Alkaloids The term alkaloid alkalilike is commonly used









































- Slides: 51

Alkaloids Ø The term “alkaloid” (alkali-like) is commonly used to designate basic heterocyclic nitrogenous compounds of plant origin that are physiologically active. 11/10/2020 Natural products 1

Introduction to Alkaloids Coca plant (Erythroxylum coca) and the molecular structure of cocaine (grey: carbon, blue: nitrogen, red: oxygen, white: hydrogen). 11/10/2020 Natural products 2

Deviation from Definition: Ø Ø Ø Basicity: Some alkaloids are not basic e. g. Colchicine, Piperine, Quaternary alkaloids. Nitrogen: The nitrogen in some alkaloids is not in a heterocyclic ring e. g. Ephedrine, Colchicine, Mescaline. Plant Origin: Some alkaloids are derived from Bacteria, Fungi, Insects, Frogs, Animals. 11/10/2020 Natural products 3

Classification: True (Typical) alkaloids that are derived from amino acids and have nitrogen in a heterocyclic ring. e. g. Atropine Ø Protoalkaloids that are derived from amino acids and do not have nitrogen in a heterocyclic ring. e. g Ephedrine Ø 11/10/2020 Natural products 4

Pseudo alkaloids that are not derived from amino acids but have nitrogen in a heterocyclic ring. Caffeine Ø False alkaloids are non alkaloids give false positive reaction with alkaloidal reagents. Ø 11/10/2020 Natural products 5

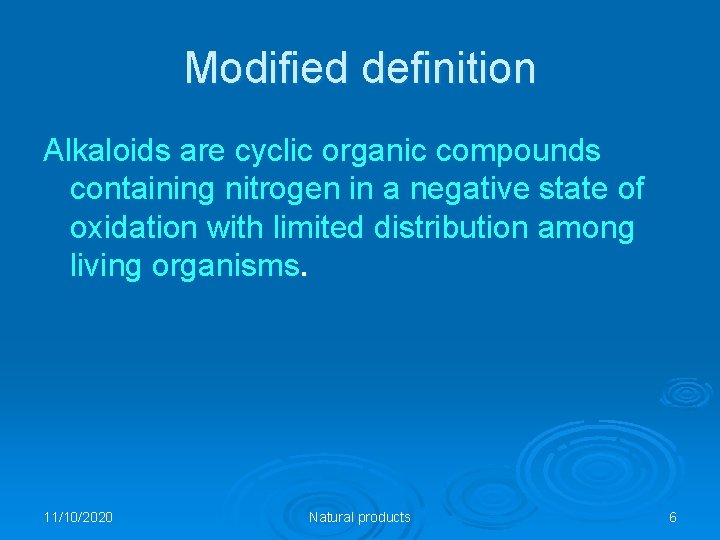
Modified definition Alkaloids are cyclic organic compounds containing nitrogen in a negative state of oxidation with limited distribution among living organisms. 11/10/2020 Natural products 6

Distribution and occurrence Rare in lower plants. Ø Dicots are more rich in alkaloids than Monocots. Ø Families rich in Alkaloids: Apocynaceae, Rubiaceae, Solanaceae and Papaveracea. Ø Families free from Alkaloids: Rosaceae, Labiatae Ø 11/10/2020 Natural products 7

Distribution in Plant: Ø All Parts e. g. Datura. Ø Barks e. g. Cinchona Ø Seeds e. g. Nux vomica Ø Roots e. g. Aconite Ø Fruits e. g. Black pepper Ø Leaves e. g. Tobacco Ø Latex e. g. Opium 11/10/2020 Natural products 8

Forms of Alkaloids: Ø Free bases Ø Salts with Organic acids e. g. Oxalic, acetic acids Ø Salts with inorganic acids e. g. HCl, H 2 SO 4. Ø Salts with special acids: e. g. Meconic acid in Opium Quinic acid in Cinchona Ø Glycosidal form e. g. Solanine in Solanum. 11/10/2020 Natural products 9

Function in Plants They may act as protective against insects and herbivores due to their bitterness and toxicity. Ø They are, in certain cases, the final products of detoxification (waste products). Ø Source of nitrogen in case of nitrogen deficiency. Ø They, sometimes, act as growth regulators in certain metabolic systems. Ø They may be utilized as a source of energy in case of deficiency in carbon dioxide assimilation. 11/10/2020 Natural products 10 Ø

Nomenclature: Trivial names should end by "ine". These names may refer to: Ø The genus of the plant, such as Atropine from Atropa belladona. Ø The plant species, such as Cocaine from Erythroxylon coca. The common name of the drug, such as Ergotamine from ergot. Ø A prominent physical character, such as Hygrine that is hygroscopic. Ø 11/10/2020 Natural products 11

Ø The name of the discoverer, such as Pelletierine that was discovered by Pelletier. Ø The physiological action, such as Emetine that acts as emetic, Morphine acts as narcotic. 11/10/2020 Natural products 12

Prefixes and suffixes: Prefixes: Ø "Nor-" designates N-demethylation or Ndemethoxylation, e. g. norpseudoephedrine and nornicotine. "Apo-" designates dehydration e. g. apomorphine. Ø "Iso-, pseudo-, neo-, and epi-" indicate different types of isomers. Ø 11/10/2020 Natural products 13

Suffixes: Ø "-dine" designates isomerism as quinidine and cinchonidine. Ø "-ine" indicates, in case of ergot alkaloids, a lower pharmacological activity e. g. ergotaminine is less potent than ergotamine. 11/10/2020 Natural products 14

Physical Properties: Condition: Ø Ø Ø Most alkaloids are crystalline solids. Few alkaloids are amorphous solids e. g. Emetine. Some are liquids that are either: Volatile e. g. nicotine and coniine, or Non-volatile e. g. pilocarpine and hyoscine. 11/10/2020 Natural products 15

II- Color: The majority of alkaloids are colorless but some are colored e. g. : Ø Colchicine and berberine are yellow. Ø Canadine is orange. Ø The salts of sanguinarine are copper-red. 11/10/2020 Natural products 16

Physical Properties: III- Solubility: Ø Both alkaloidal bases and their salts are soluble in alcohol. Ø Generally, the bases are soluble in organic solvents and insoluble in water Exceptions: Ø Bases soluble in water: caffeine, ephedrine, codeine, colchicine, pilocarpine and quaternary ammonium bases. Ø Bases insoluble or sparingly soluble in certain organic solvents: morphine in ether, theobromine and theophylline in benzene. 11/10/2020 Natural products 17

Ø Salts are usually soluble in water and, insoluble or sparingly soluble in organic solvents. Exceptions: Ø Salts insoluble in water: quinine monosulphate. Ø Salts soluble in organic solvents: lobeline and apoatropine hydrochlorides are soluble in chloroform. 11/10/2020 Natural products 18

IV- Isomerization: Ø Optically active isomers may show different physiological activities. Ø l-ephedrine is 3. 5 times more active than dephedrine. Ø l-ergotamine is 3 -4 times more active than dergotamine. 11/10/2020 Natural products 19

Ø d- Tubocurarine is more active than the corresponding l- form. Ø Quinine (l-form) is antimalarial and its disomer quinidine is antiarrythmic. Ø The racemic (optically inactive) dl-atropine is physiologically active. 11/10/2020 Natural products 20

Chemical Properties: I- Nitrogen: Ø Ø Primary amines R-NH 2 Secondary amines R 2 -NH Tertiary amines R 3 -N Quaternary ammonium salts R 4 -N e. g Norephedrine Ephedrine Atropine d-Tubocurarine II- Basicity: Ø R 2 -NH > R-NH 2 > R 3 -N Ø Saturated hexacyclic amines is more basic than aromatic amines. 11/10/2020 Natural products 21

According to basicity Alkaloids are classified into: Weak bases e. g. Caffeine Ø Strong bases e. g. Atropine Ø Amphoteric * Phenolic Alkaloids e. g. Morphine *Alkaloids with Carboxylic groups e. g. Narceine Ø Neutral alkaloids e. g. Colchicine Ø 11/10/2020 Natural products 22

III- Oxygen: Ø Most alkaloids contain Oxygen and are solid in nature e. g. Atropine. Ø Some alkaloids are free from Oxygen and are mostly liquids e. g. Nicotine, Coniine. 11/10/2020 Natural products 23

IV- Stability: Ø Effect of heat: Alkaloids are decomposed by heat, except Strychnine and caffeine (sublimable). Ø Reaction with acids: 1 - Salt formation. 2 - Dil acids hydrolyze Ester Alkaloids e. g. Atropine 11/10/2020 Natural products 24

3 - Conc. acids may cause: Ø Dehydration: Atropine → Apoatropine Morphine → Apomorphine Ø Demethoxylation: e. g. Codeine 11/10/2020 Natural products 25

Effect of Alkalies: 1 - Dil alkalis liberate most alkaloids from their salts e. g. NH 3. 2 - They may cause isomerization (racemization) of alkaloid as the conversion of hyoscyamine to atropine. 3 - They also can form salts with alkaloids containing a carboxylic group e. g. narceine. 4 - Strong alkalis: such as aqueous Na. OH and KOH form salts with phenolic alkaloids. 5 - Strong alkalis cause hydrolysis of Ester alkaloids (e. g. atropine, cocaine and physostigmine) and Amide alkaloids ( colchicines). 6 - Strong alkalis cause opening of lactone ring. Ø 11/10/2020 Natural products 26

Ø Effect of light and Oxygen: Some alkaloids are unstable when exposed to light and Oxygen: 11/10/2020 Natural products 27

Qualitative test for Alkaloids: Precipitation Reagents: They are used to: 1 - Indicate the absence or presence of Alkaloids 2 - Test for complete of extraction Ø Disadvantages: Some non alkaloids interfere such as Proteins, lactones, coumarins 11/10/2020 Natural products 28

Ø Classification of Alkaloidal precipitating agents: 1 - Reagents that form double salts: a- Mayer’s Reagent: Potassium Mercuric Iodide. b- Dragendorff’s Reagents: Potassium Iodobismethate. c- Gold Chloride. 2 - Reagents Containing Halogens: a- Wagner’s Reagent: Iodine/ Potassium Iodide. 3 -Organic Acids: a- Hager’s Reagent: Picric Acid b- Tannic Acid. 4 - Oxygenated High Molecular Weight Acids: a- Phosphomolybdic acid b- Phosphotungestic acid c-11/10/2020 Silicotungestic Acid Natural products 29

Ø Colour Reagents: 1 - Froehd’s Reagent: Phosphomolybdic acid 2 - Marqui’s Reagent: Formaldehyde/ Conc. H 2 SO 4 3 - Mandalin’s Reagent: Sulphovanidic acid 4 - Erdmann’s Reagent: Conc. HNO 3/Conc. H 2 SO 4 5 - Mecke's Reagent: Selenious acid / conc. H 2 SO 4 6 - Shaer's Reagent: Hydrogen peroxide / conc. H 2 SO 4 7 - Rosenthaler's Reagent: Potassium arsenate / conc. H 2 SO 4 8 - Conc. HNO 3 11/10/2020 Natural products 30

Extraction, Purification and Isolation of Alkaloids from Powdered plants Ø Extraction and purification Method I: The powder is treated with alkalis to liberates the free bases that can then be extracted with water immiscible organic solvents. Method II: The powdered material is extracted with water or aqueous alcohol containing dilute acid. Alkaloids are extracted as their salts together with accompanying soluble impurities. Method III: The powder is extracted with water soluble organic solvents such as Me. OH or Et. OH which are good solvents for both salts and free bases. 11/10/2020 Natural products 31

11/10/2020 Natural products 32

Ø Liberation of the free bases: Alkalis are used to liberate free bases. Alkalis must be strong enough to liberate free bases. However, choice of strong alkalis must be avoided in some cases: 1 - Ester Alkaloids e. g. Solanaceous Alkaloids 2 - Amide Alkaloids e. g. Colchicine 3 - Phenolic Alkaloids e. g. Morphine 4 - Lactone Alkaloids e. g. Pilocarpine 5 - Fatty Drugs due to saponification and emulsion formation. 11/10/2020 Natural products 33

Ø NH 4 OH: Most widely used due to many advantages: 1 - Strong enough to liberate most of alkaloids from their salts. 2 - Milder than fixed alkalis so more safe. 3 - Volatile so easy to get rid of it. Ø Other Alkalis: Na 2 CO 3, Na. HCO 3, Ca(OH)2, Mg. O. 11/10/2020 Natural products 34

Ø Extraction of the free bases: Ø CHCl 3: Strong solvent can extract most of the alkaloids. Extracts contain more impurities. Carcinogenic. Ø Ether: Gives cleaner Extract but have some disadvantages: 1 - High volatility 2 - Peroxide formation 3 - High water miscibility 11/10/2020 Natural products 35

Volatile Alkaloids Ø The best way for their extraction is steam distillation. Ø Plant material + water + Fixed alkali Heat 11/10/2020 steam contain alkaloids received in acidic sloution. Natural products 36

ØPurification of the Crude Alkaloidal Fractions: Ø Repeated Acid-Base procedures: Render extract Acidic, extract with organic solvent (dissolve non alkaloidal impurities), Alkalinize and extract again with organic solvents (Dissolve Alkaloids). Ø Precipitation with alkaloidal precipitating agent. Ø Convert to crystalline salts. 11/10/2020 Natural products 37

ØSeparation of Alkaloidal Mixtures: Ø Fractional Crystallization: 11/10/2020 Natural products 38

11/10/2020 Natural products 39

Ø Preparation of Derivatives: Separation of Primary, Secondary and Tertiary Alkaloids. 11/10/2020 Natural products 40

Ø Fractional Liberation: 11/10/2020 Natural products 41

Ø Fractional Distillation: e. g. Separation of Nicotine and Anabasine Ø Chromatographic Separation. 11/10/2020 Natural products 42

Identification of Alkaloids: Ø Melting point Ø Colour test Ø Optical Rotation Ø Microcrystal test Ø HPLC, GC-MS Ø UV, IR, NMR, MS. 11/10/2020 Natural products 43

Quantitative Determination of Alkaloids: Ø Ø Volumetric methods: These are based on reaction of alkaloidal bases with acids (Acid-Base titration). They include: Aqueous titration: This is carried by either: 1 - Direct titration of the alcoholic solution of the alkaloidal residue with standard acid, or 2 - Back titration by dissolving the residue in a known amount of standard acid and back titration of residual acid against standard alkali. Non-aqueous titration: This method is suitable for determination of weak bases e. g. Caffeine. 11/10/2020 Natural products 44

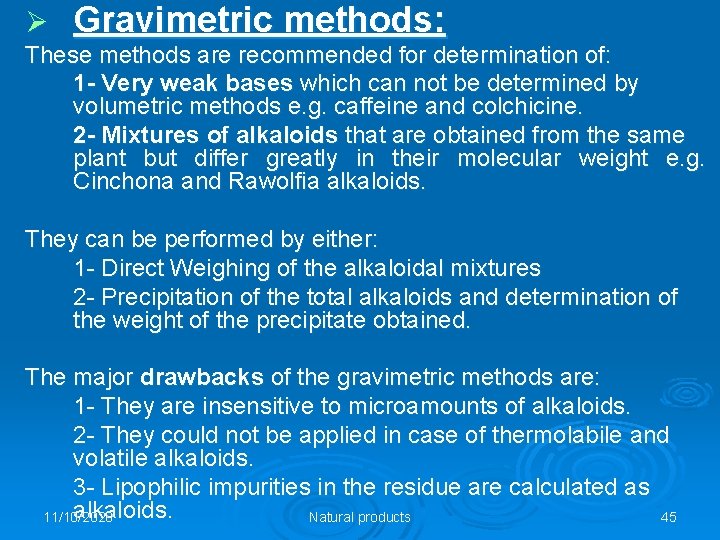
Ø Gravimetric methods: These methods are recommended for determination of: 1 - Very weak bases which can not be determined by volumetric methods e. g. caffeine and colchicine. 2 - Mixtures of alkaloids that are obtained from the same plant but differ greatly in their molecular weight e. g. Cinchona and Rawolfia alkaloids. They can be performed by either: 1 - Direct Weighing of the alkaloidal mixtures 2 - Precipitation of the total alkaloids and determination of the weight of the precipitate obtained. The major drawbacks of the gravimetric methods are: 1 - They are insensitive to microamounts of alkaloids. 2 - They could not be applied in case of thermolabile and volatile alkaloids. 3 - Lipophilic impurities in the residue are calculated as alkaloids. 11/10/2020 Natural products 45

Ø Colourimetric Method: e. g. Morphine + Na. NO 2/HCl Ergot + p-dimethylaminobenzaldehyde Ø Spectrophotometric Methods. Ø Polarimetric Method. Ø Fluorimetric Method. Ø Chromatographic Methods 11/10/2020 Natural products 46

ØClassification of Alkaloids Ø Biogenetic. Based on the biogenetic pathway that form the alkaloids. Ø Botanical Source. According to the plant source of alkaloids. Ø Type of Amines. Primary, Secondary, Tertiary alkaloids. Ø Basic Chemical Skeleton 11/10/2020 Natural products 47

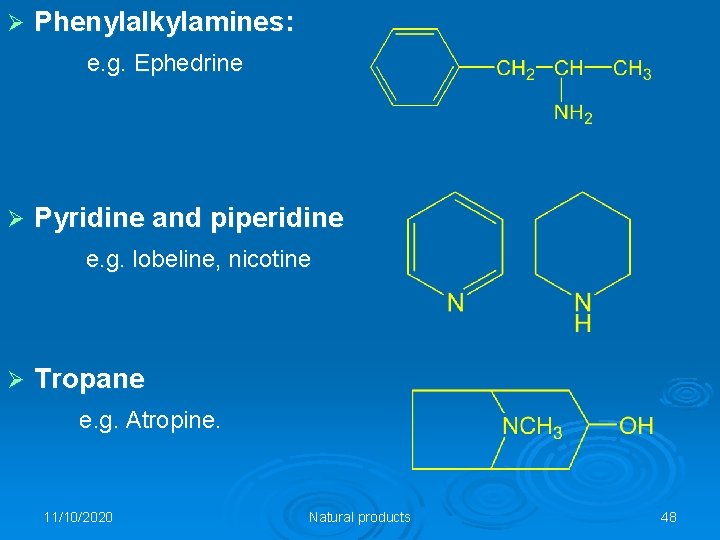
Ø Phenylalkylamines: e. g. Ephedrine Ø Pyridine and piperidine e. g. lobeline, nicotine Ø Tropane e. g. Atropine. 11/10/2020 Natural products 48

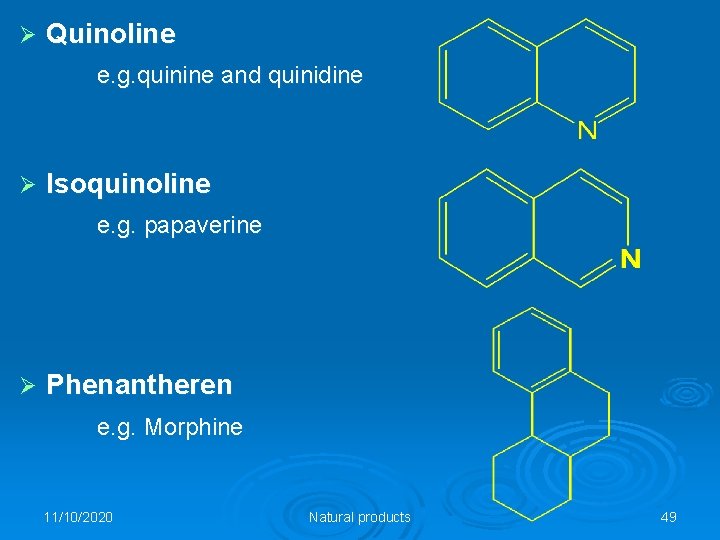
Ø Quinoline e. g. quinine and quinidine Ø Isoquinoline e. g. papaverine Ø Phenantheren e. g. Morphine 11/10/2020 Natural products 49

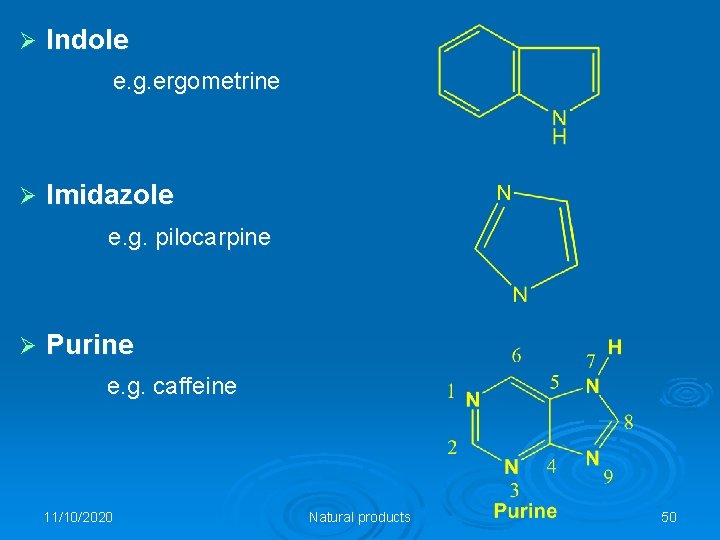
Ø Indole e. g. ergometrine Ø Imidazole e. g. pilocarpine Ø Purine e. g. caffeine 11/10/2020 Natural products 50

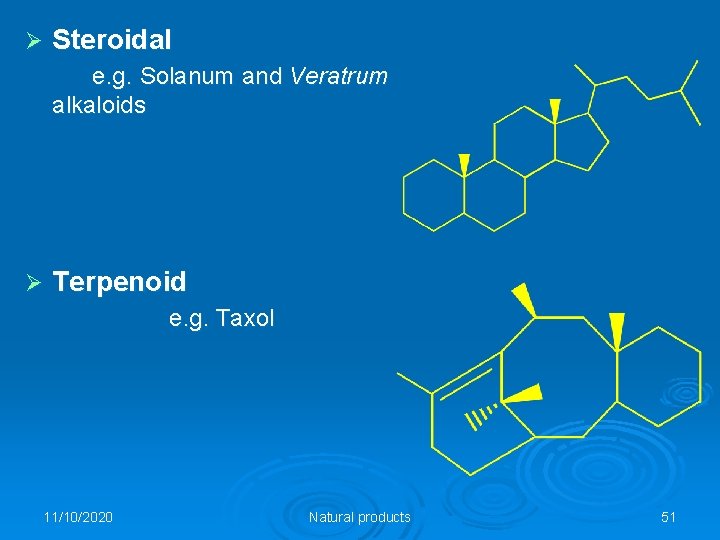
Ø Steroidal e. g. Solanum and Veratrum alkaloids Ø Terpenoid e. g. Taxol 11/10/2020 Natural products 51
 Two types of pattern development
Two types of pattern development Constrains two faces, edges, points, or axes together.
Constrains two faces, edges, points, or axes together. Hose appliances
Hose appliances 3 components of narrative paragraph
3 components of narrative paragraph Computer graphics models are now commonly used for making
Computer graphics models are now commonly used for making Classification of clutch
Classification of clutch Most commonly used system
Most commonly used system Methods are commonly used to:
Methods are commonly used to: Chapter 7 alcohol other drugs and driving
Chapter 7 alcohol other drugs and driving Viewing plane line symbol
Viewing plane line symbol A pneumatic drill is commonly used
A pneumatic drill is commonly used Closed center curls
Closed center curls Fibrojen
Fibrojen Struktur piridin
Struktur piridin Alkaloid čaju
Alkaloid čaju Ergoline alkaloids
Ergoline alkaloids Agustina setiawati
Agustina setiawati Biological importance of alkaloids
Biological importance of alkaloids Otrovan biljni alkaloid
Otrovan biljni alkaloid Alkaloid xantina
Alkaloid xantina Alkaloid
Alkaloid Alkaloid nikotin
Alkaloid nikotin True alkaloid
True alkaloid Alkaloid caju
Alkaloid caju Tabiatda õsadigan dorivor õsimliklar
Tabiatda õsadigan dorivor õsimliklar Norlupinane
Norlupinane Ephedra
Ephedra Pyrimidine examples
Pyrimidine examples Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Serpentiana
Serpentiana Purine alkaloids
Purine alkaloids Belladonna alkaloids drugs
Belladonna alkaloids drugs Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Purine alkaloids
Purine alkaloids Tropane alkaloids
Tropane alkaloids Alkaloids definition pharmacognosy
Alkaloids definition pharmacognosy Peomus boldus
Peomus boldus Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Spongocel
Spongocel Commonly misused homonyms
Commonly misused homonyms Collection of web pages
Collection of web pages Signing naturally homework 5:8
Signing naturally homework 5:8 Effected vs affected
Effected vs affected What are optical storage devices are most commonly known as
What are optical storage devices are most commonly known as Commonly misspelled homophones
Commonly misspelled homophones Sodium hydroxide relaxers are commonly called
Sodium hydroxide relaxers are commonly called Relaxer strengths
Relaxer strengths Virtual memory is commonly implemented by
Virtual memory is commonly implemented by Abused children commonly exhibit
Abused children commonly exhibit The circle graphs are commonly called
The circle graphs are commonly called Section 17-3 note taking guide answer key
Section 17-3 note taking guide answer key