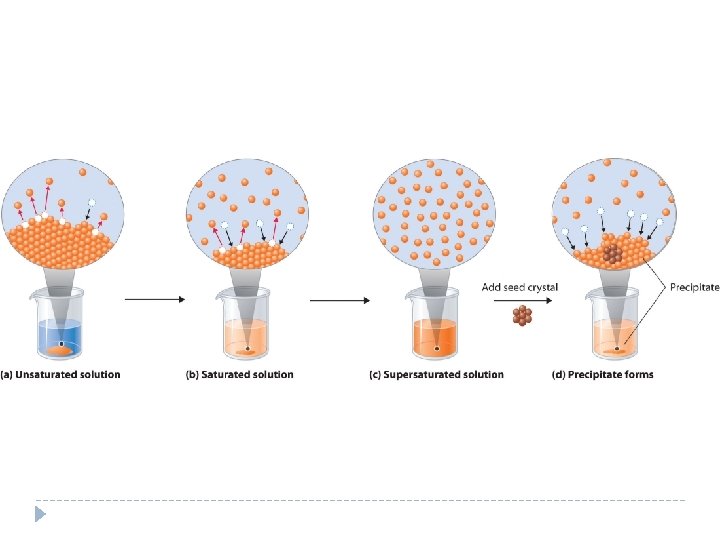
Solubility (How much is dissolved) Solubility – The maximum amount of solute that will dissolve in a given amount of solvent at a specified temperature and pressure. Solubility is usually expressed in grams of solute per 100 g of solvent or in grams/liter.

Solubility cont. . . Saturated solution contains the maximum amount of dissolved solute for a given amount of solvent at a specific pressure and temperature. In a saturated solution, solvation and crystallization are in equilibrium (They are happening at equal rates at the same time. ) Unsaturated solution contains less dissolved solute for a given temperature and pressure than a saturated solution Supersaturated solution contains more dissolved solute than a saturated solution at the same temperature A supersaturated solution is made at high temperatures, cooled slowly, and is unstable


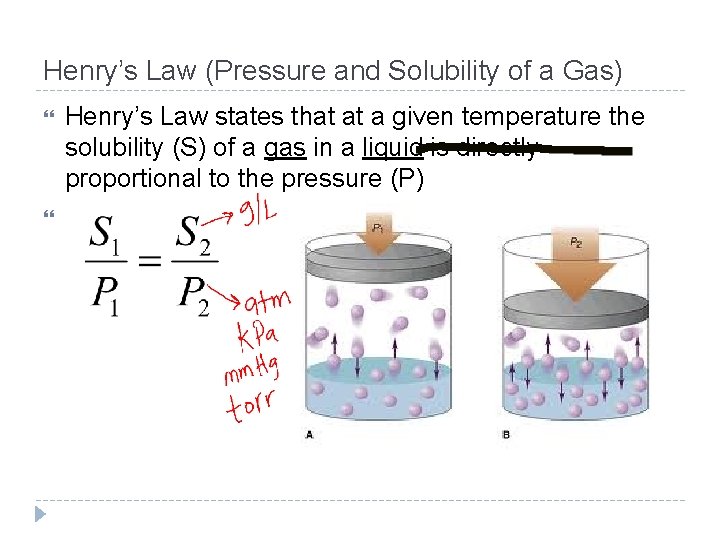
Factors That Affect Solubility (How much is dissolved) Temperature 1. Most substances as temperature increases solubility increases Gases are the exception and solubility tends to decrease as temperature increases, because they are moving quickly escaping the solvent Pressure (For Gases Only) 2. The solubility of a gas in any solvent increases as the pressure above the solution increases, keeping the gas from escaping in the solvent




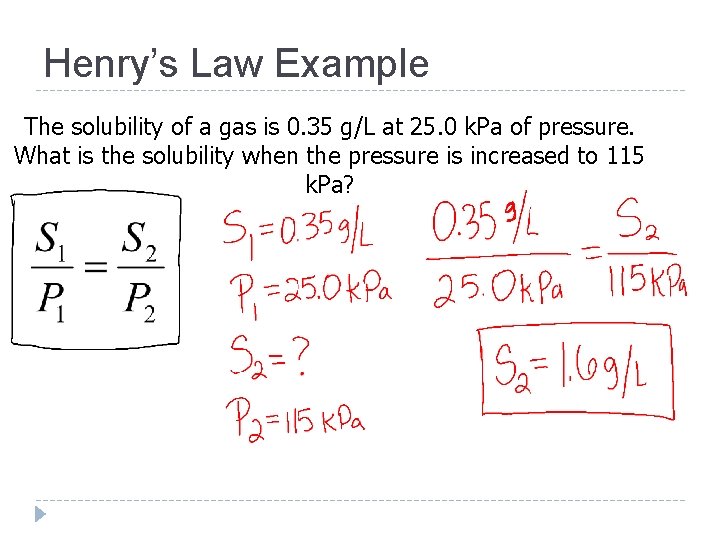
Henry’s Law (Pressure and Solubility of a Gas) Henry’s Law states that at a given temperature the solubility (S) of a gas in a liquid is directly proportional to the pressure (P)

Describing Solutions - Electrolytes are compounds that dissociate or ionize in water to form a solution that conducts an electric current To dissociate or ionize means to break apart into individual ions Ionic compounds are electrolytes because they dissociate into ions When Na. Cl is dissolved in water it breaks up into Na+ and Cl- ions.

Electrolytes dissociate into individual ions and conduct an electric current

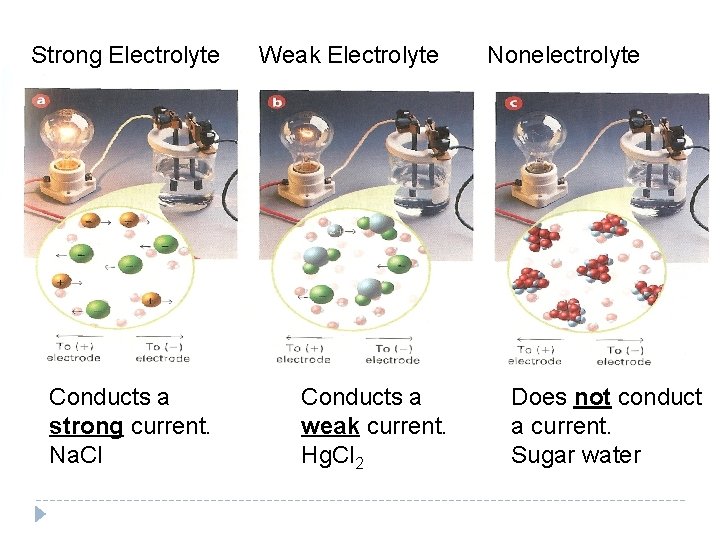
Strong Electrolyte Conducts a strong current. Na. Cl Weak Electrolyte Conducts a weak current. Hg. Cl 2 Nonelectrolyte Does not conduct a current. Sugar water

Strong and Weak Electrolytes A strong electrolyte conducts a strong current and the compound has completely dissociated into ions A weak electrolyte conducts a poor current because only part of the solute exists as ions. Na. Cl NH 3 (Ammonia) A nonelectrolyte does not dissociate or form ions, thus does not conduct a current. Most molecular compounds are nonelectrolytes Sucrose (sugar)

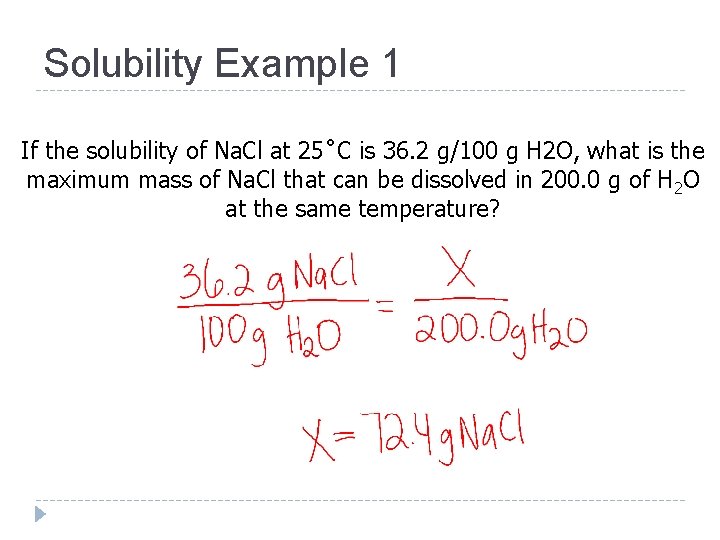
Solubility Example 1 If the solubility of Na. Cl at 25˚C is 36. 2 g/100 g H 2 O, what is the maximum mass of Na. Cl that can be dissolved in 200. 0 g of H 2 O at the same temperature?

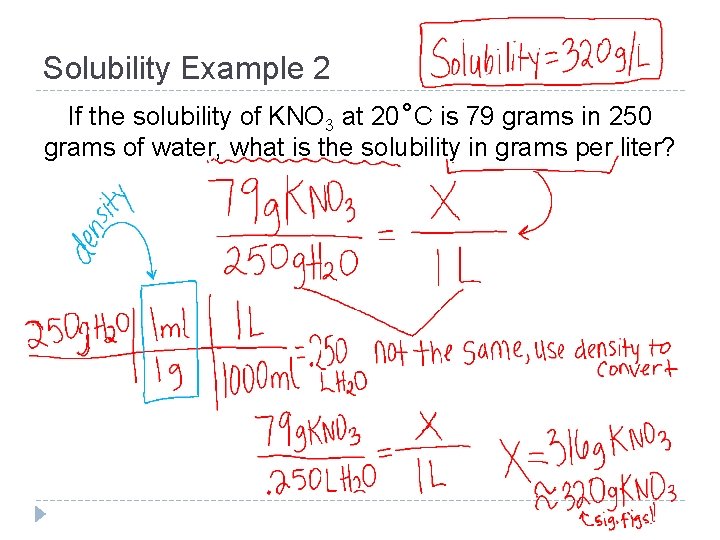
Solubility Example 2 If the solubility of KNO 3 at 20˚C is 79 grams in 250 grams of water, what is the solubility in grams per liter?

Henry’s Law Example The solubility of a gas is 0. 35 g/L at 25. 0 k. Pa of pressure. What is the solubility when the pressure is increased to 115 k. Pa?