Chemical Formulas Chemical Compounds Chapter 7 Chemical formulas











- Slides: 23

Chemical Formulas & Chemical Compounds Chapter 7

Chemical formulas • Common names give no info about chemical composition. • Chemists use systematic methods for naming compounds & writing formulas.

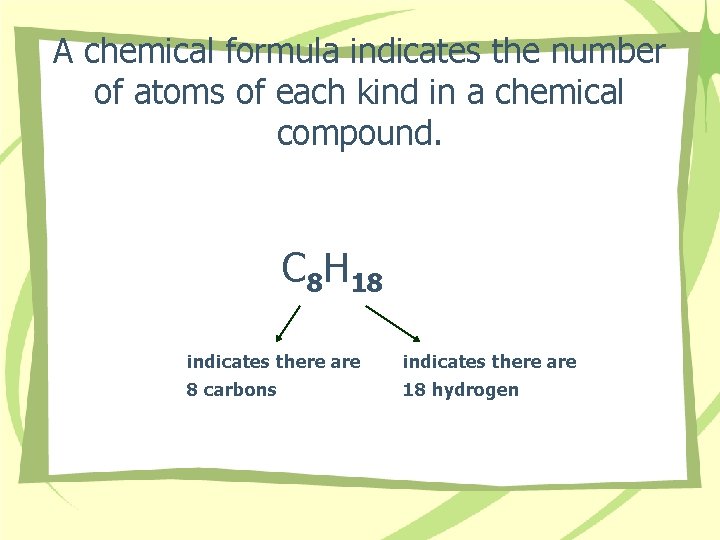
A chemical formula indicates the number of atoms of each kind in a chemical compound. C 8 H 18 indicates there are 8 carbons 18 hydrogen

Monatomic Ions • Ions formed from a single ion • Formed by gaining or losing electrons

Cont’d… • Monatomic cations are identified by the elements name. • Monatomic anions are named by changing the element’s ending to –ide. Cations Mg Magnesium Mg+2 Magnesium cation Anions O Oxygen O-2 Oxide

Binary compounds • Compounds composed of two different elements • Total number of positive & negative charges must be equal • The positive & negative charges can be crossed over

Rules for binary compounds 1. Write the symbols for the ions side by side, using the cation first. 2. Cross over the charges. 3. Write the formula without the charges.

Problem Write the formula for the binary ionic compounds formed between the following elements: a) magnesium & iodine b) magnesium & sulfur c) magnesium & nitrogen

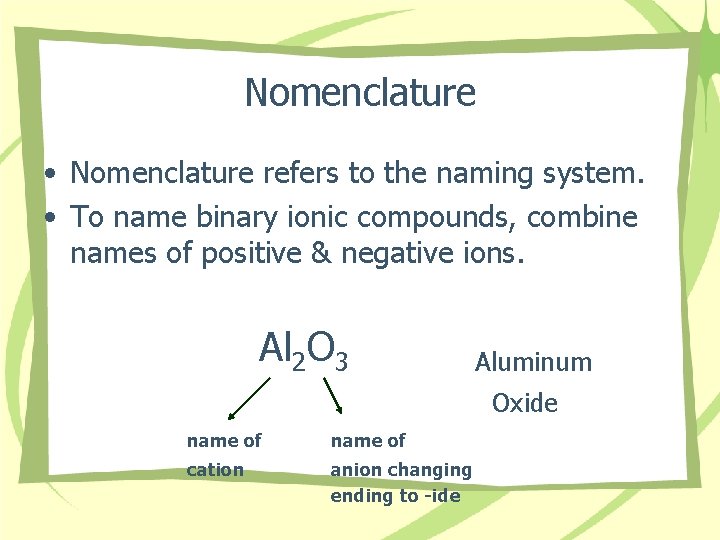
Nomenclature • Nomenclature refers to the naming system. • To name binary ionic compounds, combine names of positive & negative ions. Al 2 O 3 Aluminum Oxide name of cation anion changing ending to -ide

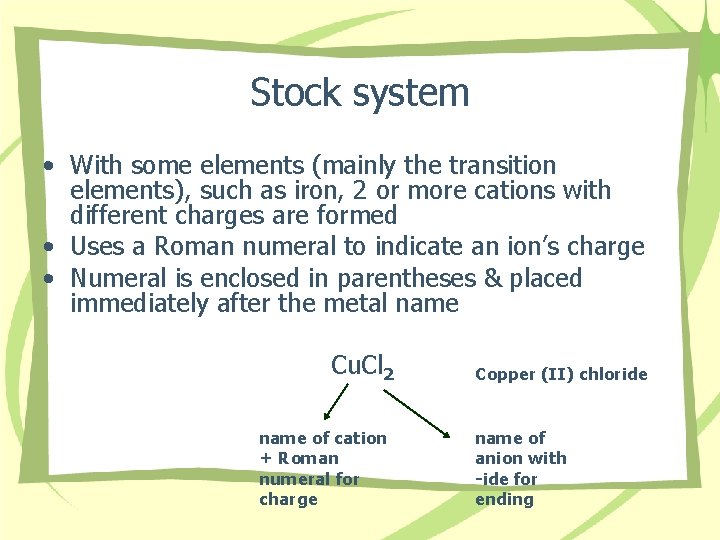
Stock system • With some elements (mainly the transition elements), such as iron, 2 or more cations with different charges are formed • Uses a Roman numeral to indicate an ion’s charge • Numeral is enclosed in parentheses & placed immediately after the metal name Cu. Cl 2 name of cation + Roman numeral for charge Copper (II) chloride name of anion with -ide for ending

Oxyanions • Oxyanions are polyatomic ions containing oxygen. • The most common oxyanions end in –ate. NO 3 - nitrate NO 2 - nitrite

Cont’d… • Sometimes more than 2 oxyanions are formed: Cl. O 4 - perchlorate Cl. O 3 - chlorate Cl. O 2 - chlorite Cl. O- hypochlorite

Prefix system • If only one atom of first element in formula, then no prefix on first element • If more than one atom of the first element, the first name begins with prefix • Second name begins with a prefix, followed by the root name, & ending in –ide P 4 O 10 tetraphosphorus decaoxide

Problem Give the name for As 2 O 5. Write the formula for oxygen difluoride.

Acids & Salts • Binary acid: consists of hydrogen & halogen • Oxyacid: contains hydrogen, oxygen, & a nonmetal • Salt: ionic compound composed of a cation & the anion from an acid • Table 7 -5 on Page 214: Common acids!

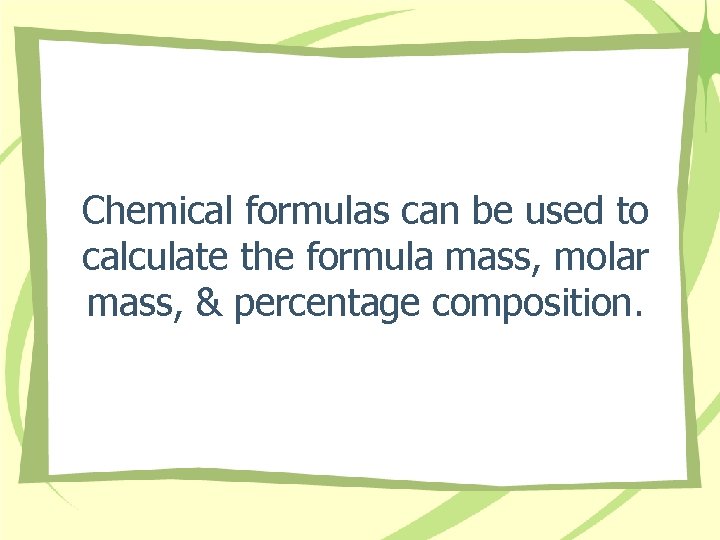
Chemical formulas can be used to calculate the formula mass, molar mass, & percentage composition.

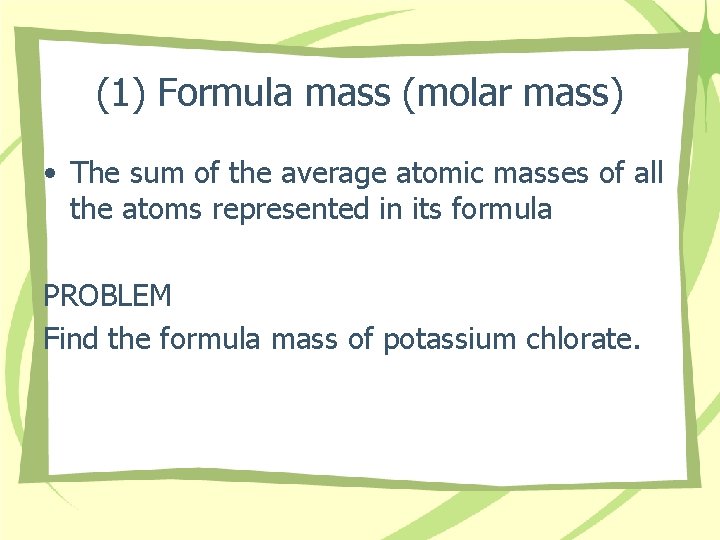
(1) Formula mass (molar mass) • The sum of the average atomic masses of all the atoms represented in its formula PROBLEM Find the formula mass of potassium chlorate.

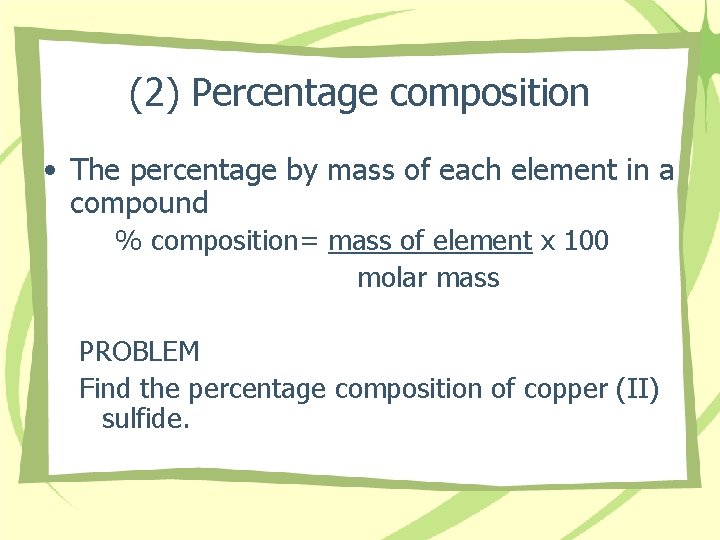
(2) Percentage composition • The percentage by mass of each element in a compound % composition= mass of element x 100 molar mass PROBLEM Find the percentage composition of copper (II) sulfide.

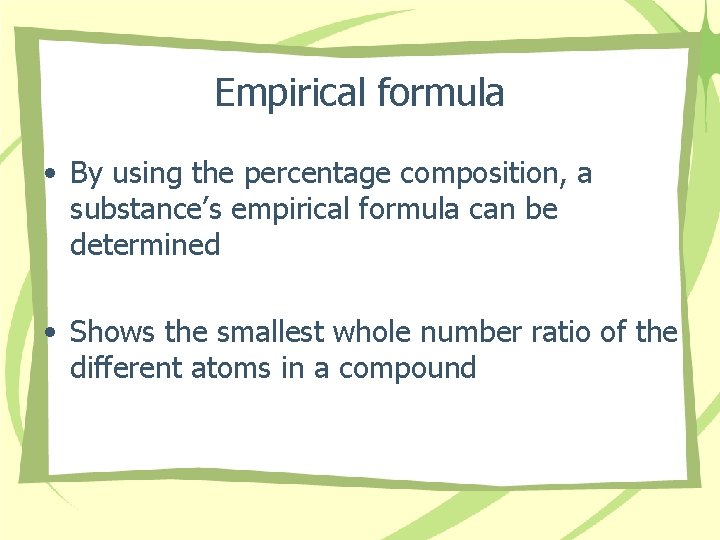
Empirical formula • By using the percentage composition, a substance’s empirical formula can be determined • Shows the smallest whole number ratio of the different atoms in a compound

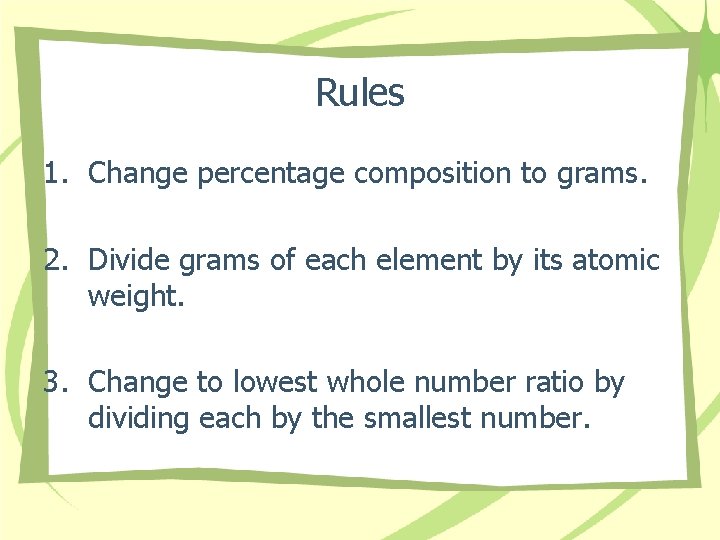
Rules 1. Change percentage composition to grams. 2. Divide grams of each element by its atomic weight. 3. Change to lowest whole number ratio by dividing each by the smallest number.

Problem Quantitative analysis shows that a compound contains 32. 38% Na, 22. 65% S, & 44. 99% O. Find the empirical formula of this compound.

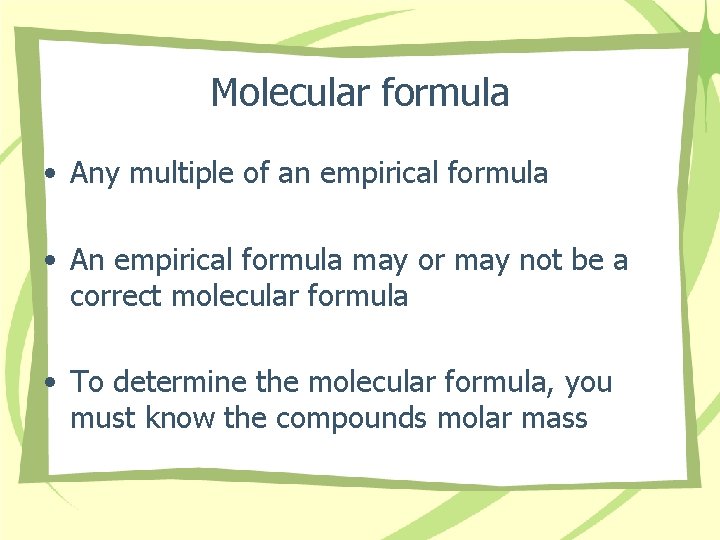
Molecular formula • Any multiple of an empirical formula • An empirical formula may or may not be a correct molecular formula • To determine the molecular formula, you must know the compounds molar mass

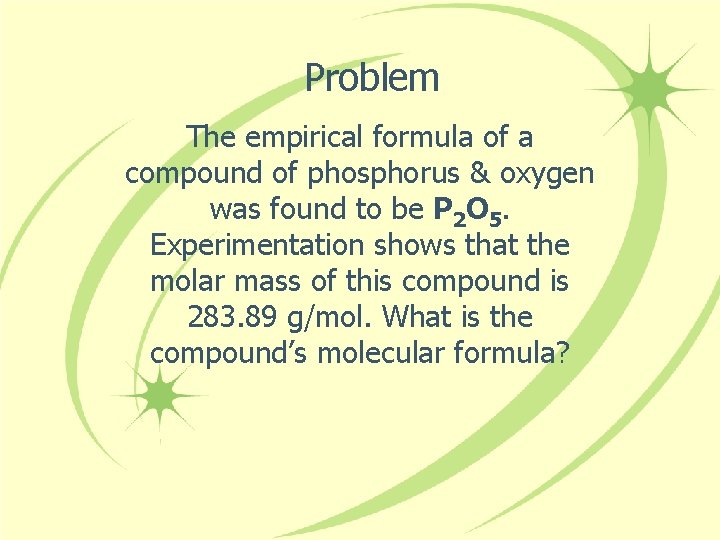
Problem The empirical formula of a compound of phosphorus & oxygen was found to be P 2 O 5. Experimentation shows that the molar mass of this compound is 283. 89 g/mol. What is the compound’s molecular formula?
 Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Love formula
Love formula Prefixes for hydrates
Prefixes for hydrates Dichlorine octoxide formula
Dichlorine octoxide formula Venn diagram of ionic, covalent and metallic bonds
Venn diagram of ionic, covalent and metallic bonds Naming compounds and writing formulas
Naming compounds and writing formulas Ionic naming rules
Ionic naming rules Which two formulas represent compounds
Which two formulas represent compounds Writing formulas and naming compounds section 3
Writing formulas and naming compounds section 3 Lesson 17 technicolor atoms flame test
Lesson 17 technicolor atoms flame test Monoatomic ion
Monoatomic ion Tetraiodine nonaoxide formula
Tetraiodine nonaoxide formula Section 3 writing formulas and naming compounds
Section 3 writing formulas and naming compounds Nhmno4
Nhmno4 Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Ionic compounds properties
Ionic compounds properties 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems Chemical names and formulas worksheet chapter 9
Chemical names and formulas worksheet chapter 9 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas A chemical formula shows
A chemical formula shows Writing formulas (crisscross method)
Writing formulas (crisscross method) Writing names for ionic compounds
Writing names for ionic compounds Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2