1 Chem465 11282020 2 Tadeusz Aniszewski Chem465 ALKALOIDS

























- Slides: 31

1 Chem=465 11/28/2020

2 Tadeusz Aniszewski Chem=465 ALKALOIDS – SECRETS OF LIFE ALKALOID CHEMISTRY, BIOLOGICAL SIGNIFICANCE, APPLICATIONS AND ECOLOGICAL ROLE 11/28/2020

3 Alkaloids Group of molecules with a relatively large occurrence in nature around the Globe. They are very diverse chemicals and biomolecules, but they are all secondary compounds and they are derived from amino acids or from the transamination process. Transamination The transfer of an amino group from one molecule to another, especially from an amino acid to a keto acid. Chem=465 11/28/2020

4 Alkaloids are classified according to the amino acids that provide their nitrogen atom and part of their skeleton. Similar alkaloids can have quite different biosynthetic pathways and different bio impacts. Alkaloids are derived from l-lysine, l-ornithine, l-tyrosine, ltryptophan, l-histidine, l-phenylalanine, nicotinic acid, anthranilic acid or acetate. Chem=465 11/28/2020

5 The terpenoid, steroid and purine alkaloids are also important. Millions of people around the Globe use purine alkaloids every day whether starting the day with a cup of coffee or drinking a cup of tea in the afternoon. Alkaloids also occur in the animal kingdom. Differently from plants, the source of these molecules in an animal’s body can be endogenous or exogenous. Alkaloids are molecules participating in both producer and consumer chains in nature. Chem=465 11/28/2020

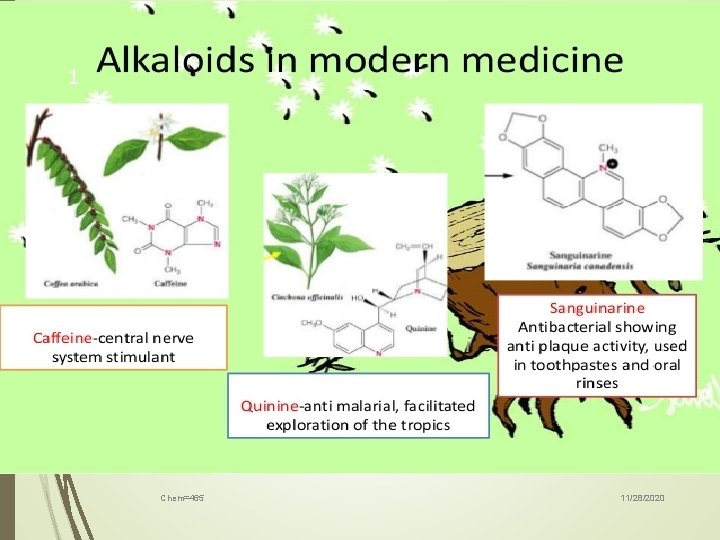
6 History Friedrich Sertürner, an apothecary’s assistant from Westphalia, first isolated morphine of the most important alkaloids in the applied sense[1]. This was in 1805, and proved a significant step forward in chemistry and pharmacology [2, 3, 4]. Chem=465 11/28/2020

7 Using the method developed by Friedrich Sertürner, the pharmacists Pierre Joseph Pelletier and Joseph Benaimé Caventou isolated, from 1817 to 1821, a remarkable range of other alkaloids, such as brucine (a close relative of strychnine), febrifuge, quinine, caffeine and veratrine [1, 5] Chem=465 11/28/2020

8 The term “alkaloid” was first mentioned in 1819 by W. Meißner, an apothecary from Halle. He observed that these compounds appeared “like alkali”, and so named them alkaloids [6]. Chem=465 11/28/2020

9 For the biologist, the alkaloid is a pure and perfect natural product. From the biological point of view, the alkaloid is any biologically active and heterocyclic chemical compound which contains nitrogen and may some pharmacological activity and, in many cases, medicinal or ecological use [7]. Chem=465 11/28/2020

10 I. With limited distribution in nature. II. Present in; Plant, Fungi, Bacteria, Marine III. It has physiological action or Poisonous effect. Chem=465 11/28/2020

11 Alkaloids are generally classified by their common molecular precursors, based on the biological pathway used to construct the molecule. From a structural point of view, alkaloids are divided according to their shapes and origins. There are three main types of alkaloids: Chem=465 11/28/2020

12 Hagnauer system of classification A. True alkaloids. B. Proto alkaloids. C. Pseudo alkaloids Chem=465 11/28/2020

13 True alkaloids and protoalkaloids are derived from amino acids, whereas pseudoalkaloids are not derived from these compounds Chem=465 11/28/2020

14 True Alkaloid True alkaloids derive from amino acid and they share a heterocyclic ring with nitrogen. These alkaloids are highly reactive substances with biological activity even in low doses. All true alkaloids have a bitter taste and appear as a white solid, with the exception of nicotine which has a brown liquid. Chem=465 11/28/2020

15 True alkaloids form water-soluble salts. Moreover, most of them are well-defined crystalline substances which unite with acids to form salts. True alkaloids may occur in plants (1) in the free state (2) as salts (3) as N-oxides Chem=465 11/28/2020

16 The primary precursors of true alkaloids are such amino acids as L-ornithine, L-lysine L-phenylalanine L-tyrosine L-tryptophan L-histidine [2, 3, 3, 2]. Chem=465 11/28/2020

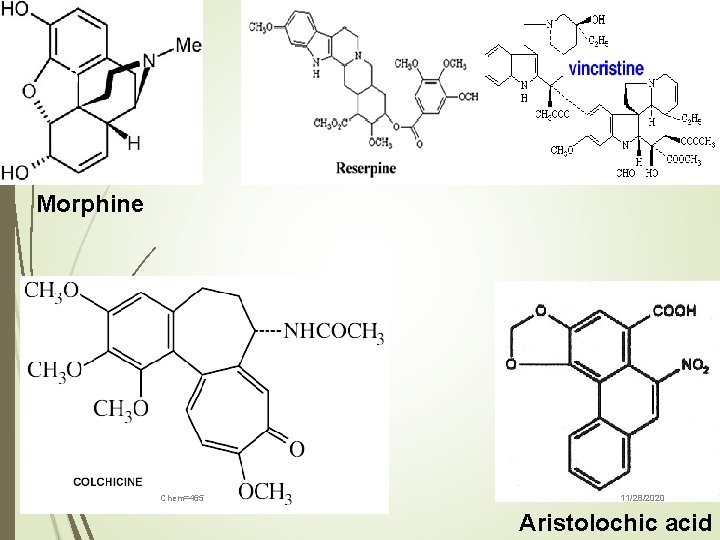
17 Examples of true alkaloids include such biologically active alkaloids as Cocaine Quinine, Cocaine Dopamine Morphine Usambarensine Chem=465 11/28/2020

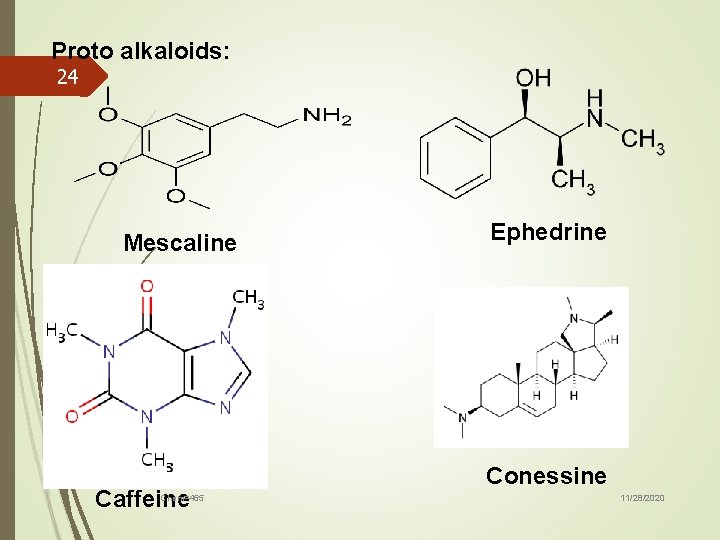
18 Protoalkaloids are compounds, in which the N atom derived from an amino acid is not a part of the heterocyclic[31]. Such kinds of alkaloid include compounds derived from L-tyrosine and L-tryptophan Protoalkaloids are those with a closed ring, being perfect but structurally simple alkaloids Chem=465 11/28/2020

19 Hordenine, mescaline and yohimbine are good examples of these kinds of alkaloid. Chini et al. [33] have found new alkaloids, Stachydrine and 4 -hydroxystachydrine, derived from Boscia angustifolia, a plant belonging to the Capparidacea family. Chem=465 11/28/2020

20 These alkaloids have a pyrroline nucleus and are basic alkaloids in the genus Boscia. The species from this genus have been used in folk medicine in East and South Africa. Boscia angustifolia is used for the treatment of mental illness, and occasionally to combat pain and neuralgia. Chem=465 11/28/2020

21 Pseudoalkaloids are compounds, the basic carbon skeletons of which are not derived from amino acids [31]. In reality, pseudoalkaloids are connected with amino acid pathways. They are derived from the precursors or postcursors (derivatives the indegradation process) of amino acids. Chem=465 11/28/2020

22 These alkaloids can also be derived from nonamin oacid precursors. Pseudoalkaloids can be acetate and phenylalanine derived or terpenoid, as well as steroidal alkaloids. Examples of pseudoalkaloids include such compounds as coniine, capsaicin, ephedrine, solanidine, caffeine, theobromine and pinidine Chem=465 11/28/2020

23 Morphine Chem=465 11/28/2020 Aristolochic acid

Proto alkaloids: 24 Mescaline Caffeine Chem=465 Ephedrine Conessine 11/28/2020

25 Chemical properties of alkaloids: Present in the Plant as Salt, ester, N-oxide, quaternary compound. ) Generally very toxic compound. Have bitter taste. Unstable compound in (Heat, Light, p. H changes) Chem=465 11/28/2020

26 Physicochemical properties: Solid crystalline compound (exception are: coniine and Nicotine are liquid (It doesn't have Oxygen in their structure). Colorless compound (exception are berberine (yellow), Betaine (red). Sharp melting Point because it’s pure compound in crystal form. Chem=465 11/28/2020

27 Can be either 1º, 2º, 3º or 40 alkaloid: Basicity depends on availability of lone pair of electrons: 1. Electron donating or electron withdrawing neighbors. 2. Type of hybridization. 3. Aromaticity. Chem=465 11/28/2020

- 28 Detection of alkaloid: Wagner's test: (I 2/k. I): Reddish brown precipitate Mayer’s Test: Hg. Cl 2 Creamy precipitate with True alkaloid. -Hagger's test: Chem=465 (Picric acid) Yellow precipitate with True alkaloid. 11/28/2020

29 Dragendroff: Tannic acid solution: Chem=465 (Potassium Bismuth Iodide) Reddish Brown precipitate Different alkaloid colored precipitate. 11/28/2020

30 Extraction: The extraction is fractional extraction (From less Polar to more Polar). -Defeating by non polar solvent (Petroleum Ether, benzene, alkane, …. ) To get red of Chlorophyll, Wax, Volatile oil, Fixed oil. -Filtration, For marc use methanol or ethanol 95% Evaporate by rotary evaporator (to Concentrate) -Add Tartaric acid 2% and Ethyl acetate will separate into two layer: Chem=465 11/28/2020

31 Organic layer (For week or neutral alkaloid) Aqueous layer (acidic layer, Tartaric acid) which have alkaloidal salt. To break the salt add NH 3 or Sodium bicarbonate. then add ethyl acetate again so will it separate into two layer again: Aqueous layer (Quaternary alkaloids 4º ) Organic layer (For basic alkaloid 10, 2º, 3º) Chem=465 11/28/2020
 Capsicum
Capsicum Tocolytic drugs
Tocolytic drugs Alkaloid examples
Alkaloid examples Purine alkaloids
Purine alkaloids Pharmacognosy alkaloids
Pharmacognosy alkaloids Solanacease
Solanacease Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Peomus
Peomus Serpentiana
Serpentiana Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Ephedra
Ephedra Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Purine examples
Purine examples Lysergic acid hydroxyethylamide
Lysergic acid hydroxyethylamide Protoalkaloids
Protoalkaloids Pharmacogosy
Pharmacogosy Zośka
Zośka Właściciel charta kusego w panu tadeuszu
Właściciel charta kusego w panu tadeuszu Tadeusz makowski zima
Tadeusz makowski zima Ubiór tadeusza soplicy
Ubiór tadeusza soplicy Tadeusz krajniak świnoujście
Tadeusz krajniak świnoujście Tadeusz kociuszko
Tadeusz kociuszko Tadeusz majewski
Tadeusz majewski Pan tadeusz epopeja
Pan tadeusz epopeja Kniaziewicz pan tadeusz
Kniaziewicz pan tadeusz Tadeusz szumlicz
Tadeusz szumlicz Skrzydlate słowa pan tadeusz
Skrzydlate słowa pan tadeusz Taniec pierwowzor poloneza
Taniec pierwowzor poloneza Topos oblężonego miasta
Topos oblężonego miasta Cechy dobrej prezentacji multimedialnej
Cechy dobrej prezentacji multimedialnej Vertical respuesta
Vertical respuesta Tadeusz makulski
Tadeusz makulski



















































