NMR Lecture 5 Factors affecting 13 C NMR










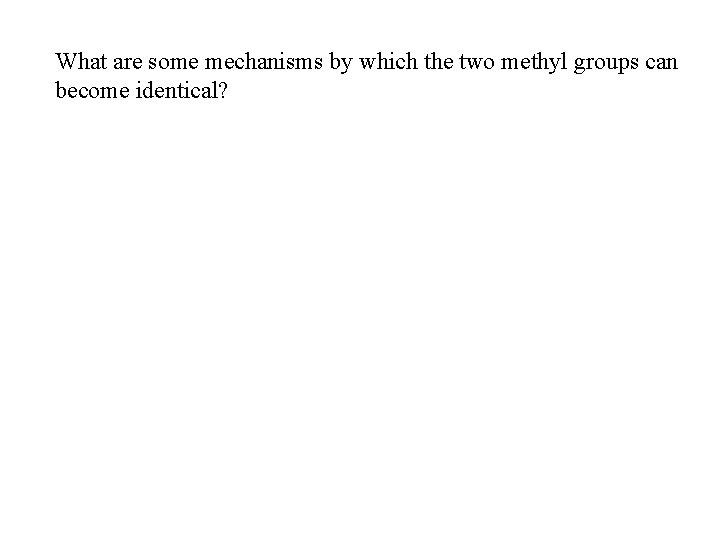






- Slides: 38

NMR Lecture 5

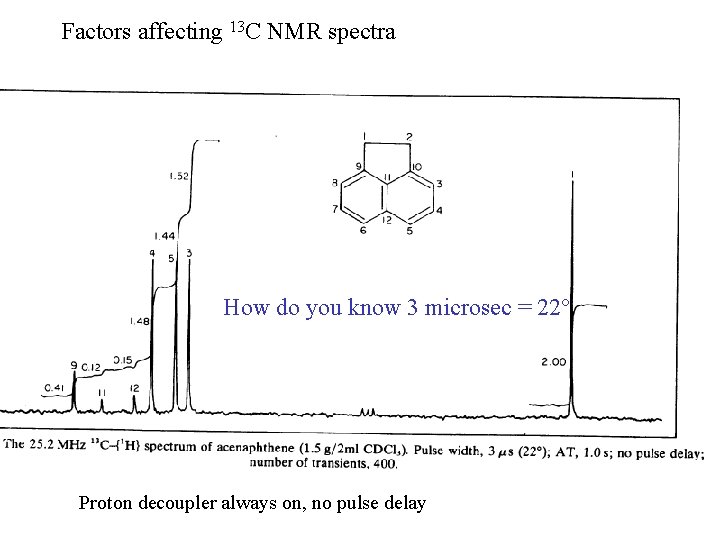
Factors affecting 13 C NMR spectra How do you know 3 microsec = 22° Proton decoupler always on, no pulse delay

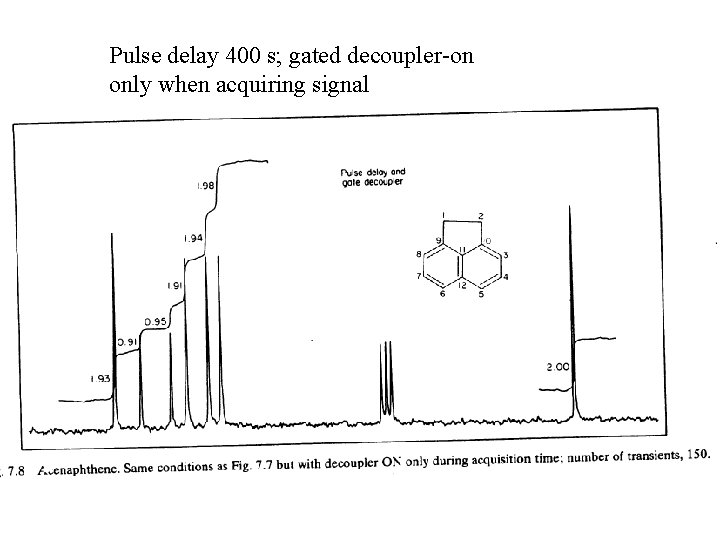
Pulse delay 400 s; gated decoupler-on only when acquiring signal

Effects of the pulse delay between pulses, 400 s Proton decoupler always on (NOE)

Information obtained by examining nmr spectra 1. Chemical shift (identifies nature of nucleus 2. Area (identifies relative number of nuclei) 3. Multiplicity ( (NOE) 2 D NMR basically provides information about connectivity and proximity

Applications of 1 H and 13 C NMR

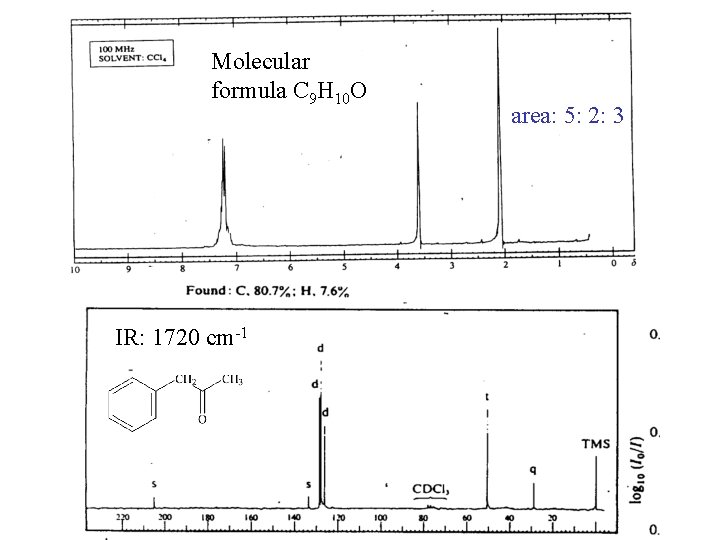
Molecular formula C 9 H 10 O IR: 1720 cm-1 area: 5: 2: 3

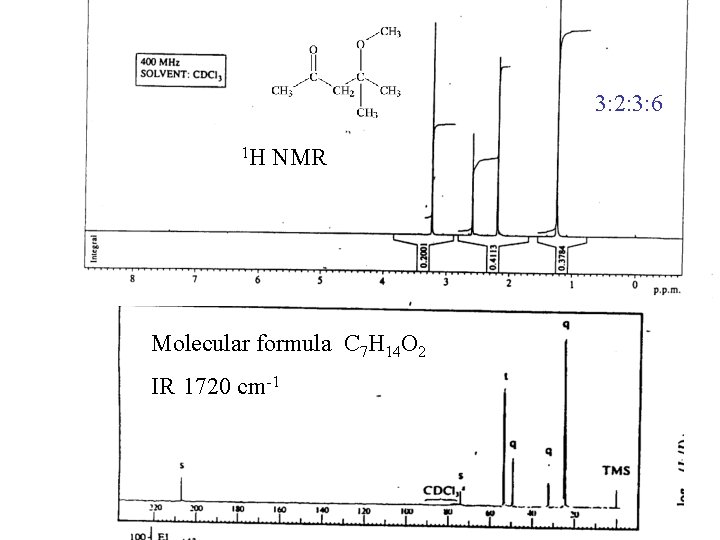
3: 2: 3: 6 1 H NMR Molecular formula C 7 H 14 O 2 IR 1720 cm-1


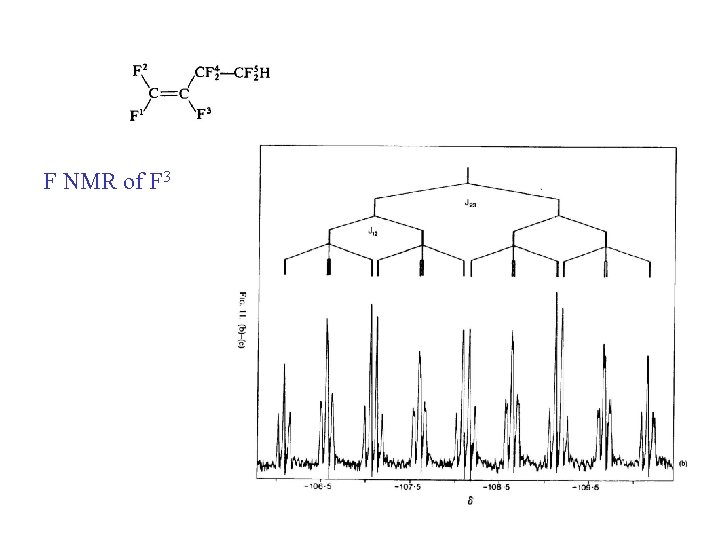
F NMR of F 3

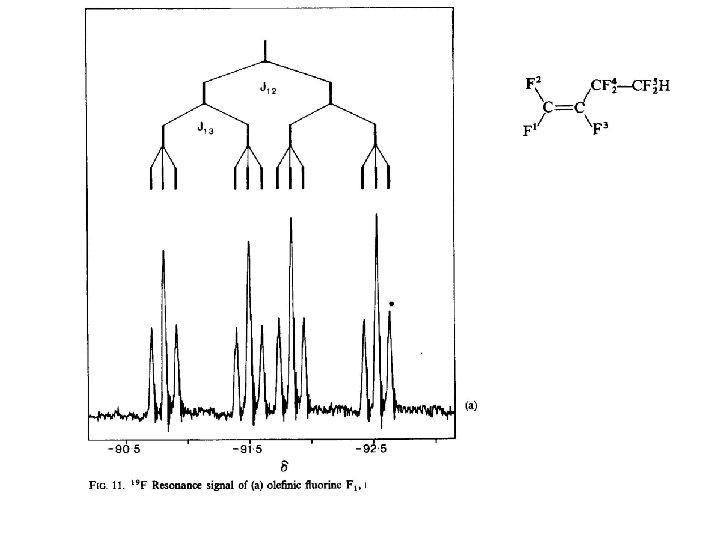
CF 24 CF 25 H =CF 3 -


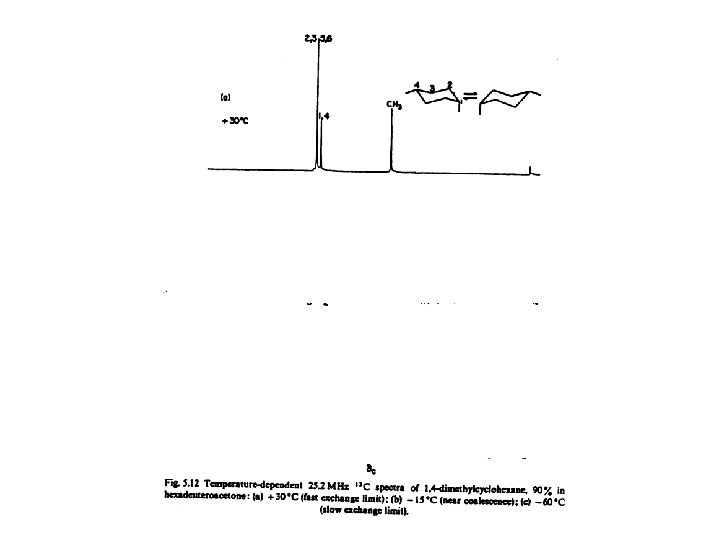
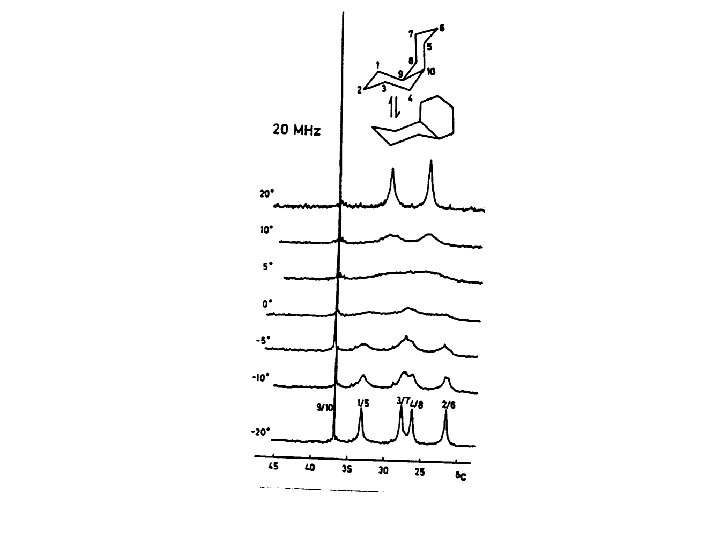
Returning to Dynamic Nuclear Magnetic Resonance For a simple exchange process coalescence /21/2 Most chemical shift differences are of the order of a few hundred Hz or less. Rate constants are of the order of few hundred sec-1. Large in comparison to k 10 -4 – 10 -5 sec-1, measured by conventional kinetics but small relative to many dynamic processes occurring in molecular systems such as rotations about bonds.






Assign the spectrum and explain the coupling observed Let’s do a thought experiment

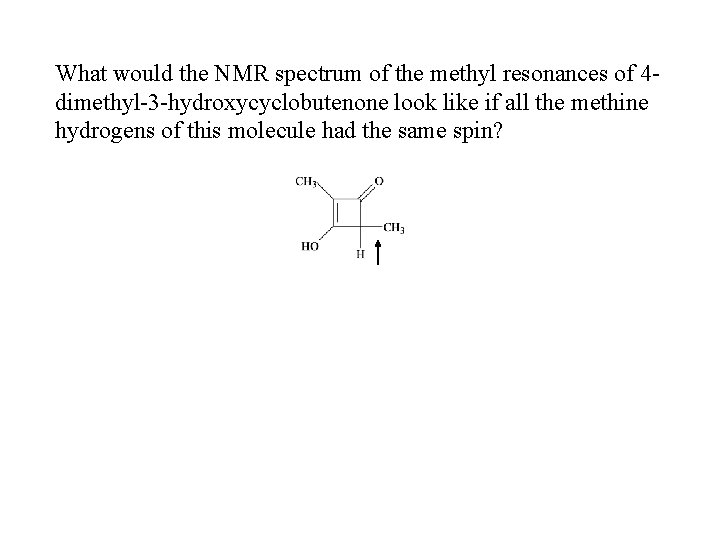
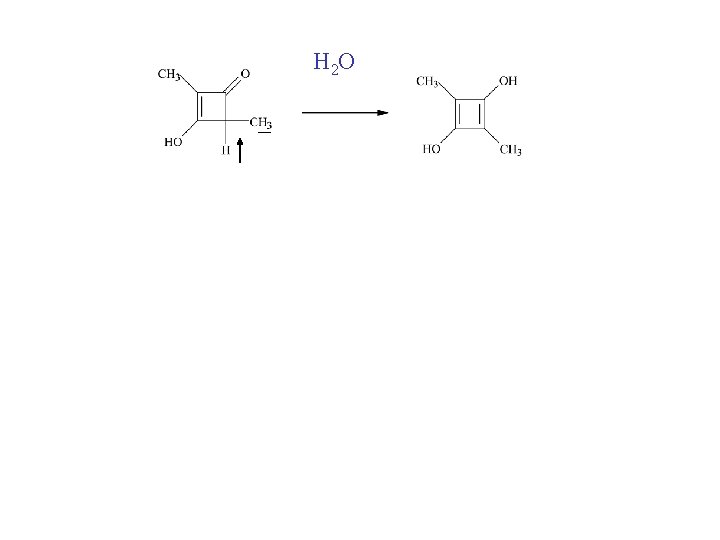
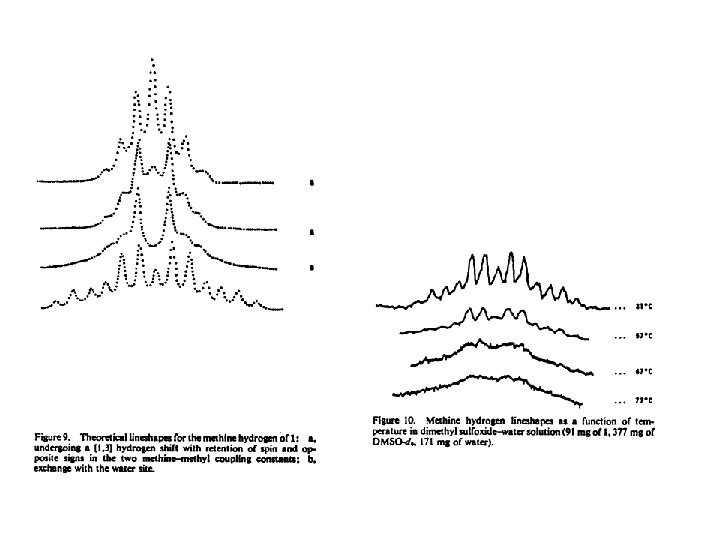
What would the NMR spectrum of the methyl resonances of 4 dimethyl-3 -hydroxycyclobutenone look like if all the methine hydrogens of this molecule had the same spin?

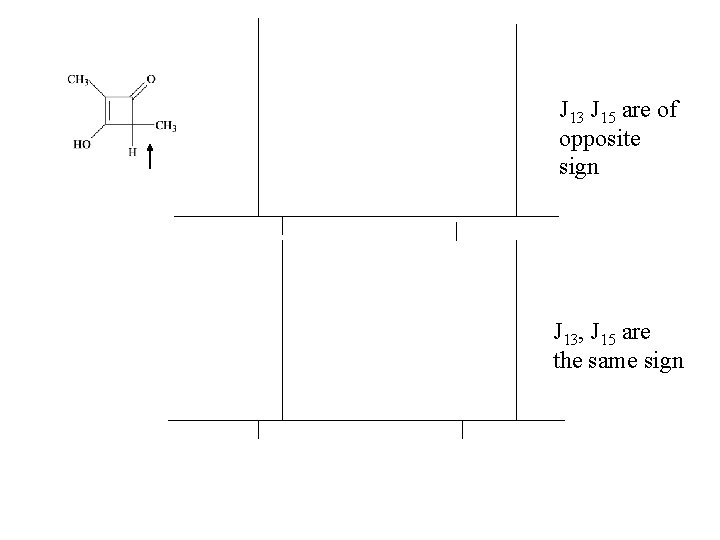
J 13 J 15 are of opposite sign J 13, J 15 are the same sign

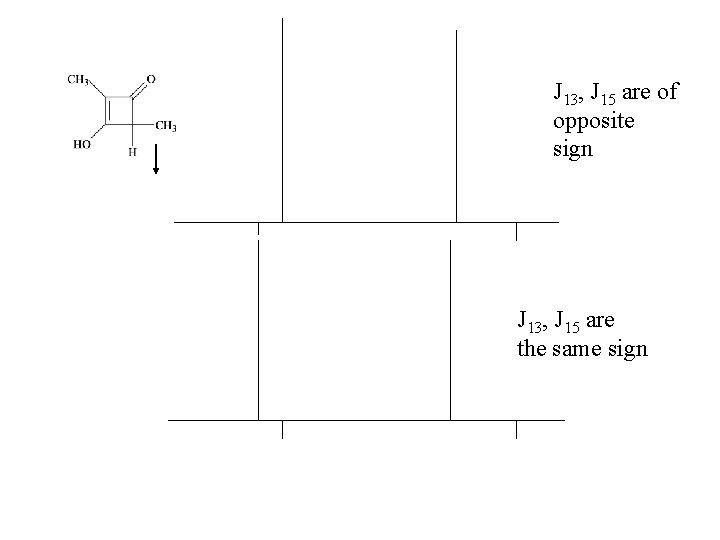
J 13, J 15 are of opposite sign J 13, J 15 are the same sign
What are some mechanisms by which the two methyl groups can become identical?

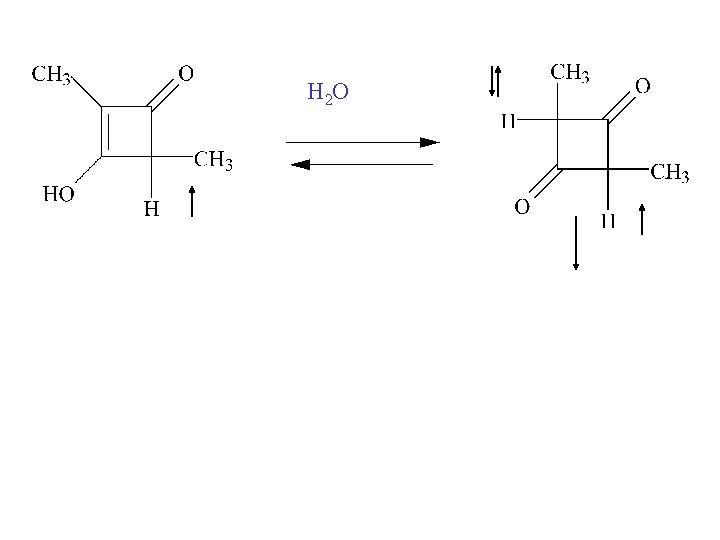
H 2 O


H 2 O


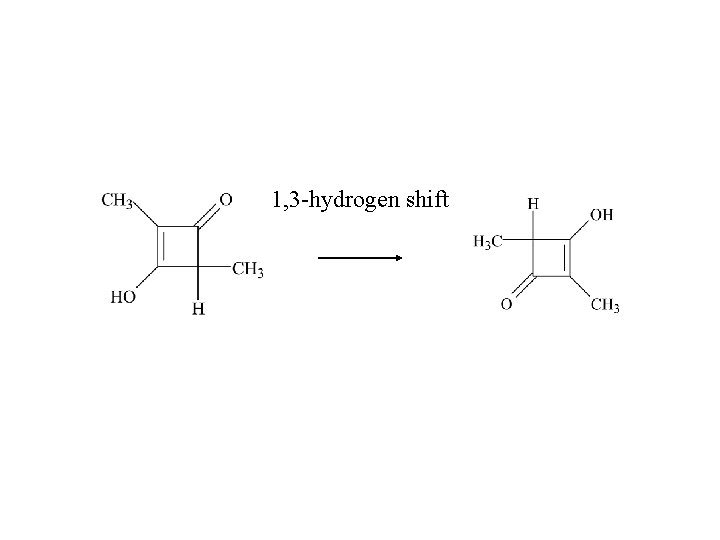
1, 3 -hydrogen shift

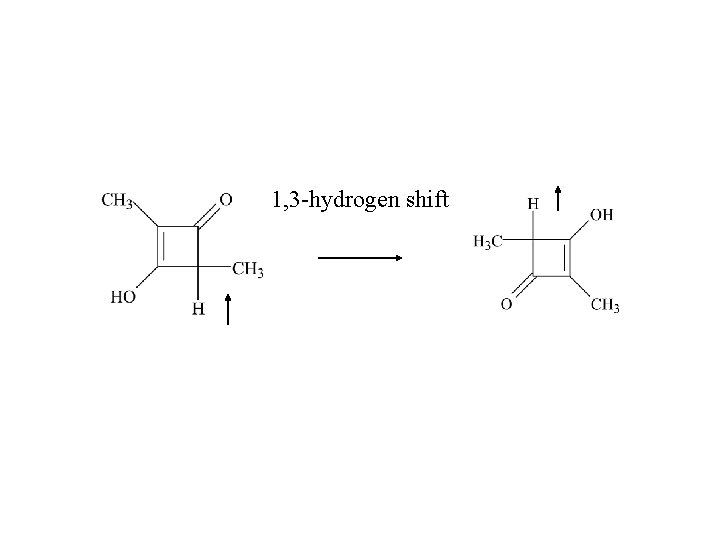
1, 3 -hydrogen shift

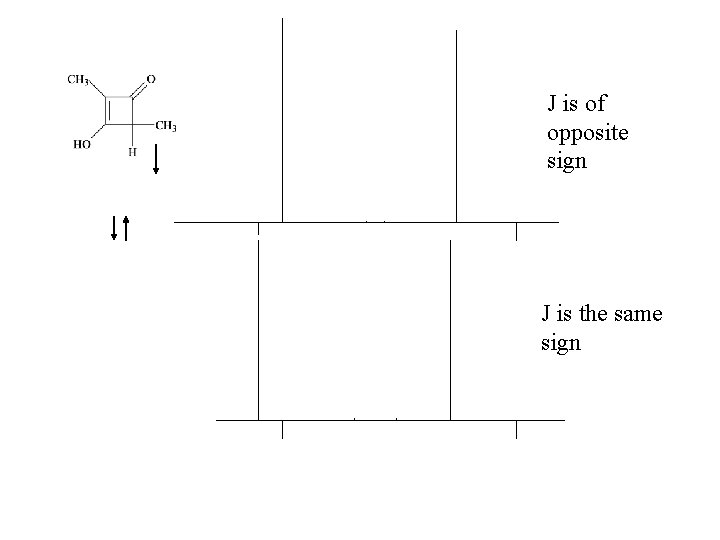
J is of opposite sign J is the same sign





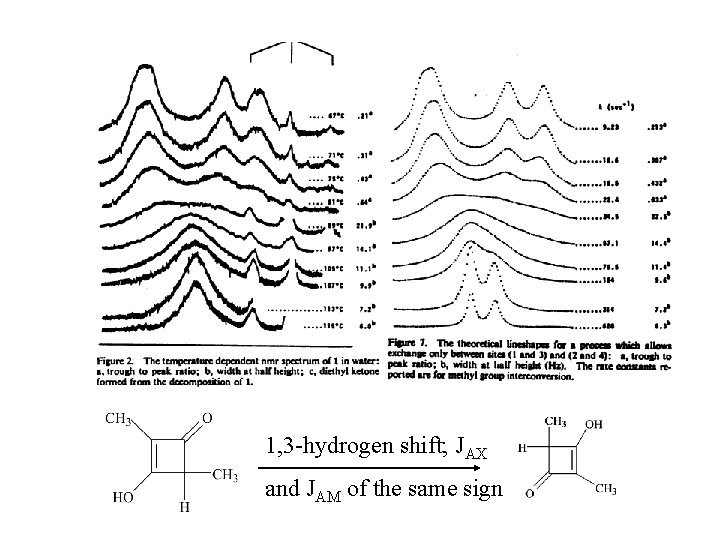
1, 3 -hydrogen shift; JAX and JAM of the same sign

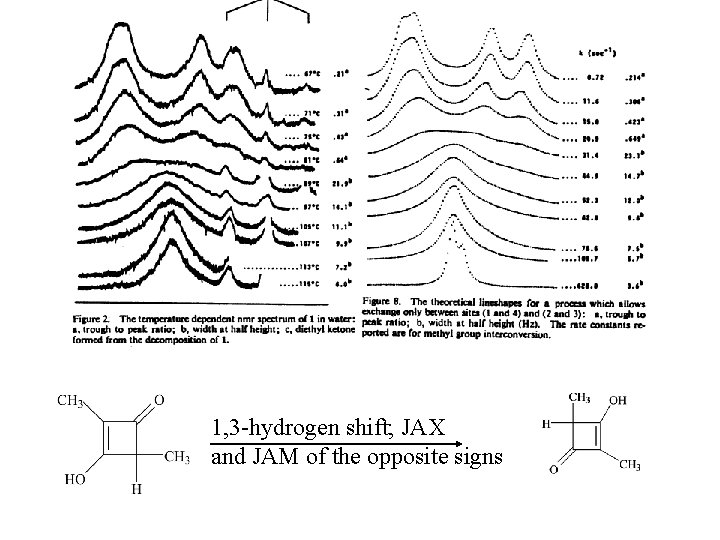
1, 3 -hydrogen shift; JAX and JAM of the opposite signs


Problem Set DNMR By line shape analysis, determine the activation energy for rotation about a carbon nitrogen bond in an amide. Go to my gateway and down load the Excel file. A plot of ln k vs 1/T should result in a straight line and the slope of the line should be - Ea/R. Your problem will be to fit the lineshapes as best you can and evaluate k for a series of temperatures. Remember that the most accurate k values will be when the line shapes change the most. k = rate constant