Chemical Equations Represent Chemical Reactions An equation must























- Slides: 39

Chemical Equations Represent Chemical Reactions

An equation must: § Represent known facts (be chemically possible) § Contain CORRECTLY written formulas § Comply with the Law of Conservation of Mass This is why we must balance them!

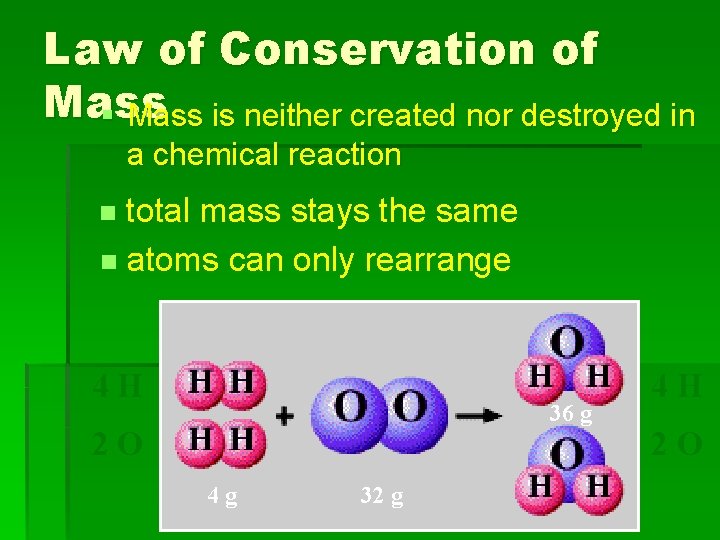
Law of Conservation of Mass § Mass is neither created nor destroyed in a chemical reaction total mass stays the same n atoms can only rearrange n 4 H 36 g 2 O 4 g 32 g 4 H 2 O

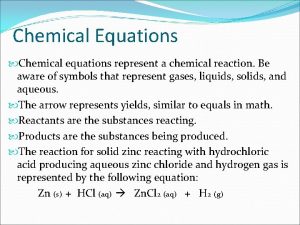
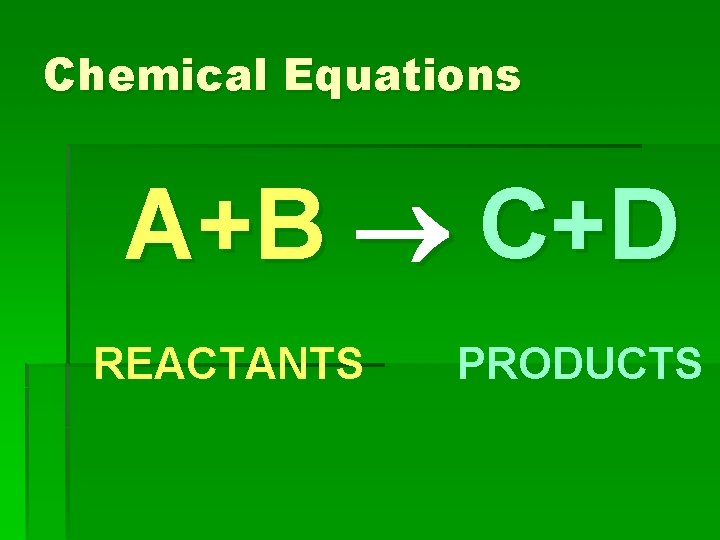
Chemical Equations A+B C+D REACTANTS PRODUCTS

Chemical Equations § Reactants – substances you begin with and are written on the LEFT side of the arrow. § Products are the substances you end up with, and are written on the RIGHT side of the arrow.

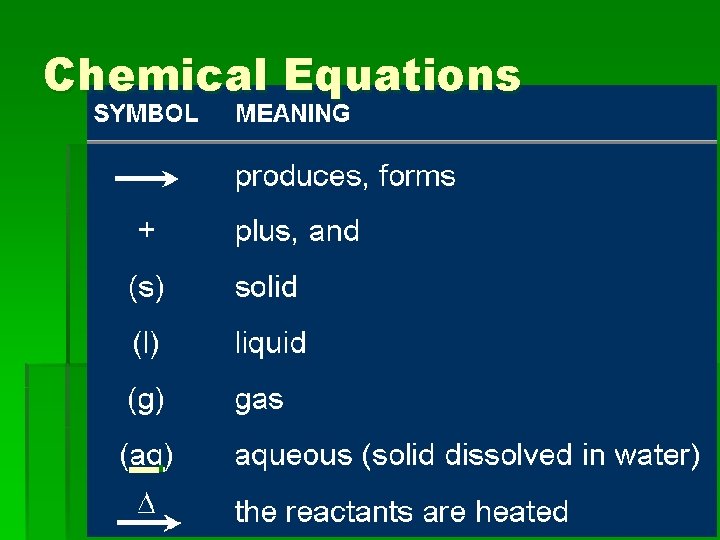
Chemical Equations

Synthesis § Two substances come together to form one product. § A + B AB § Examples: § 2 H 2 + O 2 2 H 2 O § 2 Na + Cl 2 2 Na. Cl

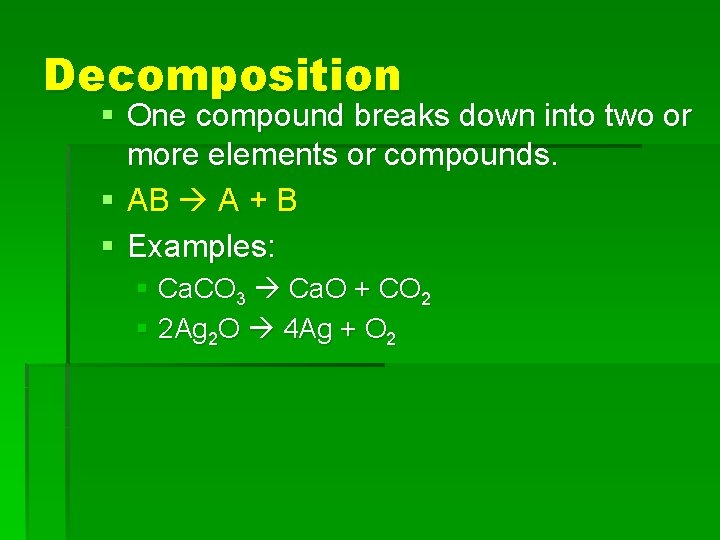
Decomposition § One compound breaks down into two or more elements or compounds. § AB A + B § Examples: § Ca. CO 3 Ca. O + CO 2 § 2 Ag 2 O 4 Ag + O 2

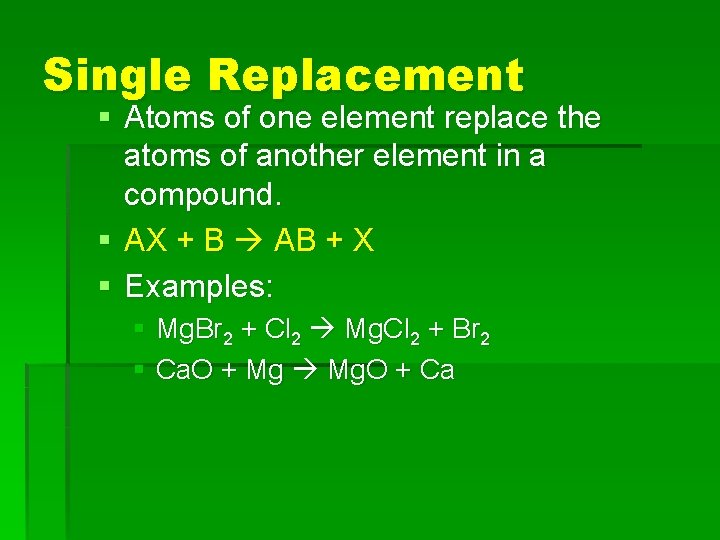
Single Replacement § Atoms of one element replace the atoms of another element in a compound. § AX + B AB + X § Examples: § Mg. Br 2 + Cl 2 Mg. Cl 2 + Br 2 § Ca. O + Mg Mg. O + Ca

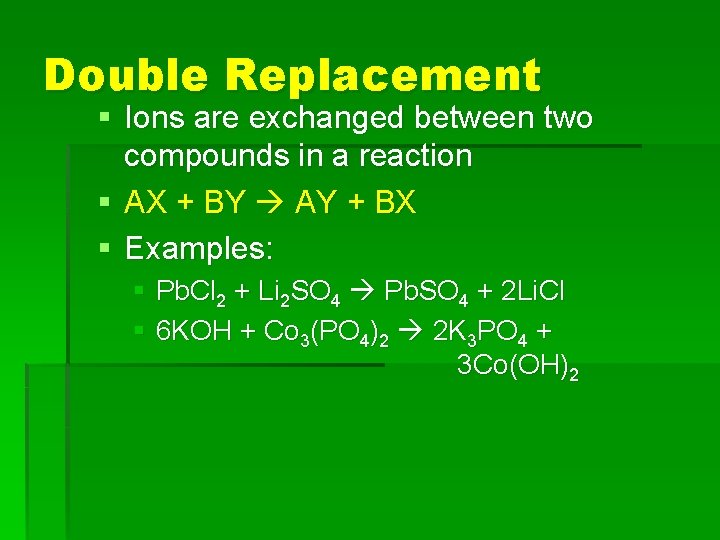
Double Replacement § Ions are exchanged between two compounds in a reaction § AX + BY AY + BX § Examples: § Pb. Cl 2 + Li 2 SO 4 Pb. SO 4 + 2 Li. Cl § 6 KOH + Co 3(PO 4)2 2 K 3 PO 4 + 3 Co(OH)2

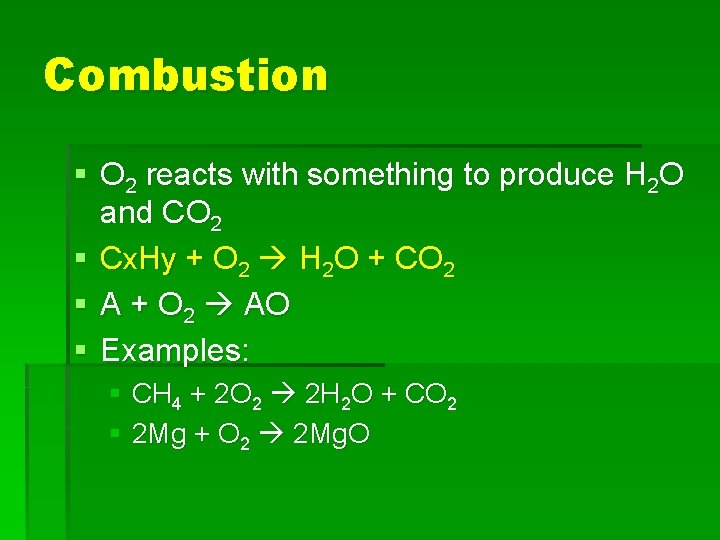
Combustion § O 2 reacts with something to produce H 2 O and CO 2 § Cx. Hy + O 2 H 2 O + CO 2 § A + O 2 AO § Examples: § CH 4 + 2 O 2 2 H 2 O + CO 2 § 2 Mg + O 2 2 Mg. O

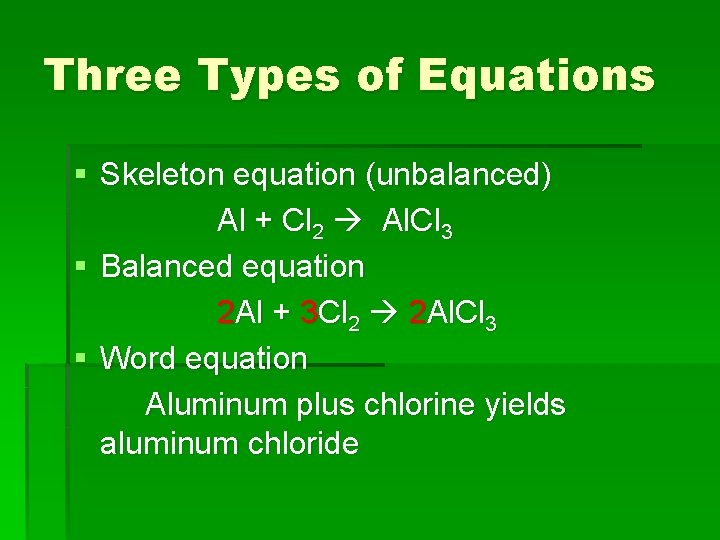
Three Types of Equations § Skeleton equation (unbalanced) Al + Cl 2 Al. Cl 3 § Balanced equation 2 Al + 3 Cl 2 2 Al. Cl 3 § Word equation Aluminum plus chlorine yields aluminum chloride

Diatomic Elements 7 elements that, when not part of a compound, ALWAYS exist as a “pair” Br I N Cl H O F (write with a 2 subscript) Ex: Br 2

Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make # of atoms on both sides equal. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

Helpful Tips § Balance one element at a time. § Update ALL atom counts after adding a coefficient. § If an element appears more than once per side, balance it last. § Balance polyatomic ions as single units. § “ 1 SO 4” instead of “ 1 S” and “ 4 O”

Helpful Tips § If there is no way to balance with a whole number, put a decimal in as the coefficient and then double everything. § When balancing a chemical equation you may add coefficients but you may NOT change subscripts. Subscripts are part of the compound and cannot be changed.

Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6

Examples: Al + Cl 2 Al. Cl 3

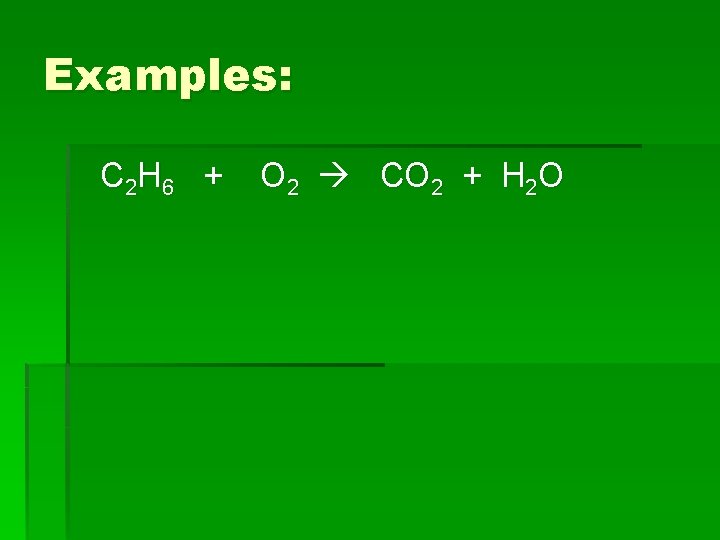
Examples: C 2 H 6 + O 2 CO 2 + H 2 O

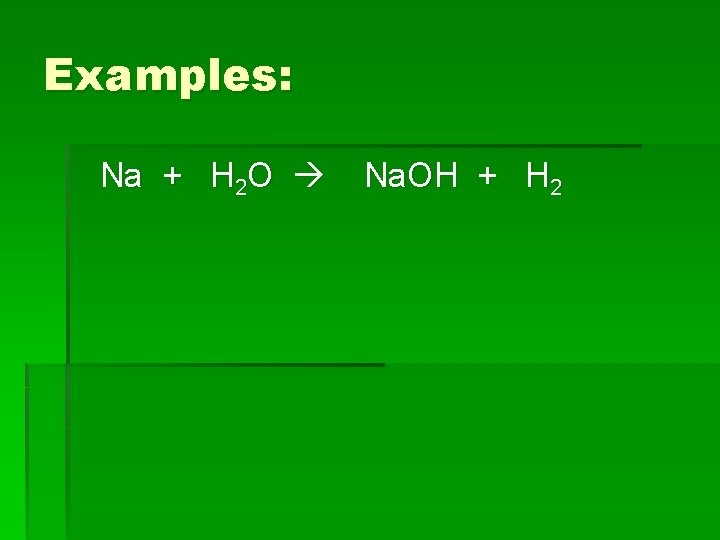
Examples: Na + H 2 O Na. OH + H 2

Examples: Barium chloride reacts with sulfuric acid to produce barium sulfate and hydrochloric acid.

Examples: Hydrogen peroxide (H O ) decomposes to 2 2 produce oxygen and dihydrogen monoxide.

Examples: Iron (II) phosphate and lithium sulfide yields lithium phosphate and iron (II) sulfide.

More Categories of Reactions: *Reactions can be classified further into 3 categories (in addition to S, D, SR, DR, C) 1) acid - base reaction 2) precipitation reaction 3) reduction - oxidation reaction

Acid - Base Reactions: *Is a type of DOUBLE REPLACEMENT reaction. *Is called Neutralization: The reaction between a traditional acid and base to produce a salt and water.

*Traditional Acid: A compound that STARTS WITH HYDROGEN, and when dissolved in water, provides a H +1 ion. *Traditional Base: A compound that ENDS WITH HYDROXIDE, and when dissolved in water, provides a OH -1 ion. *Salt: A compound consisting of the cation from a base and the anion from an acid. HX (aq) + ZOH (aq) ZX (aq) + H 2 O (l) ( ) + ( )

Acid - Base Reactions: Example: nitrous acid reacts with sodium hydroxide to produce sodium nitrite and water

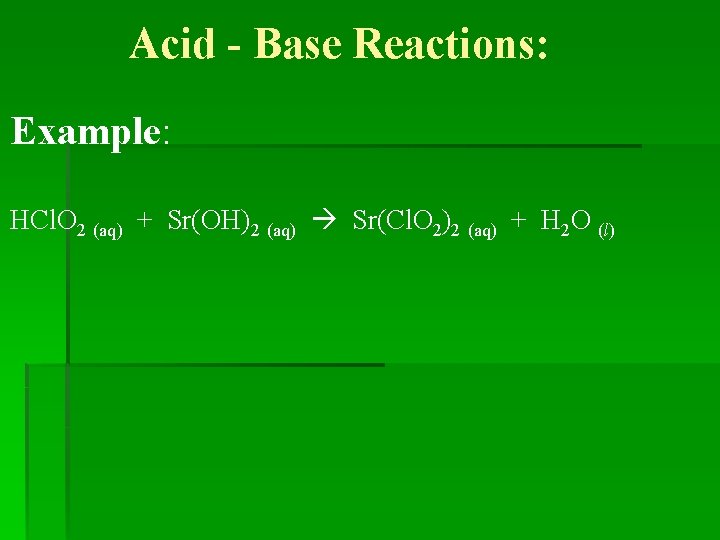
Acid - Base Reactions: Example: HCl. O 2 (aq) + Sr(OH)2 (aq) Sr(Cl. O 2)2 (aq) + H 2 O (l)

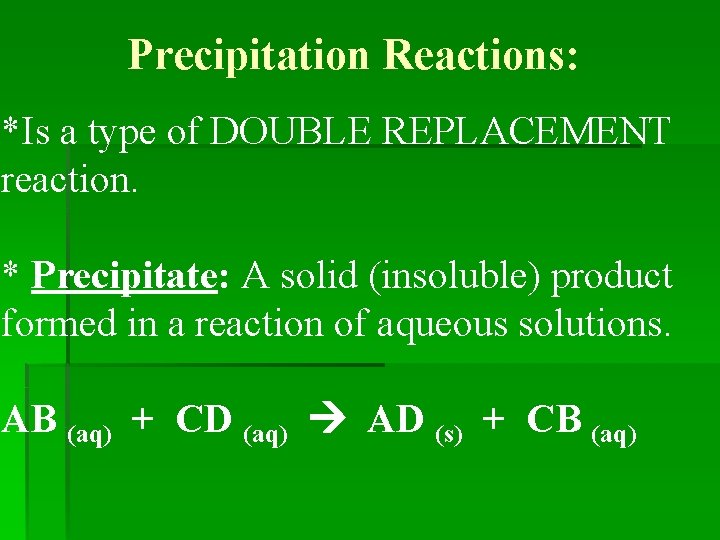
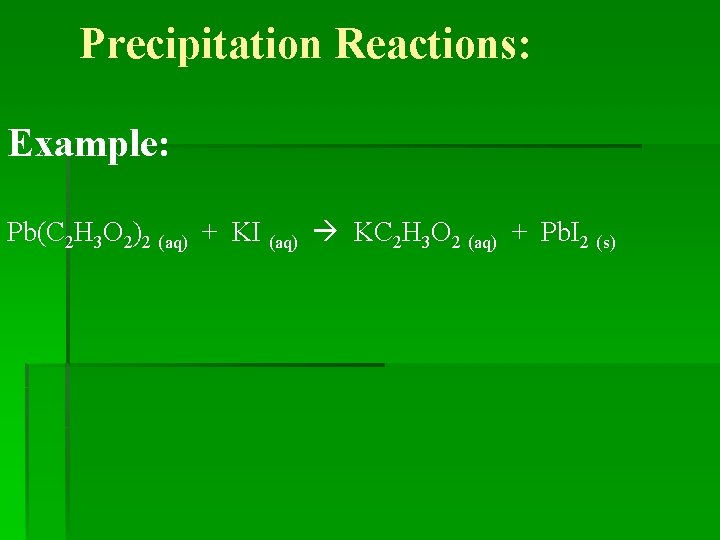
Precipitation Reactions: *Is a type of DOUBLE REPLACEMENT reaction. * Precipitate: A solid (insoluble) product formed in a reaction of aqueous solutions. AB (aq) + CD (aq) AD (s) + CB (aq)

Precipitation Reactions: Example: Pb(C 2 H 3 O 2)2 (aq) + KI (aq) KC 2 H 3 O 2 (aq) + Pb. I 2 (s)

Precipitation Reactions: Example: A calcium chloride solution and a sodium carbonate solution are mixed and solid calcium carbonate and aqueous sodium chloride are produced.

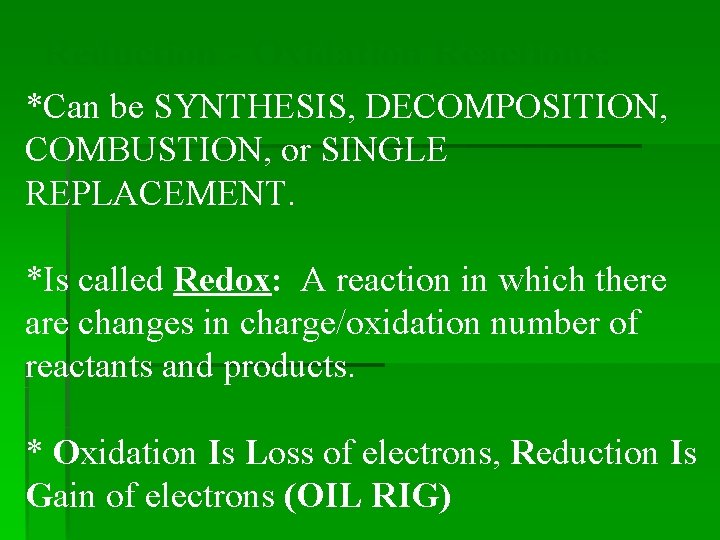
Reduction - Oxidation Reactions: *Can be SYNTHESIS, DECOMPOSITION, COMBUSTION, or SINGLE REPLACEMENT. *Is called Redox: A reaction in which there are changes in charge/oxidation number of reactants and products. * Oxidation Is Loss of electrons, Reduction Is Gain of electrons (OIL RIG)

Reduction - Oxidation Reactions: * For a Red-Ox reaction to be valid, both reduction and oxidation must occur. *When Oxidation occurs, the charge becomes more positive if you compare left to right side. *When Reduction occurs, the charge becomes more negative if you compare left to right side.

Reduction - Oxidation Reactions: *Basic rules for assigning oxidation numbers: 1) the charge/oxidation number on all elements (or diatomic elements) is zero. 2) for any monatomic ion, the oxidation number is the same as the charge on the ion. 3) the sum of the oxidation numbers in an ionic or molecular compound is zero. (this is why compounds are neutral)

Reduction - Oxidation Reactions: *HINT: The easiest way to recognize a Red-Ox reaction is if a substance is elemental (or diatomic) on one side and part of a compound on the other side of the reaction arrow.

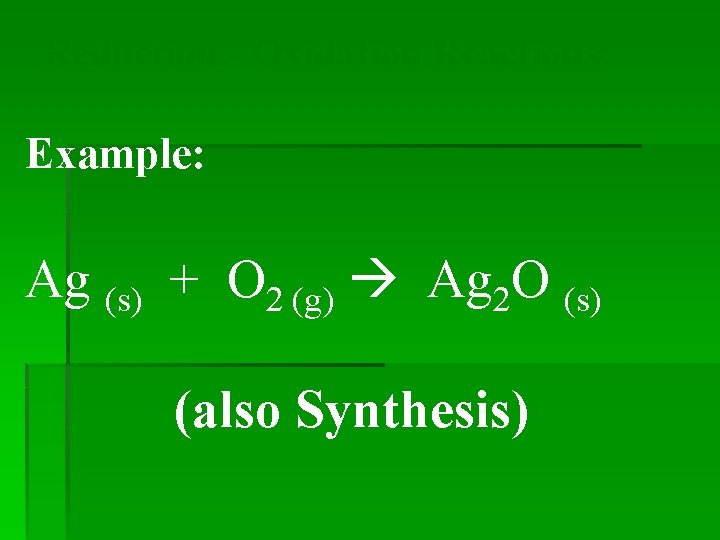
Reduction - Oxidation Reactions: Example: Ag (s) + O 2 (g) Ag 2 O (s) (also Synthesis)

Reduction - Oxidation Reactions: Example: Water, when electrolyzed, produces hydrogen and oxygen (also Decomposition)

Reduction - Oxidation Reactions: Example: Zn (s) + H 2 SO 4 (aq) Zn. SO 4 (aq) + H 2 (g) (also Single Replacement)

Reduction - Oxidation Reactions: Example: Methane burns in oxygen to produce carbon dioxide and water (also Combustion)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 4: toxins lesson 73 worksheet answers
Unit 4: toxins lesson 73 worksheet answers Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Examples of chemical change
Examples of chemical change Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations Section 1 chemical changes
Section 1 chemical changes Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Translating chemical equations
Translating chemical equations He must become greater; i must become less
He must become greater; i must become less Larry made this picture to represent a chemical reaction
Larry made this picture to represent a chemical reaction Stoichiometry island diagram
Stoichiometry island diagram Different types of redox reactions
Different types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Chemical reaction types
Chemical reaction types Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions Chemistry predicting products
Chemistry predicting products Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life 5 general types of chemical reactions
5 general types of chemical reactions Reactants and products
Reactants and products