ALKALOIDS Alkaloid d Difficult to define No definitive








- Slides: 36

ALKALOIDS

Alkaloid d: Difficult to define No definitive difference between an alkaloid and naturally occurring complex amines. Alkaloids: Nitrogen atom. They also normally have a significant physiological action on humans and animals. TRUE ALKALOIDS The basic unit in the biogenesis of the true alkaloids are AMINO ACIDS. The non-nitrogen containing rings or side chains are derived from TERPENE units and / or ACETATE, while METHIONINE is responsible for the addition of methyl groups to nitrogen atoms. Alkaloids are highly reactive substances with biological activity in low doses.

WHAT IS AN ALKALOID ? • Naturally-occurring compounds that contain nitrogen • Many have heterocyclic rings as a part of their structure • They are found mostly in plants • Many are physiologically active (often spectacularly) • Many are used by native peoples for religious or medicinal purposes. • Many are basic (“alkaline”, due to an unshared pair on N) • Those nitrogen compounds that are found in all organisms (i. e. , amino acids, nucleic acids, etc. ) are not considered alkaloids. • Alkaloids are “secondary metabolites”, they are not involved in primary metabolism.

Alkaloid Description • Contains nitrogen - usually derived from an amino acid. • Bitter tasting, generally white solids (exception - nicotine is a brown liquid). They give a precipitate with heavy metal iodides. Except Caffeine. Alkaloids are basic - they form water soluble salts. • • • In plants, they may exist in the free state, as salts or as Noxides. Occur in a limited number of plants.


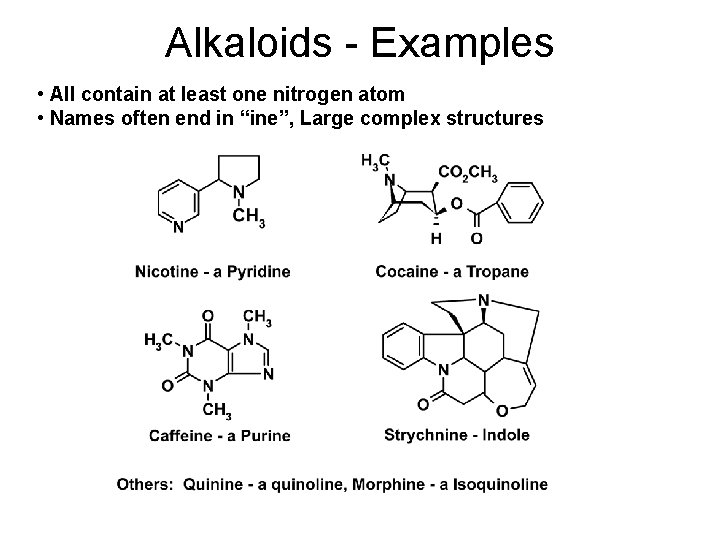
Alkaloids - Examples • All contain at least one nitrogen atom • Names often end in “ine”, Large complex structures

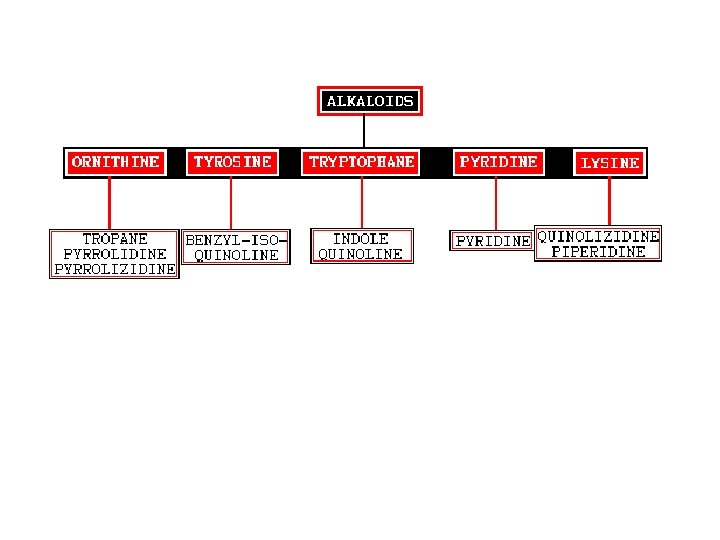
Ø Classification of Alkaloids • Biogenetic. Based on the biogenetic pathway that form the alkaloids. • Botanical Source. According to the plant source of alkaloids. • Type of Amines. Primary, Secondary, Tertiary alkaloids. • Basic Chemical Skeleton

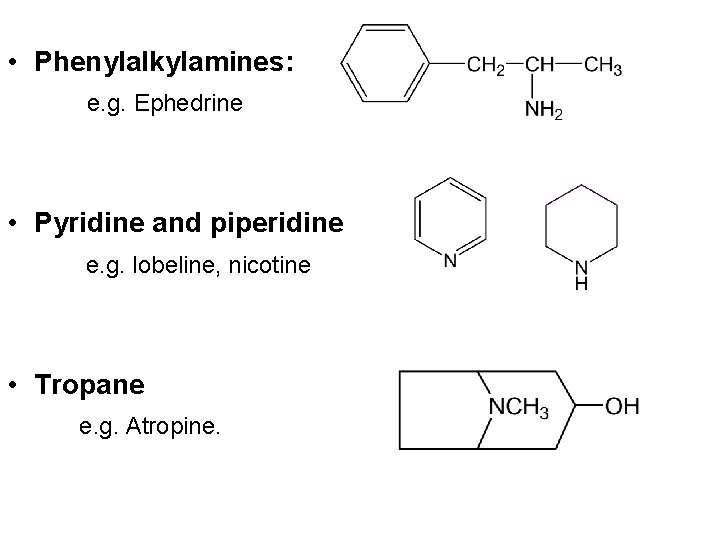
• Phenylalkylamines: e. g. Ephedrine • Pyridine and piperidine e. g. lobeline, nicotine • Tropane e. g. Atropine.

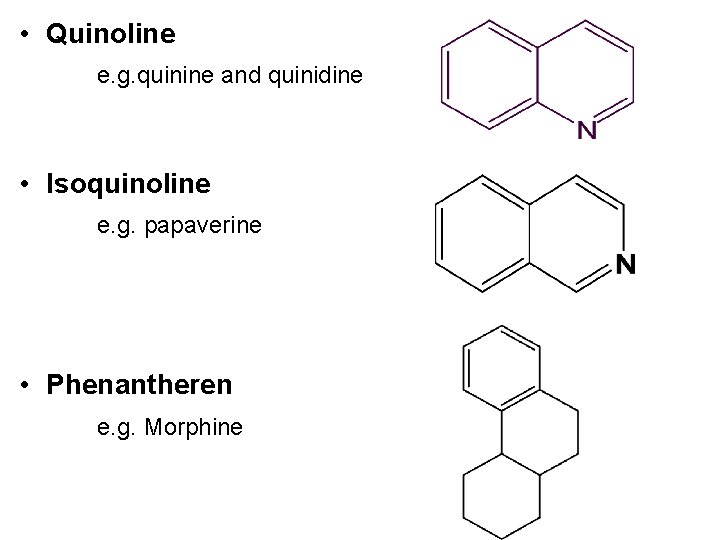
• Quinoline e. g. quinine and quinidine • Isoquinoline e. g. papaverine • Phenantheren e. g. Morphine

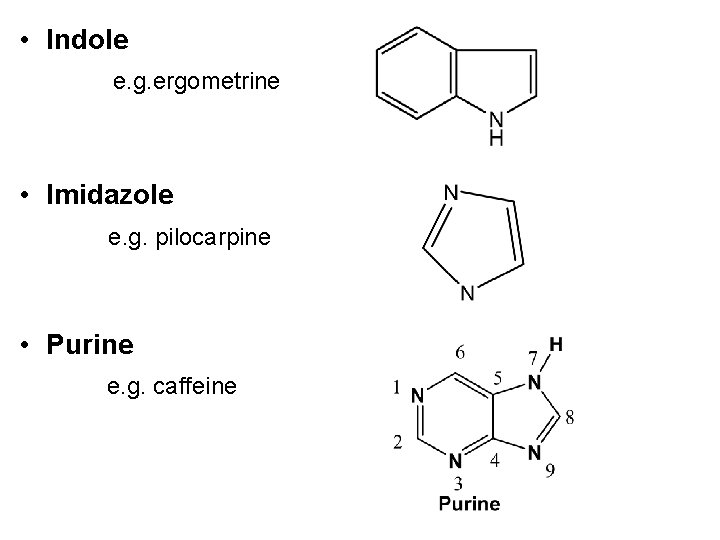
• Indole e. g. ergometrine • Imidazole e. g. pilocarpine • Purine e. g. caffeine

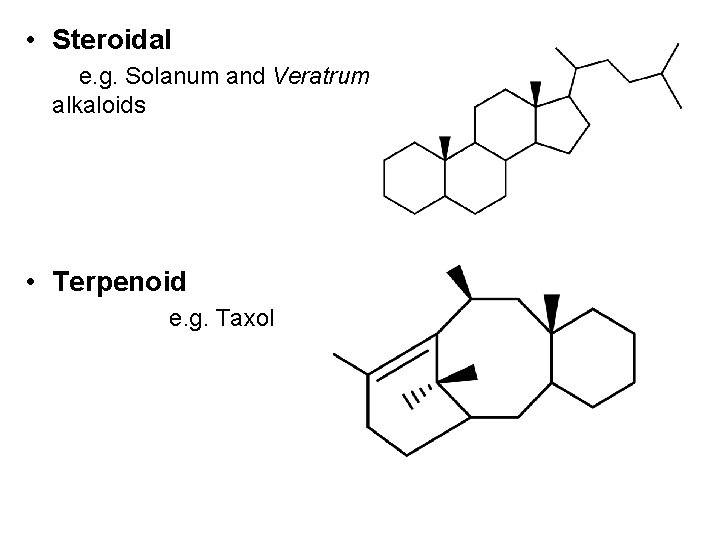
• Steroidal e. g. Solanum and Veratrum alkaloids • Terpenoid e. g. Taxol

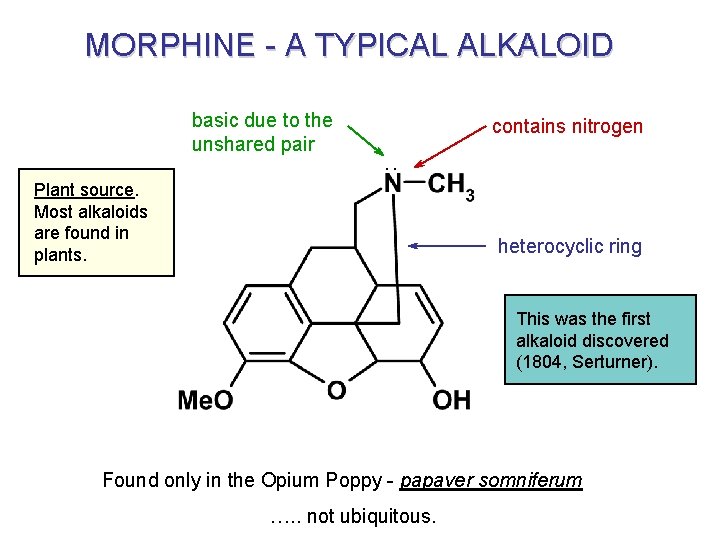
MORPHINE - A TYPICAL ALKALOID basic due to the unshared pair contains nitrogen . . Plant source. Most alkaloids are found in plants. heterocyclic ring This was the first alkaloid discovered (1804, Serturner). Found only in the Opium Poppy - papaver somniferum …. . not ubiquitous.

HOW ARE ALKALOIDS CLASSIFIED ? Common classification schemes use either: • The heterocyclic ring systems found as a part of the compound’s structure • The plant or plant family where they originate* * The majority of alkaloids (>90%) are found in plants therefore, we will speak mostly about plants and their biochemistry.

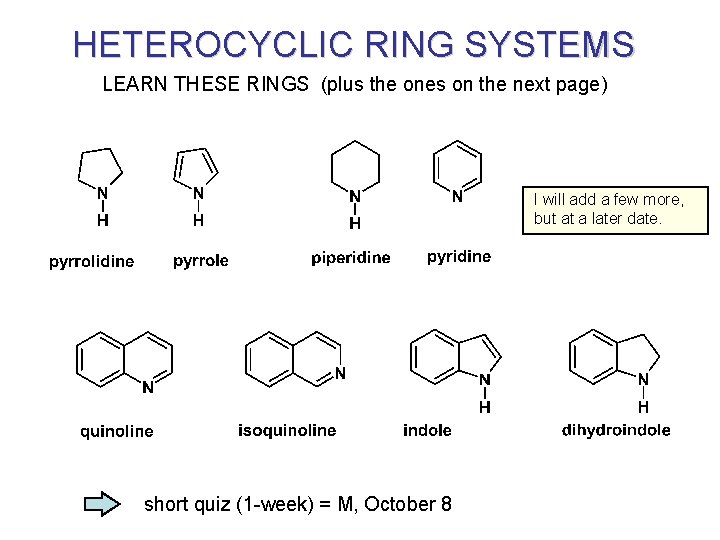
HETEROCYCLIC RING SYSTEMS LEARN THESE RINGS (plus the ones on the next page) I will add a few more, but at a later date. short quiz (1 -week) = M, October 8

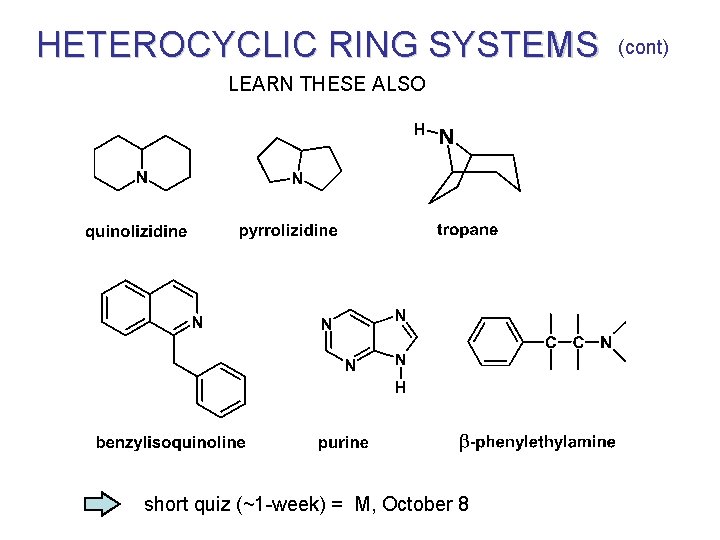
HETEROCYCLIC RING SYSTEMS LEARN THESE ALSO short quiz (~1 -week) = M, October 8 (cont)

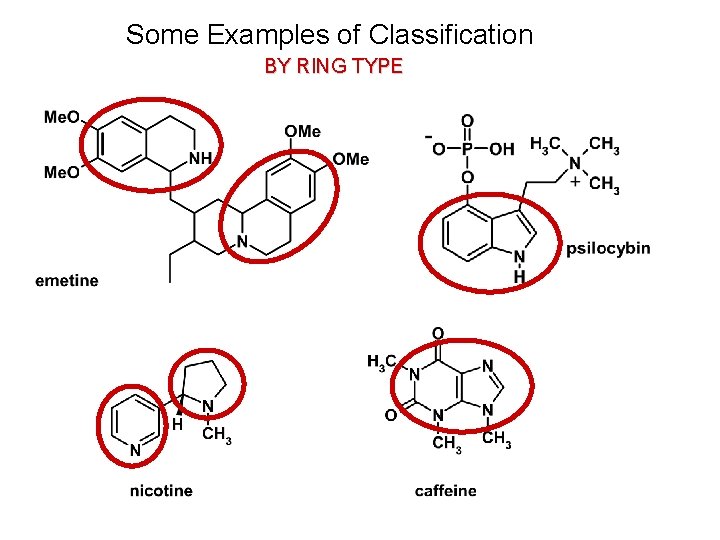
Some Examples of Classification BY RING TYPE

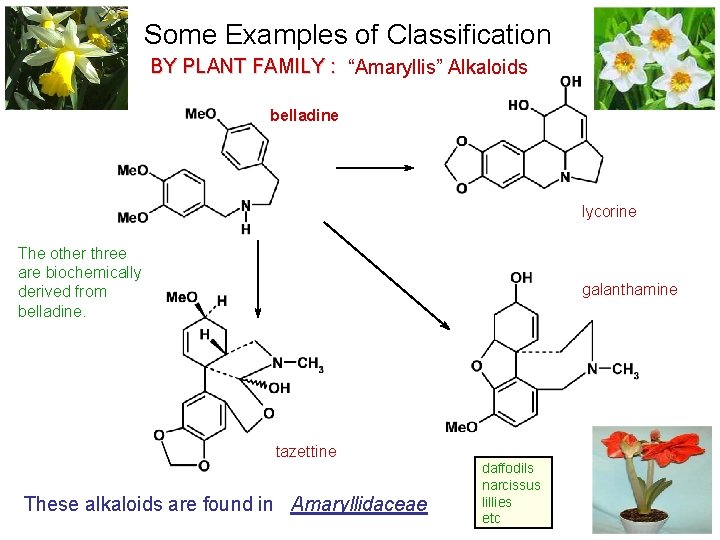
Some Examples of Classification BY PLANT FAMILY : “Amaryllis” Alkaloids belladine lycorine The other three are biochemically derived from belladine. galanthamine tazettine These alkaloids are found in Amaryllidaceae daffodils narcissus lillies etc

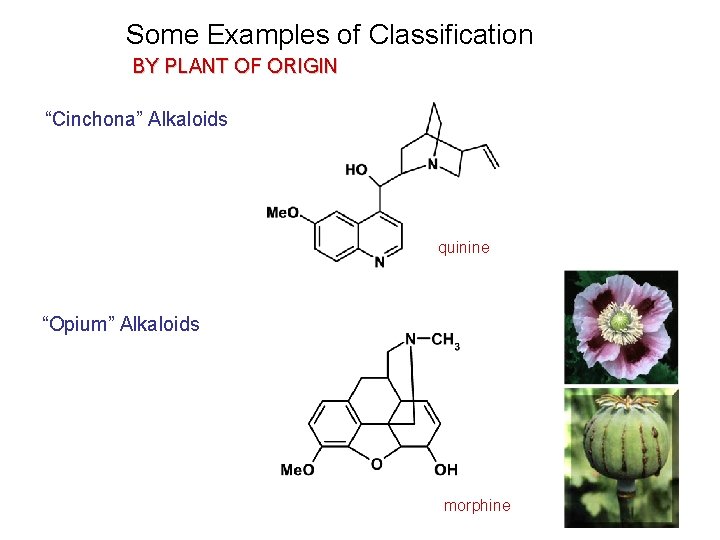
Some Examples of Classification BY PLANT OF ORIGIN “Cinchona” Alkaloids quinine “Opium” Alkaloids morphine

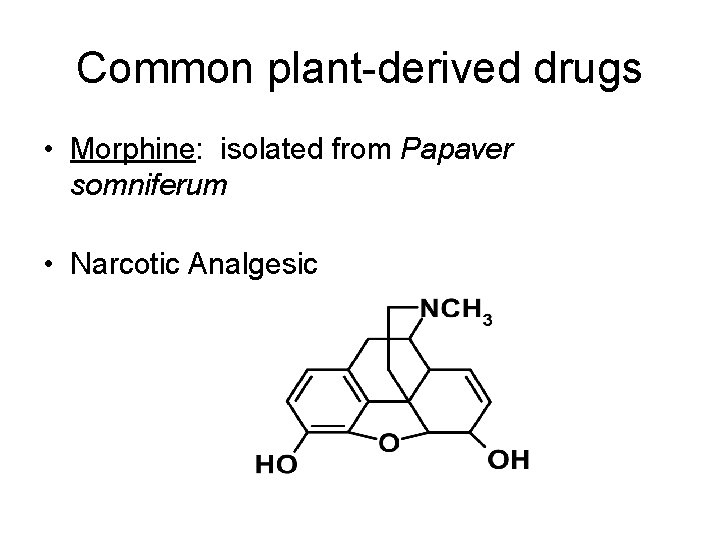
Common plant-derived drugs • Morphine: isolated from Papaver somniferum • Narcotic Analgesic

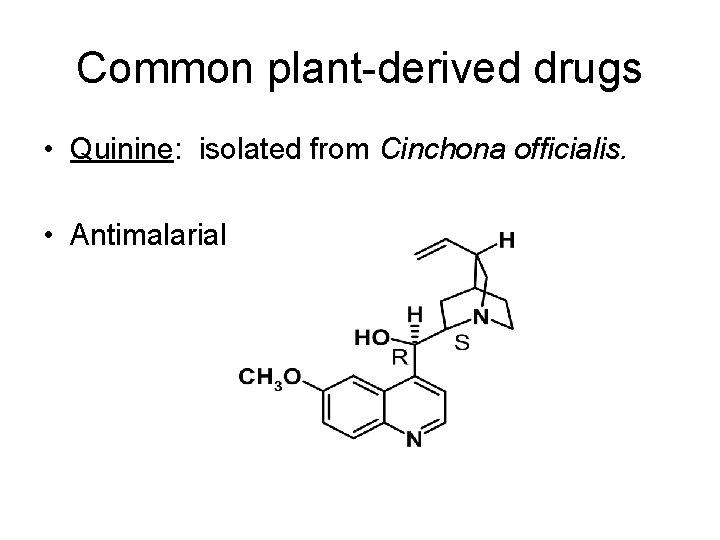
Common plant-derived drugs • Quinine: isolated from Cinchona officialis. • Antimalarial

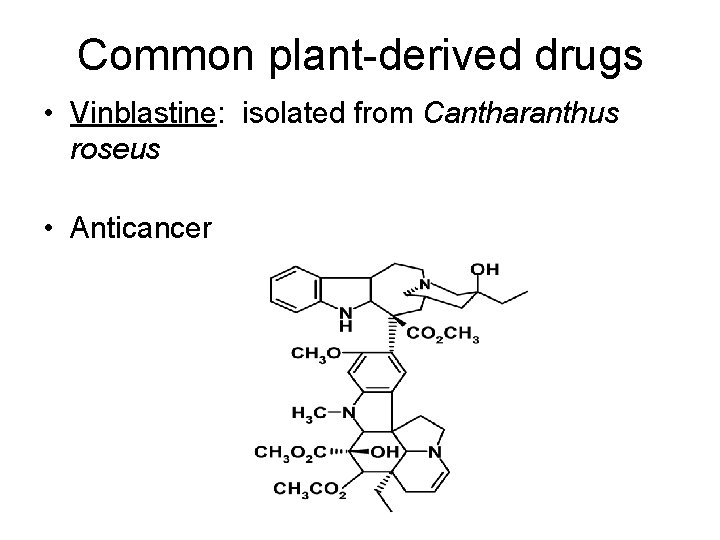
Common plant-derived drugs • Vinblastine: isolated from Cantharanthus roseus • Anticancer

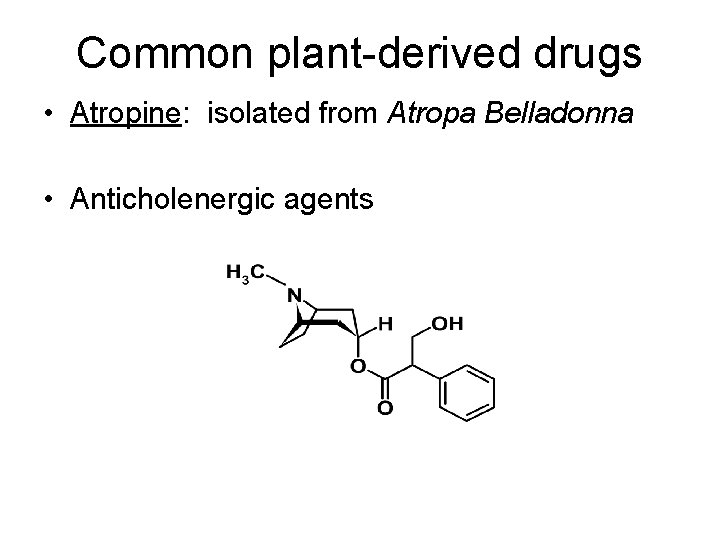
Common plant-derived drugs • Atropine: isolated from Atropa Belladonna • Anticholenergic agents

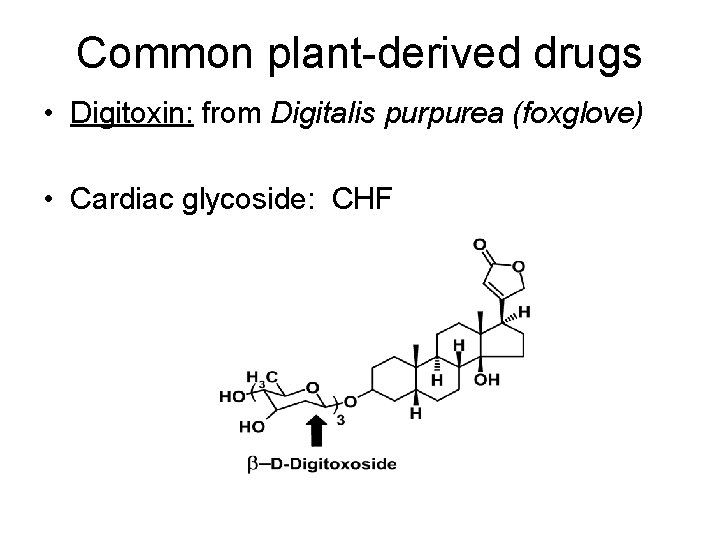
Common plant-derived drugs • Digitoxin: from Digitalis purpurea (foxglove) • Cardiac glycoside: CHF

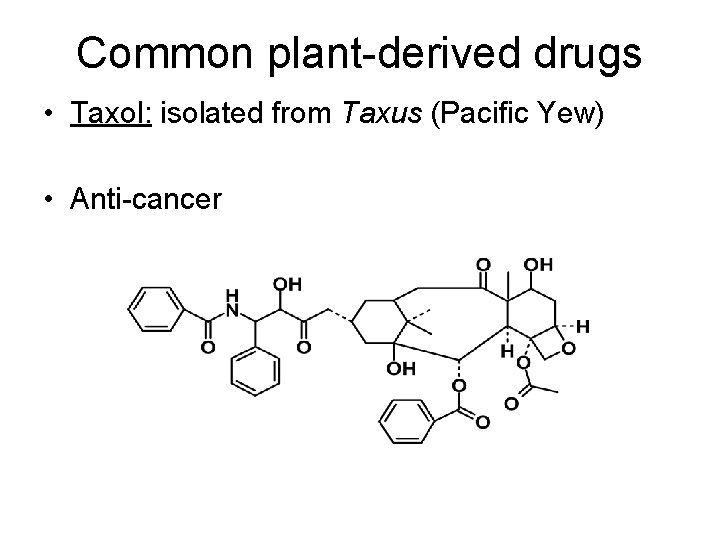
Common plant-derived drugs • Taxol: isolated from Taxus (Pacific Yew) • Anti-cancer

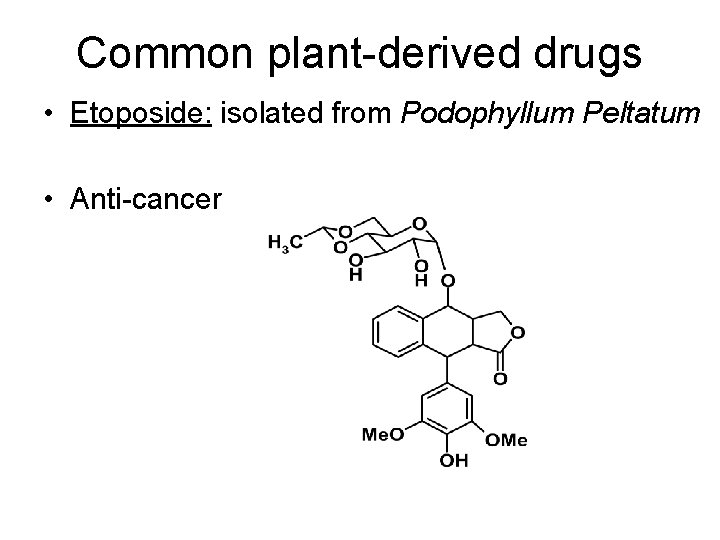
Common plant-derived drugs • Etoposide: isolated from Podophyllum Peltatum • Anti-cancer

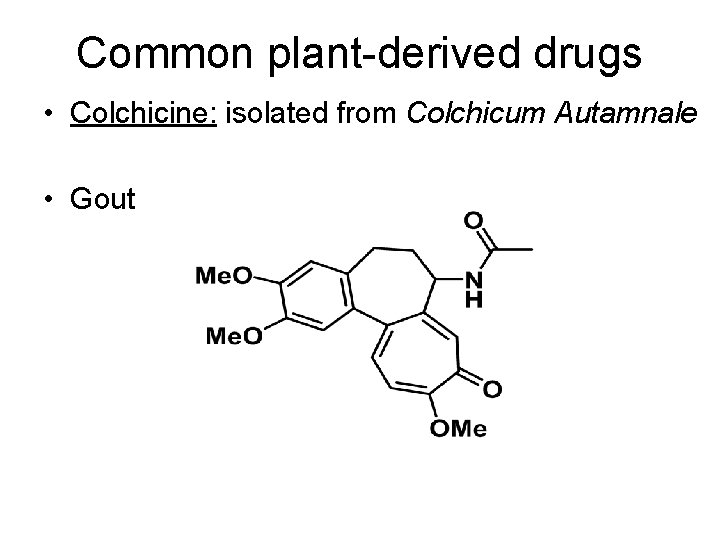
Common plant-derived drugs • Colchicine: isolated from Colchicum Autamnale • Gout

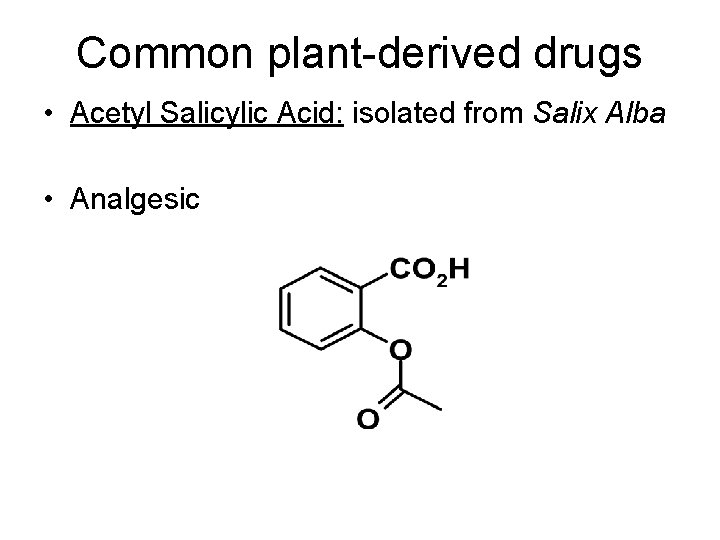
Common plant-derived drugs • Acetyl Salicylic Acid: isolated from Salix Alba • Analgesic

Natural Products Chemistry Primary metabolites 1. Direct function in primary biochemical pathways are essential for life (G-3 P, R-5 P) Secondary metabolites 1. Synthesized directly from primary metabolites 2. NOT involved in the primary life-sustaining processes important in proper biochemistry

Secondary Metabolites Primary classes • Terpenes – Cholesterol synthesis • Phenylpropanoids – Fatty acids • Polyketides – Fatty acids • Steroids – Progesterone, etc. • Glycosides – Protein glycolosys, etc. • Alkaloids – Amine containing moiety

Tests for Alkaloids – Most alkaloids are precipitated from neutral or slightly acidic solution by – Mayer's reagent (potassiomercuric iodide solution) Cream coloured precipitate. – Dragendorff's reagent (solution of potassium bismuth iodide) orange coloured precipitate. – Wagner’s reagent (iodine in potassium iodide) redbrown precipitate – Hagers reagent (picric acid) yellow precipitate – Caffeine does precipitate

Occurrence Distribution & of Alkaloids • Occur in bacteria and rarely in fungi. • Some alkaloids occur in several genera from different species, but most occur in closely related species. • Some occur in certain families • All alkaloids of one plant will have a common biogenenetic origin.

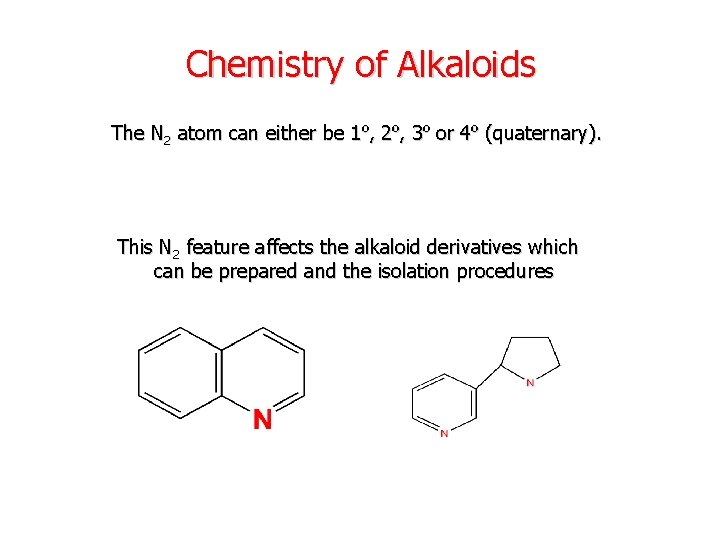
Chemistry of Alkaloids The N 2 atom can either be 1º, 2º, 3º or 4º (quaternary). This N 2 feature affects the alkaloid derivatives which can be prepared and the isolation procedures

Physical & chemical properties • The basicity of alkaloids depends on the availability of the lone pair of e- on the N 2 atoms: • Because some alkaloids have a carbonyl group on the amide, they can also be neutral (colchicine & piperine). • Basic characteristic renders complex alkaloids unstable, so that in solution they are sensitive to heat, light & oxygen. • Basic character of alkaloids also allows them to form salts with mineral acids (such as hydrochlorides, nitrates and sulphates) or inorganic acids (tartrates, sulfamates). • Solid salts can be conserved well and are a common commercial form of alkaloids.

MW: 100 – 900 , they are whit crystals. In rare cases they are coloured. Most bases which do not contain O 2 are liquid at room temperature All active except Purins Soluble in concentrated hydroalcoholic solutions They give a precipitate with heavy metal iodides. Caffeine, a purine derivative, does not precipitate like most alkaloids.

Naming of Alkaloids Numerous methods can be used to name alkaloids • Generic plant name – atropine from Atropa belladonna • Specific name of the plant – cocaine from Erythroxylum coca. • Common name of the herb – ergotamine from ergot (rye) • Physiological action of the plant – emetine producing emesis • Other – e. g. morphine derived from ancient Greek mythology – Morpheus – god of dreams

Extraction of Alkaloids Extraction is based on the basicity of alkaloids ( they normally occur in plants as salts). Herbs often contain other materials which interfere with extraction by forming emulsions. ( waxes, terpenes, pigments and other lipophilic substances) avoided by defatting Extraction method normally depends on the raw material, the purpose of extraction & the scale on which is to be performed. For research purposes: chromatography allows for quick and reliable results. TLC plate