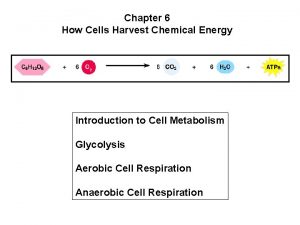
Chapter 6 How Cells Harvest Chemical Energy Power






- Slides: 83

Chapter 6 How Cells Harvest Chemical Energy Power. Point Lectures for Campbell Biology: Concepts & Connections, Seventh Edition Reece, Taylor, Simon, and Dickey © 2012 Pearson Education, Inc. Lecture by Edward J. Zalisko

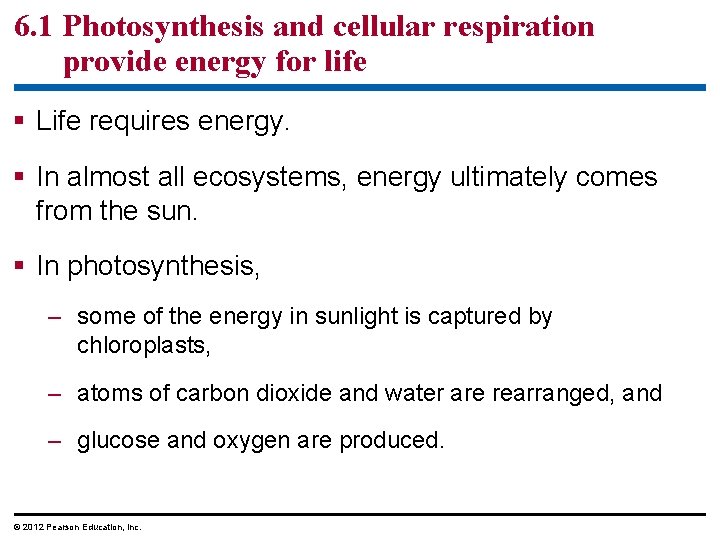
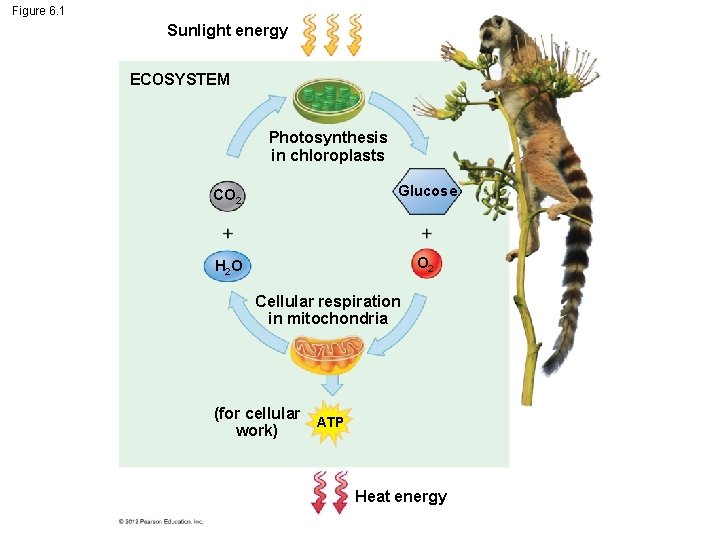
6. 1 Photosynthesis and cellular respiration provide energy for life Life requires energy. In almost all ecosystems, energy ultimately comes from the sun. In photosynthesis, – some of the energy in sunlight is captured by chloroplasts, – atoms of carbon dioxide and water are rearranged, and – glucose and oxygen are produced. © 2012 Pearson Education, Inc.

Cellular Respiration Process that allows – harvesting energy from food, – yields large amounts of ATP, and – Uses ATP to drive cellular work. © 2012 Pearson Education, Inc.

6. 1 Photosynthesis and cellular respiration provide energy for life In cellular respiration – glucose is broken down to carbon dioxide and water and – the cell captures some of the released energy to make ATP. Cellular respiration takes place in the mitochondria of eukaryotic cells. © 2012 Pearson Education, Inc.

Figure 6. 0_1 Chapter 6: Big Ideas Cellular Respiration: Aerobic Harvesting of Energy Fermentation: Anaerobic Harvesting of Energy Stages of Cellular Respiration Connections Between Metabolic Pathways

Figure 6. 0_2

CELLULAR RESPIRATION: AEROBIC HARVESTING OF ENERGY © 2012 Pearson Education, Inc.

Figure 6. 1 Sunlight energy ECOSYSTEM Photosynthesis in chloroplasts CO 2 Glucose H 2 O O 2 Cellular respiration in mitochondria (for cellular work) ATP Heat energy

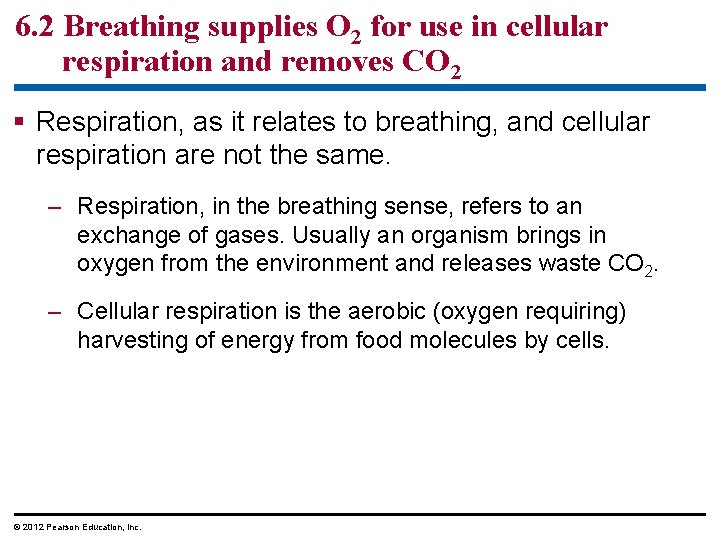
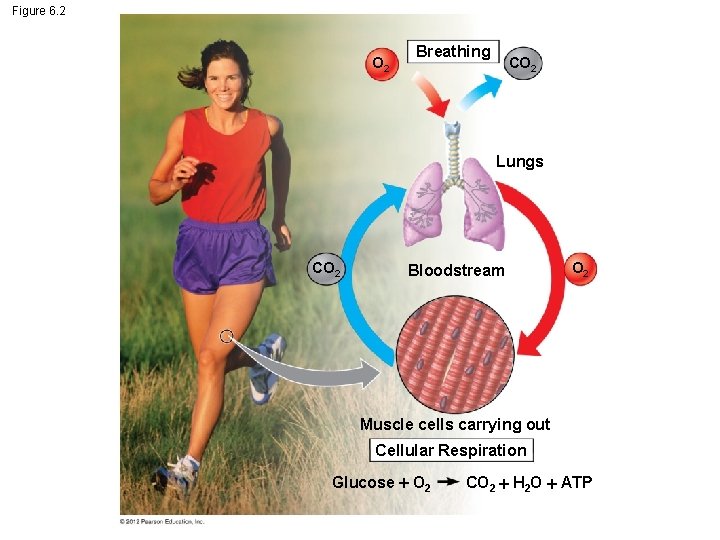
6. 2 Breathing supplies O 2 for use in cellular respiration and removes CO 2 Respiration, as it relates to breathing, and cellular respiration are not the same. – Respiration, in the breathing sense, refers to an exchange of gases. Usually an organism brings in oxygen from the environment and releases waste CO 2. – Cellular respiration is the aerobic (oxygen requiring) harvesting of energy from food molecules by cells. © 2012 Pearson Education, Inc.

Figure 6. 2 O 2 Breathing CO 2 Lungs CO 2 Bloodstream O 2 Muscle cells carrying out Cellular Respiration Glucose O 2 CO 2 H 2 O ATP

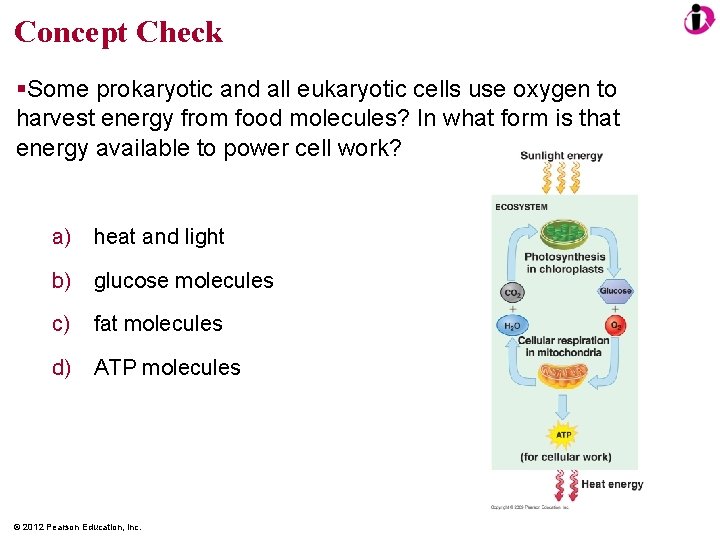
Concept Check Some prokaryotic and all eukaryotic cells use oxygen to harvest energy from food molecules? In what form is that energy available to power cell work? a) heat and light b) glucose molecules c) fat molecules d) ATP molecules © 2012 Pearson Education, Inc.

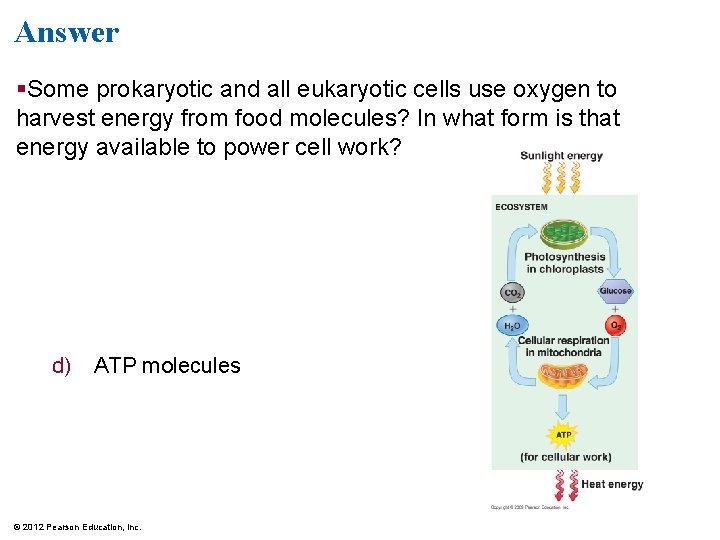
Answer Some prokaryotic and all eukaryotic cells use oxygen to harvest energy from food molecules? In what form is that energy available to power cell work? d) ATP molecules © 2012 Pearson Education, Inc.

6. 3 Cellular respiration banks energy in ATP molecules Cellular respiration is an exergonic process that transfers energy from the bonds in glucose to form ATP. Cellular respiration – produces up to 32 ATP molecules from each glucose molecule and – captures only about 34% of the energy originally stored in glucose. Other foods (organic molecules) can also be used as a source of energy. © 2012 Pearson Education, Inc.

6. 4 CONNECTION: The human body uses energy from ATP for all its activities The average adult human needs about 2, 200 kcal of energy per day. – About 75% of these calories are used to maintain a healthy body. – The remaining 25% is used to power physical activities. © 2012 Pearson Education, Inc.

6. 4 CONNECTION: The human body uses energy from ATP for all its activities A kilocalorie (kcal) is – the quantity of heat required to raise the temperature of 1 kilogram (kg) of water by 1 o. C, – the same as a food Calorie, and – used to measure the nutritional values indicated on food labels. © 2012 Pearson Education, Inc.

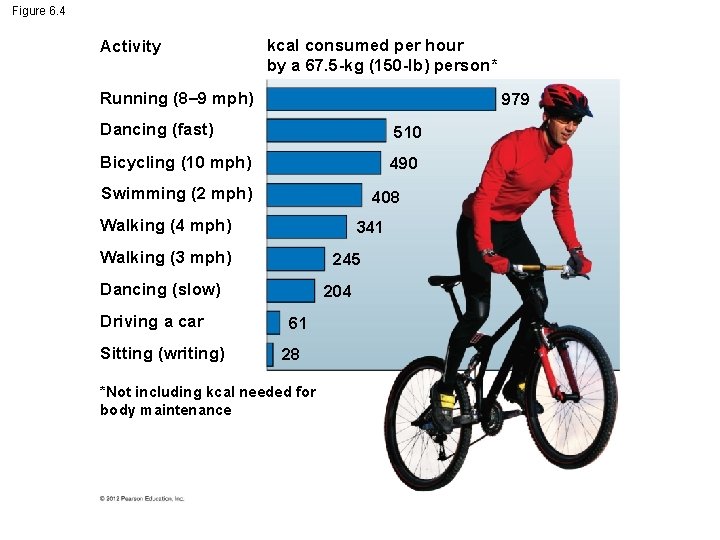
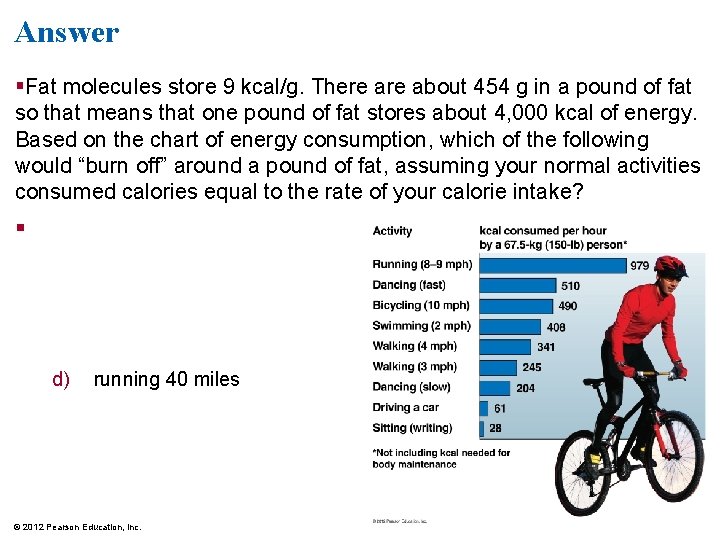
Figure 6. 4 Activity kcal consumed per hour by a 67. 5 -kg (150 -lb) person* Running (8– 9 mph) 979 Dancing (fast) 510 Bicycling (10 mph) 490 Swimming (2 mph) 408 Walking (4 mph) 341 Walking (3 mph) 245 Dancing (slow) Driving a car Sitting (writing) 204 61 28 *Not including kcal needed for body maintenance

Concept Check Fat molecules store 9 kcal/g. There about 454 g in a pound of fat so that means that one pound of fat stores about 4, 000 kcal of energy. Based on the chart of energy consumption, which of the following would “burn off” around a pound of fat, assuming your normal activities consumed calories equal to the rate of your calorie intake? a) running 7 miles b) swimming 2 miles c) walking 27 miles (3 miles per hour) d) running 40 miles © 2012 Pearson Education, Inc.

Answer Fat molecules store 9 kcal/g. There about 454 g in a pound of fat so that means that one pound of fat stores about 4, 000 kcal of energy. Based on the chart of energy consumption, which of the following would “burn off” around a pound of fat, assuming your normal activities consumed calories equal to the rate of your calorie intake? d) running 40 miles © 2012 Pearson Education, Inc.

6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen The energy necessary for life is contained in the arrangement of electrons in chemical bonds in organic molecules. An important question is how do cells extract this energy? © 2012 Pearson Education, Inc.

6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen When the carbon-hydrogen bonds of glucose are broken, electrons are transferred to oxygen. – Oxygen has a strong tendency to attract electrons. – An electron loses potential energy when it “falls” to oxygen. Energy can be released from glucose by simply burning it. The energy is dissipated as heat and light and is not available to living organisms. © 2012 Pearson Education, Inc.

6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen On the other hand, cellular respiration is the controlled breakdown of organic molecules. Energy is – gradually released in small amounts, – captured by a biological system, and – stored in ATP. © 2012 Pearson Education, Inc.

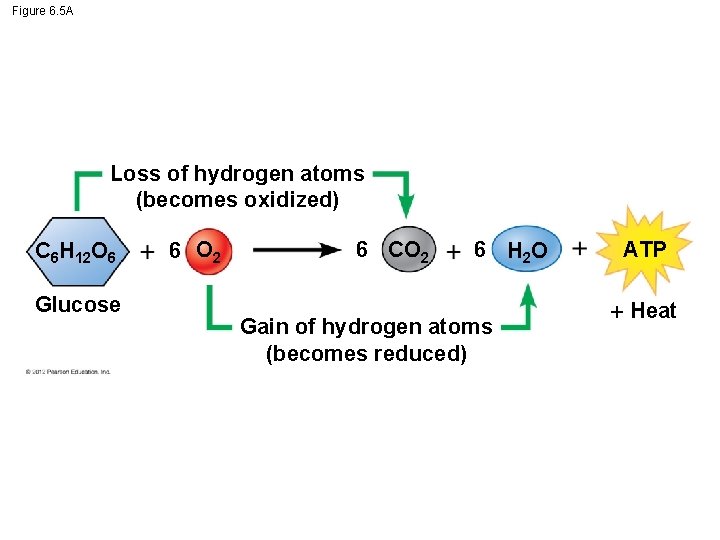
6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen The movement of electrons from one molecule to another is an oxidation-reduction reaction, or redox reaction. In a redox reaction, – the loss of electrons from one substance is called oxidation, – the addition of electrons to another substance is called reduction, – a molecule is oxidized when it loses one or more electrons, and – reduced when it gains one or more electrons. © 2012 Pearson Education, Inc.

Figure 6. 5 A Loss of hydrogen atoms (becomes oxidized) C 6 H 12 O 6 Glucose 6 O 2 6 CO 2 6 H 2 O Gain of hydrogen atoms (becomes reduced) ATP Heat

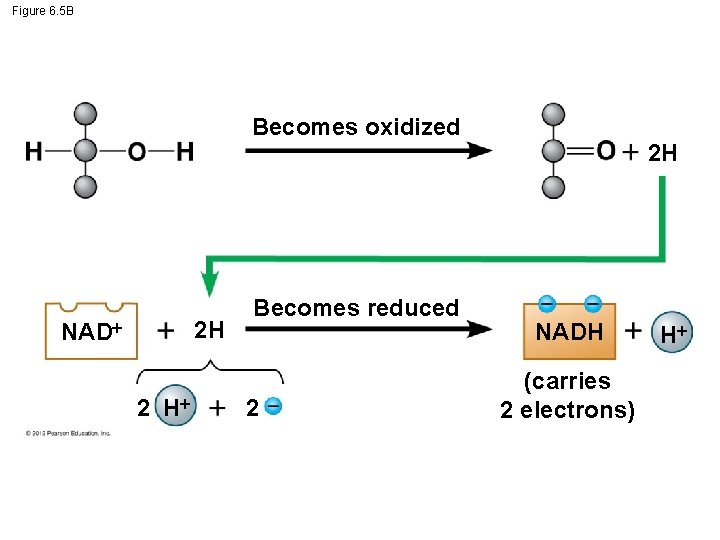
6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen Enzymes are necessary to oxidize glucose and other foods. NAD+ – is an important enzyme in oxidizing glucose, – accepts electrons, and – becomes reduced to NADH. © 2012 Pearson Education, Inc.

Figure 6. 5 B Becomes oxidized NAD 2 H 2 H Becomes reduced 2 2 H NADH (carries 2 electrons) H

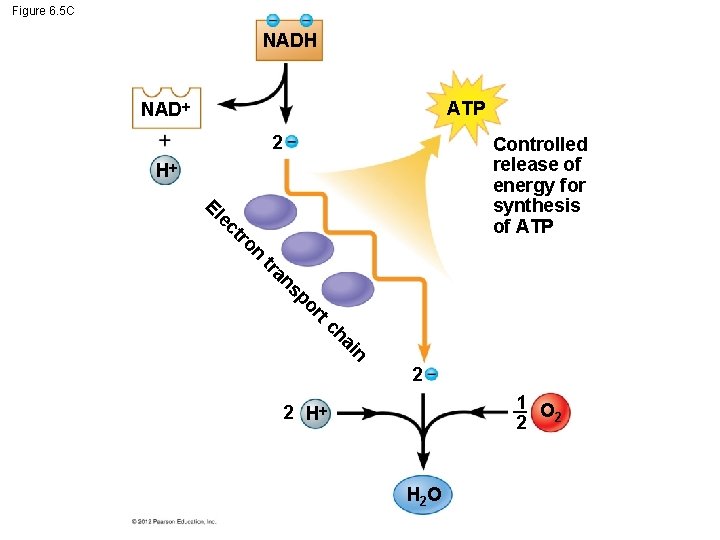
6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen There are other electron “carrier” molecules that function like NAD+. – They form a staircase where the electrons pass from one to the next down the staircase. – These electron carriers collectively are called the electron transport chain. – As electrons are transported down the chain, ATP is generated. © 2012 Pearson Education, Inc.

Figure 6. 5 C NADH ATP NAD 2 Controlled release of energy for synthesis of ATP H El ec t ro n tr an sp or tc ha in 2 1 O 2 2 2 H H 2 O

STAGES OF CELLULAR RESPIRATION © 2012 Pearson Education, Inc.

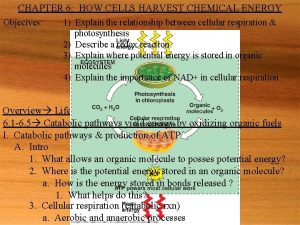
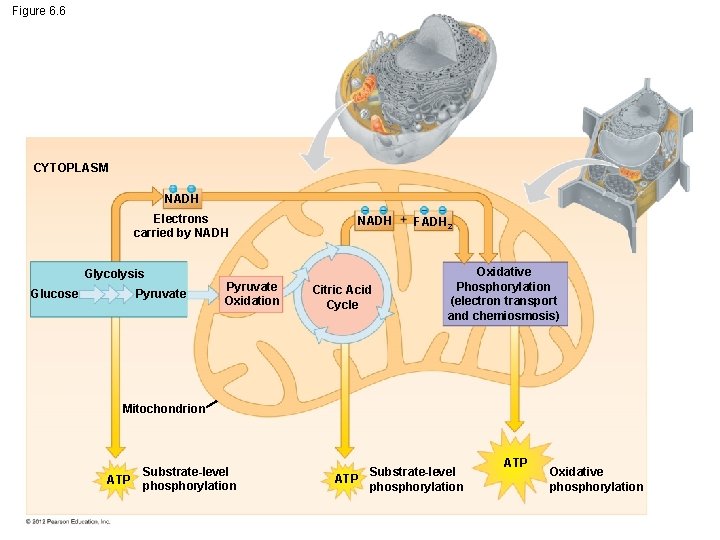
6. 6 Overview: Cellular respiration occurs in three main stages Cellular respiration consists of a sequence of steps that can be divided into three stages. – Stage 1 – Glycolysis – Stage 2 – Pyruvate oxidation and citric acid cycle – Stage 3 – Oxidative phosphorylation © 2012 Pearson Education, Inc.

Figure 6. 6 CYTOPLASM NADH Electrons carried by NADH Glycolysis Glucose Pyruvate Oxidation NADH Citric Acid Cycle FADH 2 Oxidative Phosphorylation (electron transport and chemiosmosis) Mitochondrion ATP Substrate-level phosphorylation Substrate-level ATP phosphorylation ATP Oxidative phosphorylation

6. 6 Overview: Cellular respiration occurs in three main stages Stage 1: Glycolysis – occurs in the cytoplasm, – begins cellular respiration, and – breaks down glucose into two molecules of a threecarbon compound called pyruvate. © 2012 Pearson Education, Inc.

6. 6 Overview: Cellular respiration occurs in three main stages Stage 2: The citric acid cycle – takes place in mitochondria, – oxidizes pyruvate to a two-carbon compound, and – supplies the third stage with electrons. © 2012 Pearson Education, Inc.

6. 6 Overview: Cellular respiration occurs in three main stages Stage 3: Oxidative phosphorylation – involves electrons carried by NADH and FADH 2, – shuttles these electrons to the electron transport chain embedded in the inner mitochondrial membrane, – involves chemiosmosis, and – generates ATP through oxidative phosphorylation associated with chemiosmosis. © 2012 Pearson Education, Inc.

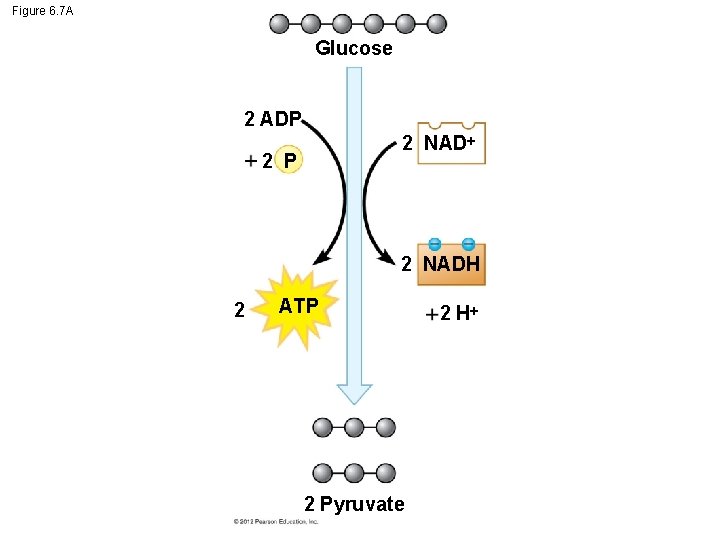
Figure 6. 7 A Glucose 2 ADP 2 NAD 2 P 2 NADH 2 ATP 2 Pyruvate 2 H

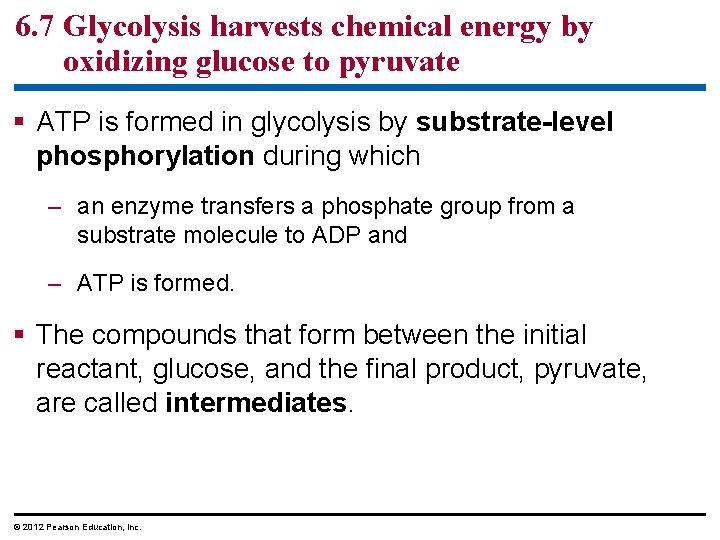
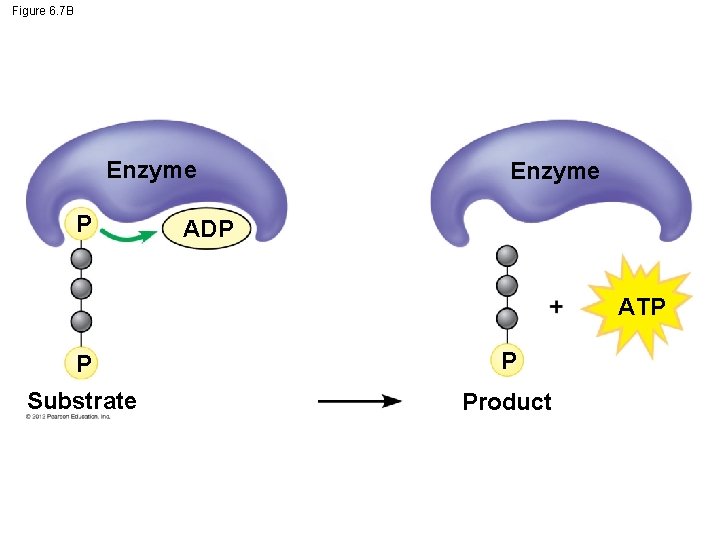
6. 7 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate ATP is formed in glycolysis by substrate-level phosphorylation during which – an enzyme transfers a phosphate group from a substrate molecule to ADP and – ATP is formed. The compounds that form between the initial reactant, glucose, and the final product, pyruvate, are called intermediates. © 2012 Pearson Education, Inc.

Figure 6. 7 B Enzyme P Enzyme ADP ATP P P Substrate Product

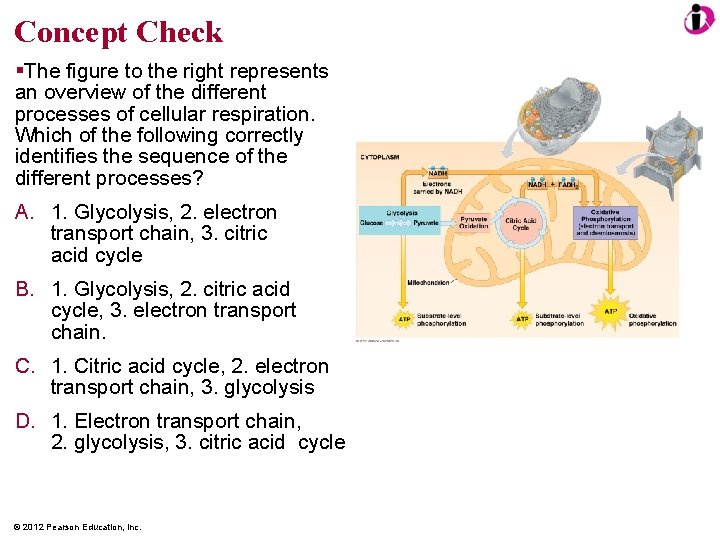
Concept Check The figure to the right represents an overview of the different processes of cellular respiration. Which of the following correctly identifies the sequence of the different processes? A. 1. Glycolysis, 2. electron transport chain, 3. citric acid cycle B. 1. Glycolysis, 2. citric acid cycle, 3. electron transport chain. C. 1. Citric acid cycle, 2. electron transport chain, 3. glycolysis D. 1. Electron transport chain, 2. glycolysis, 3. citric acid cycle © 2012 Pearson Education, Inc.

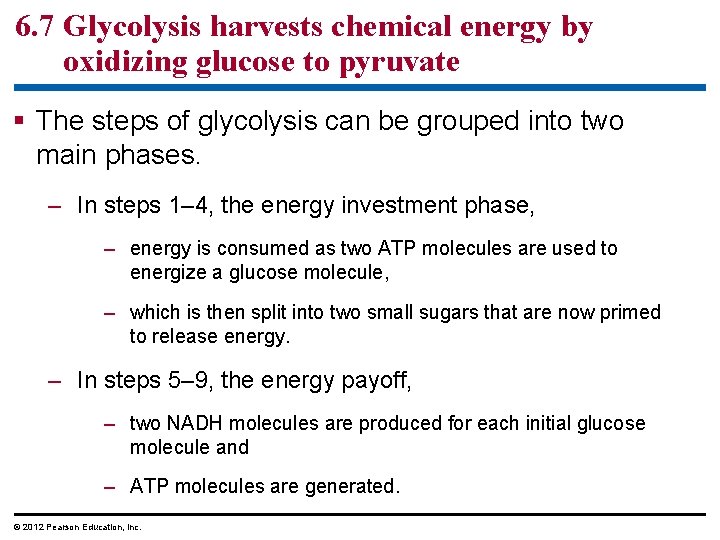
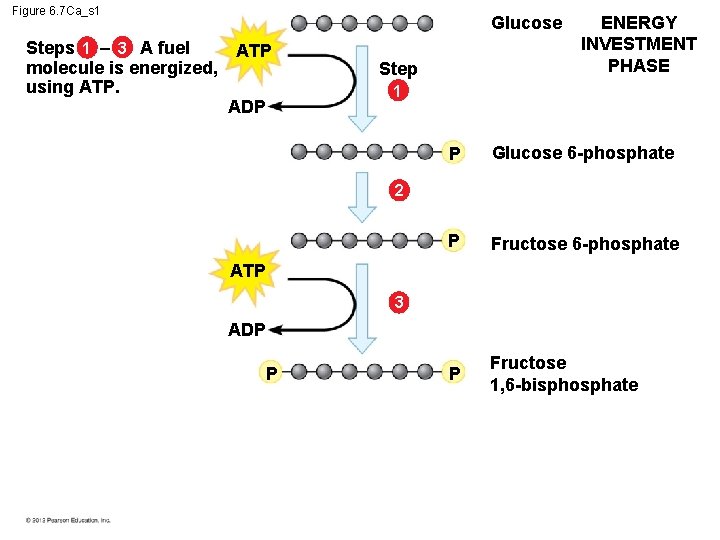
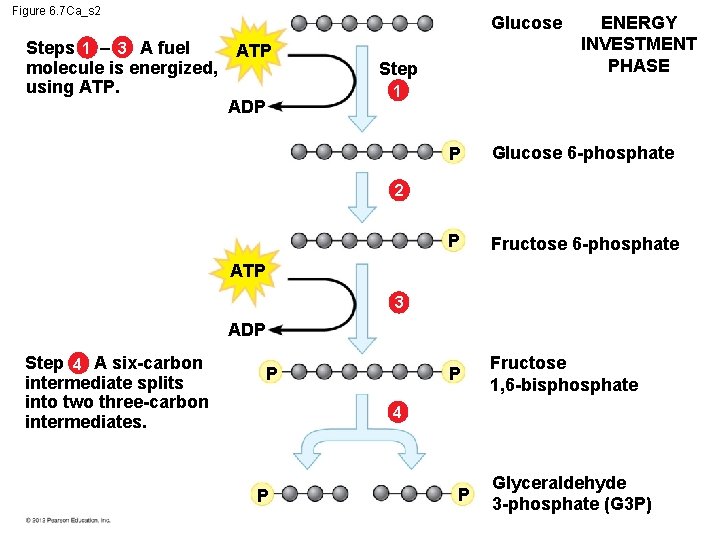
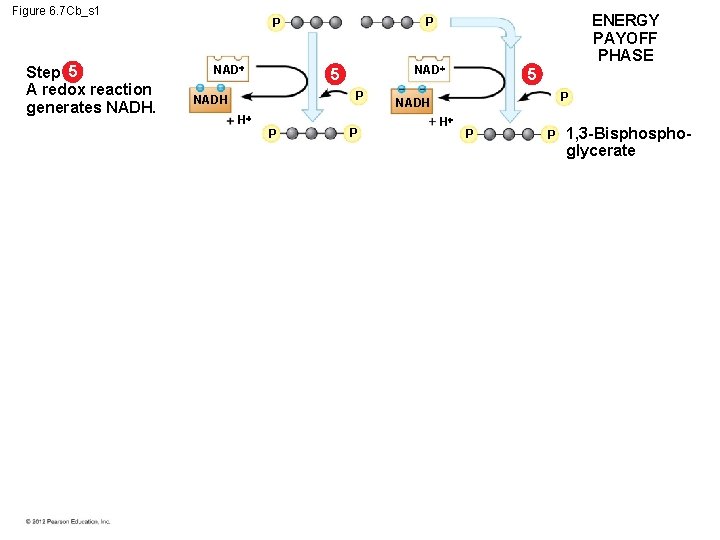
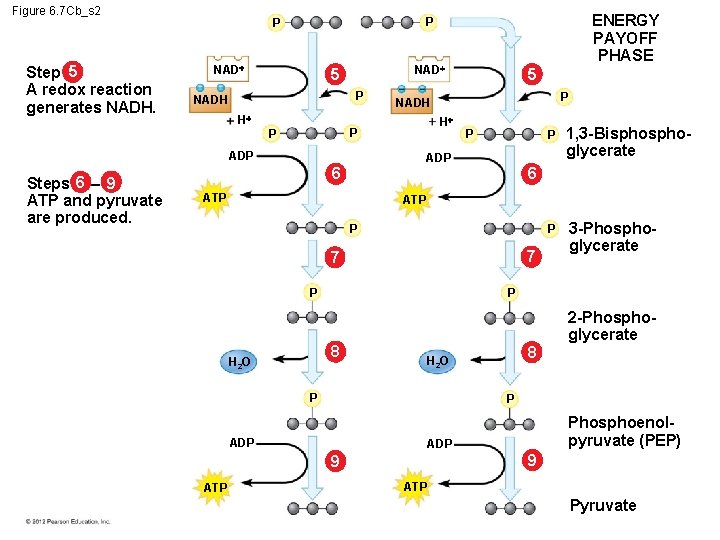
6. 7 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate The steps of glycolysis can be grouped into two main phases. – In steps 1– 4, the energy investment phase, – energy is consumed as two ATP molecules are used to energize a glucose molecule, – which is then split into two small sugars that are now primed to release energy. – In steps 5– 9, the energy payoff, – two NADH molecules are produced for each initial glucose molecule and – ATP molecules are generated. © 2012 Pearson Education, Inc.

Figure 6. 7 Ca_s 1 Steps 1 – 3 A fuel molecule is energized, using ATP. Glucose ATP ADP Step ENERGY INVESTMENT PHASE 1 P Glucose 6 -phosphate P Fructose 1, 6 -bisphosphate 2 ATP 3 ADP P

Figure 6. 7 Ca_s 2 Steps 1 – 3 A fuel molecule is energized, using ATP. Glucose ATP ADP Step ENERGY INVESTMENT PHASE 1 P Glucose 6 -phosphate P Fructose 1, 6 -bisphosphate 2 ATP 3 ADP Step 4 A six-carbon intermediate splits into two three-carbon intermediates. P 4 P P Glyceraldehyde 3 -phosphate (G 3 P)

Figure 6. 7 Cb_s 1 Step 5 A redox reaction generates NADH. NAD 5 P NADH H P ENERGY PAYOFF PHASE P P P 5 P NADH H P P 1, 3 -Bisphoglycerate

Figure 6. 7 Cb_s 2 Step 5 A redox reaction generates NADH. NAD 5 P NADH H ADP 5 P NADH H P P Steps 6 – 9 ATP and pyruvate are produced. ENERGY PAYOFF PHASE P P P 1, 3 -Bisphoglycerate P 3 -Phosphoglycerate ADP 6 6 ATP P 7 7 P P 8 H 2 O P 2 -Phosphoglycerate P ADP Phosphoenolpyruvate (PEP) ADP 9 9 ATP Pyruvate

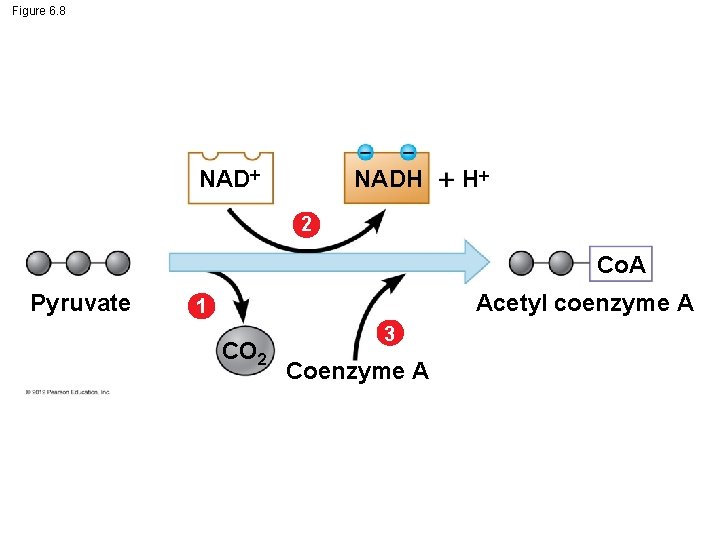
6. 8 Pyruvate is oxidized prior to the citric acid cycle Two molecules of pyruvate are produced for each molecule of glucose that enters glycolysis. Pyruvate does not enter the citric acid cycle, but undergoes some chemical grooming in which – a carboxyl group is removed and given off as CO 2, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH, – coenzyme A joins with the two-carbon group to form acetyl coenzyme A, abbreviated as acetyl Co. A, and – acetyl Co. A enters the citric acid cycle. © 2012 Pearson Education, Inc.

Figure 6. 8 NADH H 2 Co. A Pyruvate Acetyl coenzyme A 1 CO 2 3 Coenzyme A

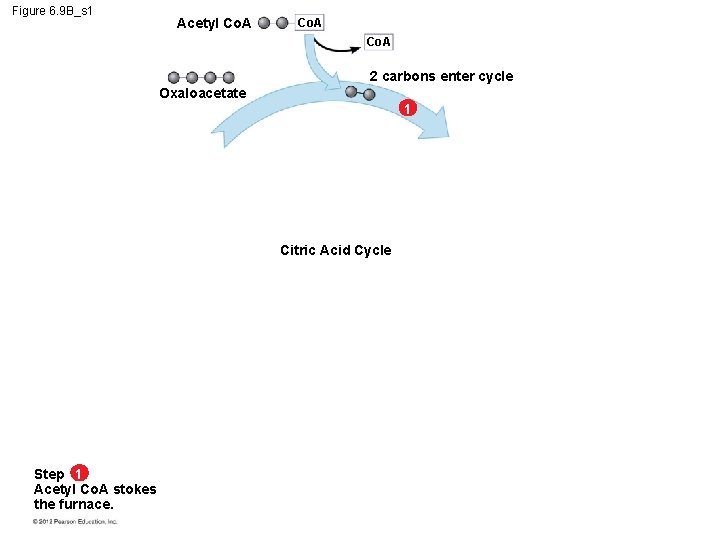
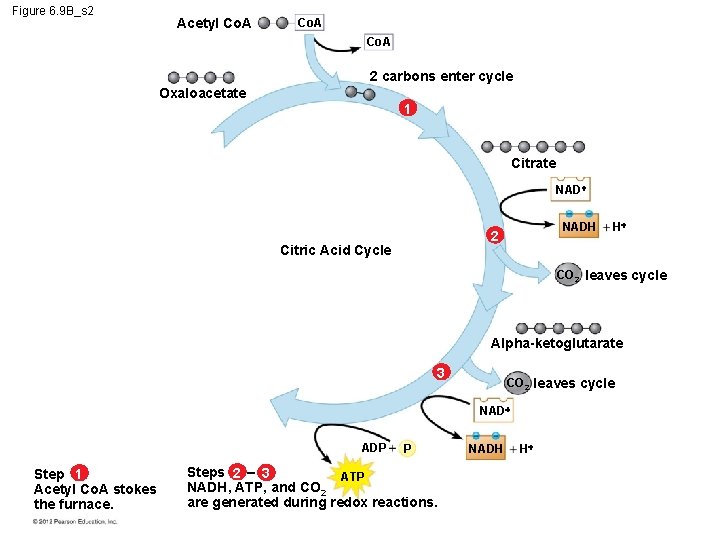
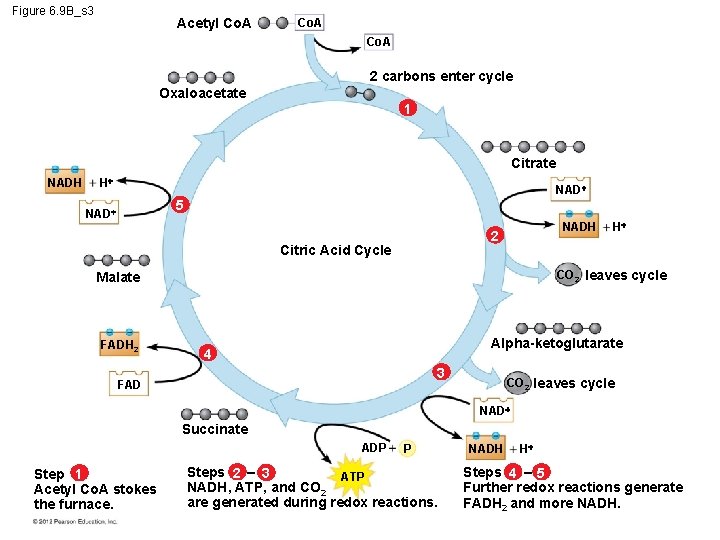
6. 9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH 2 molecules The citric acid cycle – is also called the Krebs cycle (after the German-British researcher Hans Krebs, who worked out much of this pathway in the 1930 s), – completes the oxidation of organic molecules, and – generates many NADH and FADH 2 molecules. © 2012 Pearson Education, Inc.

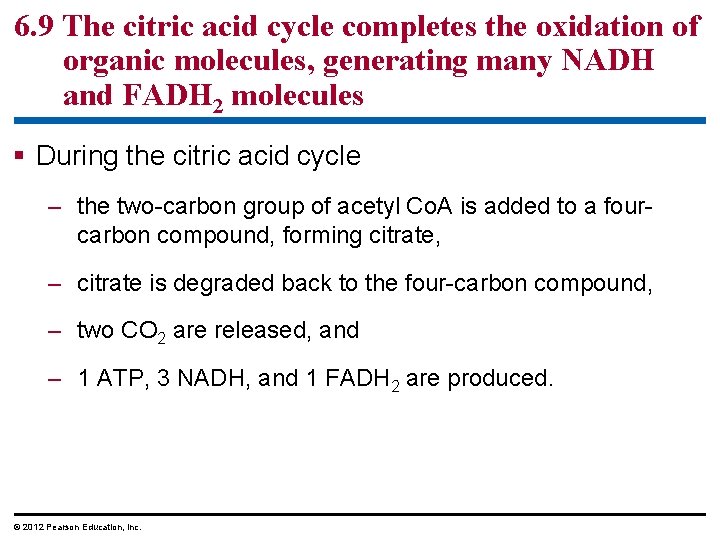
6. 9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH 2 molecules During the citric acid cycle – the two-carbon group of acetyl Co. A is added to a fourcarbon compound, forming citrate, – citrate is degraded back to the four-carbon compound, – two CO 2 are released, and – 1 ATP, 3 NADH, and 1 FADH 2 are produced. © 2012 Pearson Education, Inc.

6. 9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH 2 molecules Remember that the citric acid cycle processes two molecules of acetyl Co. A for each initial glucose. Thus, after two turns of the citric acid cycle, the overall yield per glucose molecule is – 2 ATP, – 6 NADH, and – 2 FADH 2. © 2012 Pearson Education, Inc.

Figure 6. 9 B_s 1 Acetyl Co. A 2 carbons enter cycle Oxaloacetate 1 Citric Acid Cycle Step 1 Acetyl Co. A stokes the furnace.

Figure 6. 9 B_s 2 Acetyl Co. A 2 carbons enter cycle Oxaloacetate 1 Citrate NADH 2 Citric Acid Cycle H CO 2 leaves cycle Alpha-ketoglutarate 3 CO 2 leaves cycle NAD ADP Step 1 Acetyl Co. A stokes the furnace. P Steps 2 – 3 ATP NADH, ATP, and CO 2 are generated during redox reactions. NADH H

Figure 6. 9 B_s 3 Acetyl Co. A 2 carbons enter cycle Oxaloacetate 1 Citrate NADH H NAD 5 NADH 2 Citric Acid Cycle CO 2 leaves cycle Malate FADH 2 H Alpha-ketoglutarate 4 3 FAD CO 2 leaves cycle NAD Succinate ADP Step 1 Acetyl Co. A stokes the furnace. P Steps 2 – 3 ATP NADH, ATP, and CO 2 are generated during redox reactions. NADH H Steps 4 – 5 Further redox reactions generate FADH 2 and more NADH.

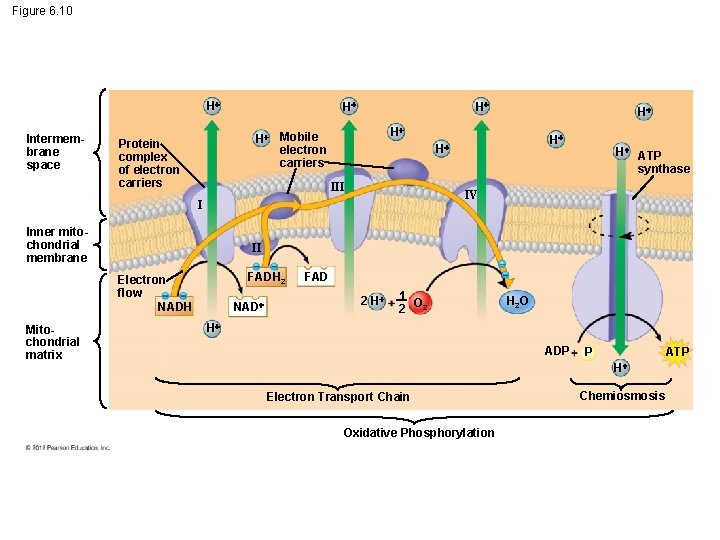
6. 10 Most ATP production occurs by oxidative phosphorylation Oxidative phosphorylation – involves electron transport and chemiosmosis and – requires an adequate supply of oxygen. © 2012 Pearson Education, Inc.

6. 10 Most ATP production occurs by oxidative phosphorylation Electrons from NADH and FADH 2 travel down the electron transport chain to O 2. Oxygen picks up H+ to form water. Energy released by these redox reactions is used to pump H+ from the mitochondrial matrix into the intermembrane space. In chemiosmosis, the H+ diffuses back across the inner membrane through ATP synthase complexes, driving the synthesis of ATP. © 2012 Pearson Education, Inc.

Figure 6. 10 H Intermembrane space H H Mobile electron carriers Protein complex of electron carriers H ATP synthase IV I II FADH 2 Electron flow NADH Mitochondrial matrix H H III Inner mitochondrial membrane H NAD FAD 2 H 1 2 O 2 H 2 O H ADP P ATP H Electron Transport Chain Oxidative Phosphorylation Chemiosmosis

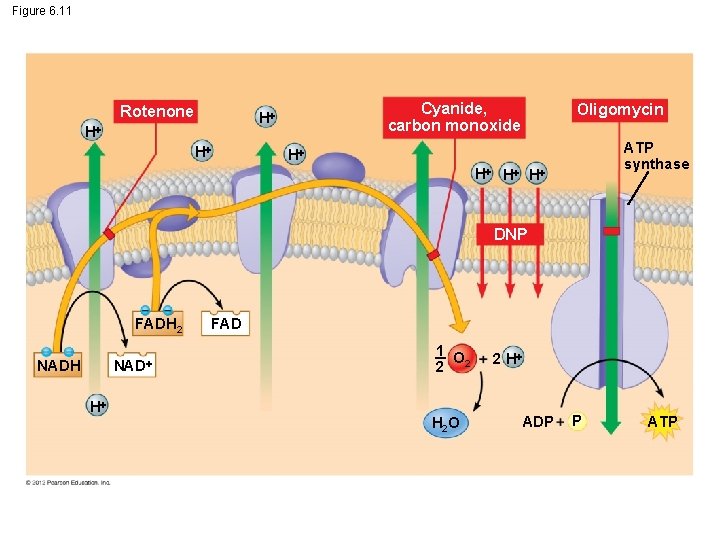
6. 11 CONNECTION: Interrupting cellular respiration can have both harmful and beneficial effects Three categories of cellular poisons obstruct the process of oxidative phosphorylation. These poisons 1. block the electron transport chain (for example, rotenone, cyanide, and carbon monoxide), 2. inhibit ATP synthase (for example, the antibiotic oligomycin), or 3. make the membrane leaky to hydrogen ions (called uncouplers, examples include dinitrophenol). © 2012 Pearson Education, Inc.

Figure 6. 11 Rotenone Cyanide, carbon monoxide H H H Oligomycin ATP synthase H H DNP FADH 2 NADH H FAD 1 O 2 2 H 2 O 2 H ADP P ATP

6. 11 CONNECTION: Interrupting cellular respiration can have both harmful and beneficial effects Brown fat – a special type of tissue associated with the generation of heat and – more abundant in hibernating mammals and newborn infants. – the cells are packed full of mitochondria, – the inner mitochondrial membrane contains an uncoupling protein, which allows H+ to flow back down its concentration gradient without generating ATP, and – ongoing oxidation of stored fats generates additional heat. © 2012 Pearson Education, Inc.

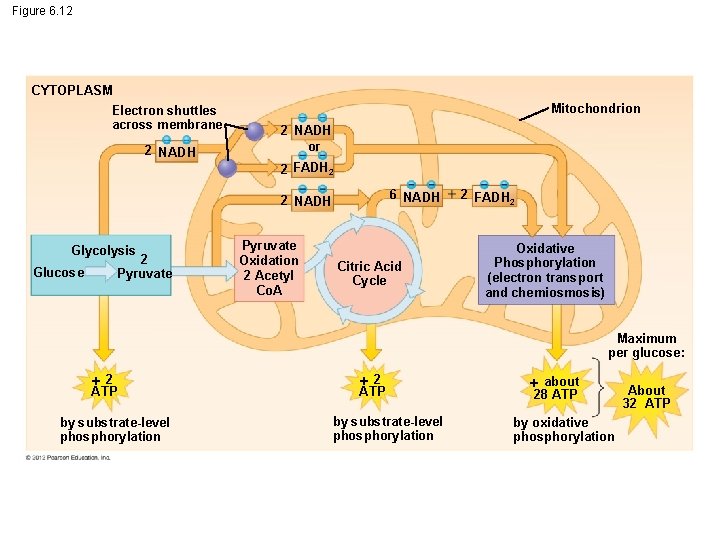
6. 12 Review: Each molecule of glucose yields many molecules of ATP The total yield is about 32 ATP molecules per glucose molecule. This is about 34% of the potential energy of a glucose molecule. In addition, water and CO 2 are produced. © 2012 Pearson Education, Inc.

Figure 6. 12 CYTOPLASM Electron shuttles across membrane 2 NADH Mitochondrion 2 NADH or 2 FADH 2 6 NADH 2 NADH Glycolysis 2 Pyruvate Glucose Pyruvate Oxidation 2 Acetyl Co. A Citric Acid Cycle 2 FADH 2 Oxidative Phosphorylation (electron transport and chemiosmosis) Maximum per glucose: 2 ATP by substrate-level phosphorylation about 28 ATP by oxidative phosphorylation About 32 ATP

FERMENTATION: ANAEROBIC HARVESTING OF ENERGY © 2012 Pearson Education, Inc.

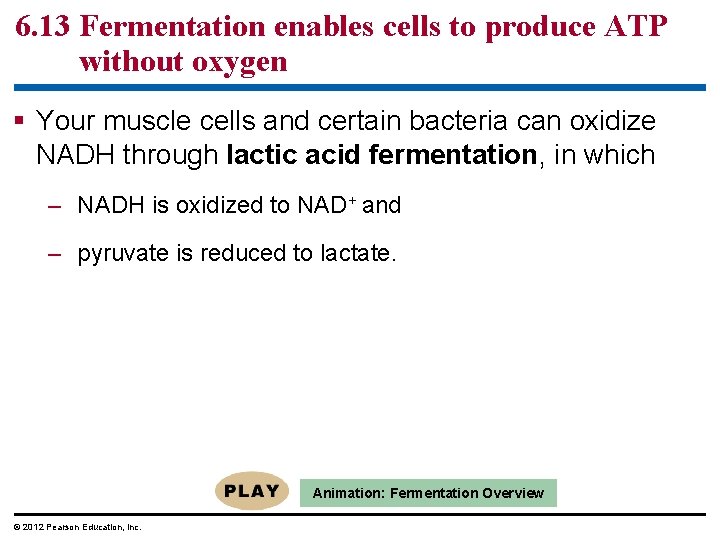
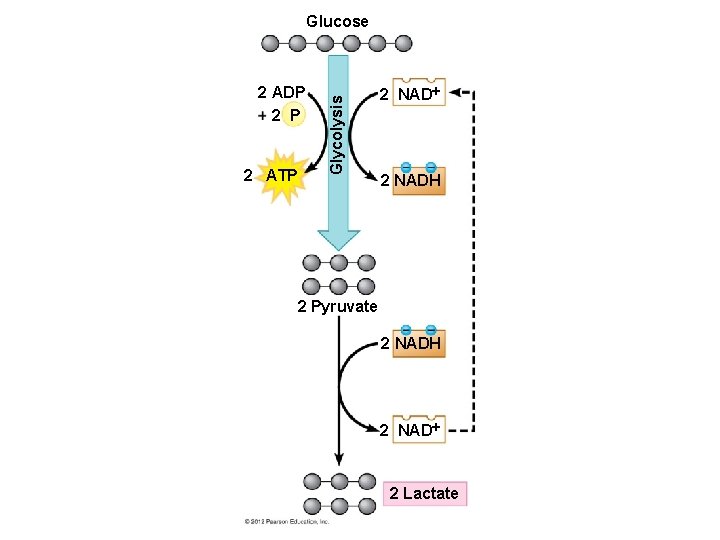
6. 13 Fermentation enables cells to produce ATP without oxygen Fermentation is a way of harvesting chemical energy that does not require oxygen. Fermentation – takes advantage of glycolysis, – produces two ATP molecules per glucose, and – reduces NAD+ to NADH. The trick of fermentation is to provide an anaerobic path for recycling NADH back to NAD+. © 2012 Pearson Education, Inc.

6. 13 Fermentation enables cells to produce ATP without oxygen Your muscle cells and certain bacteria can oxidize NADH through lactic acid fermentation, in which – NADH is oxidized to NAD+ and – pyruvate is reduced to lactate. Animation: Fermentation Overview © 2012 Pearson Education, Inc.

6. 13 Fermentation enables cells to produce ATP without oxygen Lactate is carried by the blood to the liver, where it is converted back to pyruvate and oxidized in the mitochondria of liver cells. The dairy industry uses lactic acid fermentation by bacteria to make cheese and yogurt. Other types of microbial fermentation turn – soybeans into soy sauce and – cabbage into sauerkraut. © 2012 Pearson Education, Inc.

2 ADP 2 ATP Glycolysis Glucose 2 NADH 2 Pyruvate 2 NADH 2 NAD 2 Lactate

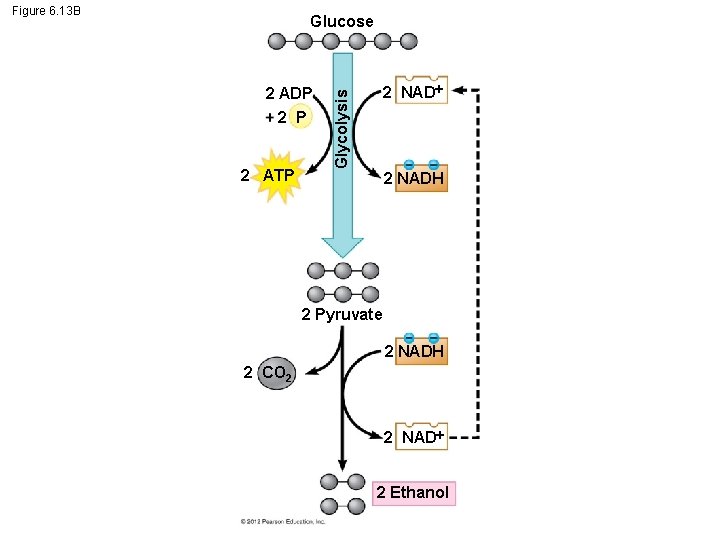
6. 13 Fermentation enables cells to produce ATP without oxygen The baking and winemaking industries have used alcohol fermentation for thousands of years. In this process yeasts (single-celled fungi) – oxidize NADH back to NAD+ and – convert pyruvate to CO 2 and ethanol. © 2012 Pearson Education, Inc.

Figure 6. 13 B Glucose 2 ATP 2 NAD Glycolysis 2 ADP 2 NADH 2 Pyruvate 2 NADH 2 CO 2 2 NAD 2 Ethanol

6. 13 Fermentation enables cells to produce ATP without oxygen Obligate anaerobes – are poisoned by oxygen, requiring anaerobic conditions, and – live in stagnant ponds and deep soils. Facultative anaerobes – include yeasts and many bacteria, and – can make ATP by fermentation or oxidative phosphorylation. © 2012 Pearson Education, Inc.

6. 14 EVOLUTION CONNECTION: Glycolysis evolved early in the history of life on Earth Glycolysis is the universal energy-harvesting process of life. The role of glycolysis in fermentation and respiration dates back to – life long before oxygen was present, – when only prokaryotes inhabited the Earth, – about 3. 5 billion years ago. © 2012 Pearson Education, Inc.

6. 14 EVOLUTION CONNECTION: Glycolysis evolved early in the history of life on Earth The ancient history of glycolysis is supported by its – occurrence in all the domains of life and – location within the cell, using pathways that do not involve any membrane-bounded organelles. © 2012 Pearson Education, Inc.

CONNECTIONS BETWEEN METABOLIC PATHWAYS © 2012 Pearson Education, Inc.

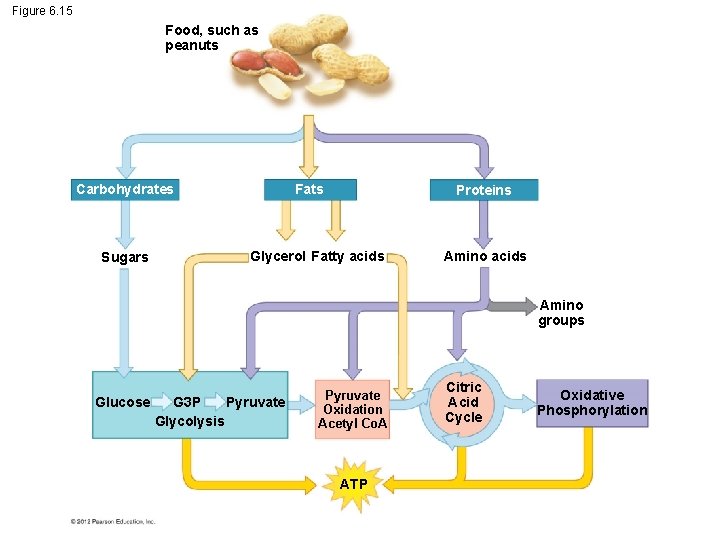
6. 15 Cells use many kinds of organic molecules as fuel for cellular respiration Although glucose is considered to be the primary source of sugar for respiration and fermentation, ATP is generated using – carbohydrates, – fats, and – proteins. © 2012 Pearson Education, Inc.

6. 15 Cells use many kinds of organic molecules as fuel for cellular respiration Fats make excellent cellular fuel because they – contain many hydrogen atoms and thus many energyrich electrons and – yield more than twice as much ATP per gram than a gram of carbohydrate or protein. © 2012 Pearson Education, Inc.

Figure 6. 15 Food, such as peanuts Carbohydrates Sugars Fats Proteins Glycerol Fatty acids Amino groups Glucose G 3 P Pyruvate Glycolysis Pyruvate Oxidation Acetyl Co. A ATP Citric Acid Cycle Oxidative Phosphorylation

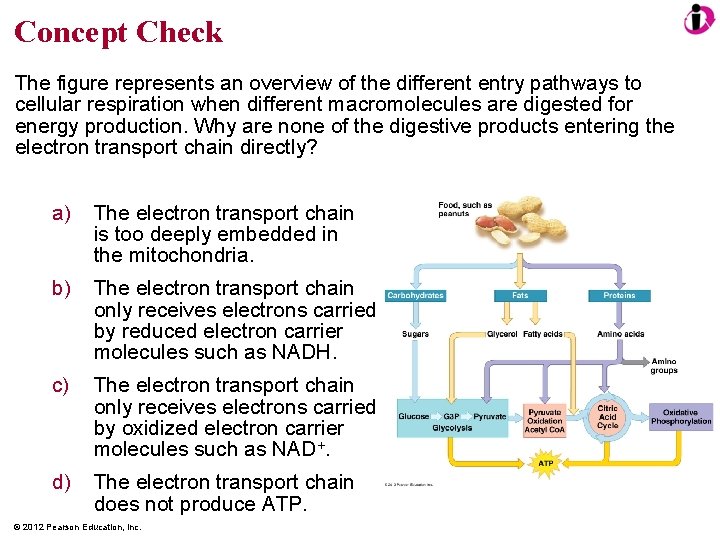
Concept Check The figure represents an overview of the different entry pathways to cellular respiration when different macromolecules are digested for energy production. Why are none of the digestive products entering the electron transport chain directly? a) The electron transport chain is too deeply embedded in the mitochondria. b) The electron transport chain only receives electrons carried by reduced electron carrier molecules such as NADH. c) The electron transport chain only receives electrons carried by oxidized electron carrier molecules such as NAD+. d) The electron transport chain does not produce ATP. © 2012 Pearson Education, Inc.

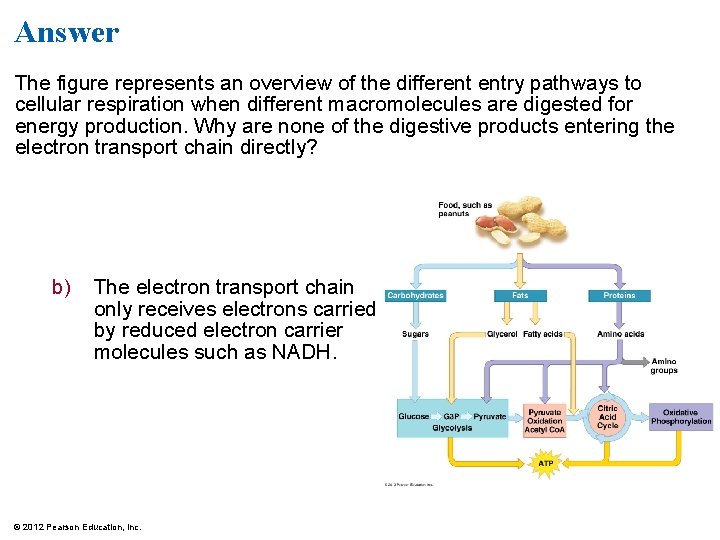
Answer The figure represents an overview of the different entry pathways to cellular respiration when different macromolecules are digested for energy production. Why are none of the digestive products entering the electron transport chain directly? b) The electron transport chain only receives electrons carried by reduced electron carrier molecules such as NADH. © 2012 Pearson Education, Inc.

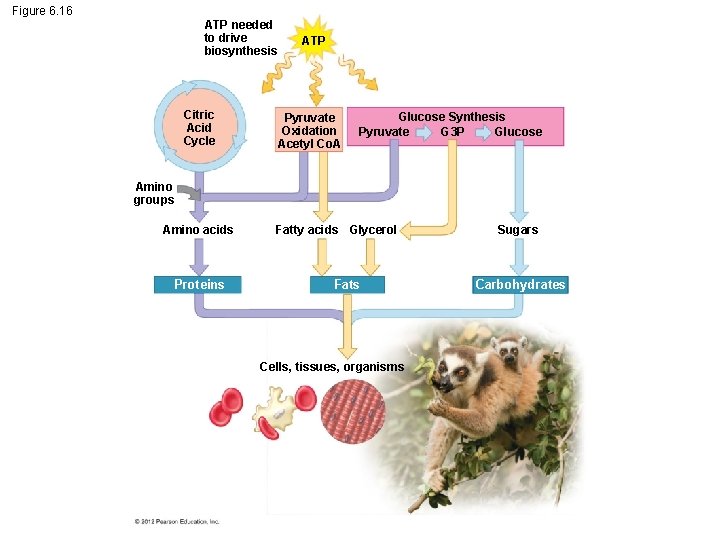
6. 16 Food molecules provide raw materials for biosynthesis Cells use intermediates from cellular respiration for the biosynthesis of other organic molecules. © 2012 Pearson Education, Inc.

Figure 6. 16 ATP needed to drive biosynthesis Citric Acid Cycle ATP Pyruvate Oxidation Acetyl Co. A Glucose Synthesis Pyruvate G 3 P Glucose Amino groups Amino acids Proteins Fatty acids Glycerol Fats Cells, tissues, organisms Sugars Carbohydrates

6. 16 Food molecules provide raw materials for biosynthesis Metabolic pathways are often regulated by feedback inhibition in which an accumulation of product suppresses the process that produces the product. © 2012 Pearson Education, Inc.

You should now be able to 1. Compare the processes and locations of cellular respiration and photosynthesis. 2. Explain how breathing and cellular respiration are related. 3. Provide the overall chemical equation for cellular respiration. 4. Explain how the human body uses its daily supply of ATP. © 2012 Pearson Education, Inc.

You should now be able to 5. Explain how the energy in a glucose molecule is released during cellular respiration. 6. Explain how redox reactions are used in cellular respiration. 7. Describe the general roles of dehydrogenase, NADH, and the electron transport chain in cellular respiration. 8. Compare the reactants, products, and energy yield of the three stages of cellular respiration. © 2012 Pearson Education, Inc.

You should now be able to 9. Explain how rotenone, cyanide, carbon monoxide, oligomycin, and uncouplers interrupt critical events in cellular respiration. 10. Compare the reactants, products, and energy yield of alcohol and lactic acid fermentation. 11. Distinguish between strict anaerobes and facultative anaerobes. 12. Explain how carbohydrates, fats, and proteins are used as fuel for cellular respiration. © 2012 Pearson Education, Inc.

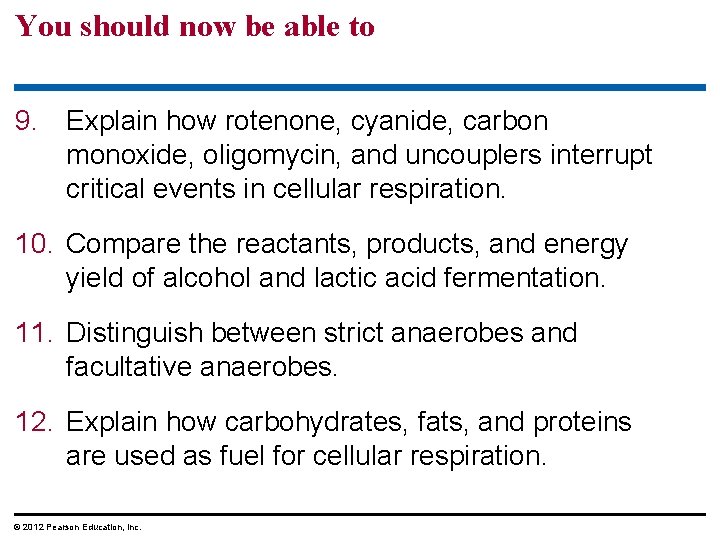
Figure 6. UN 01 CYTOPLASM Electrons carried by NADH Glycolysis Glucose Mitochondrion NADH Pyruvate Oxidation Substratelevel ATP phosphorylation NADH FADH 2 Citric Acid Cycle Oxidative Phosphorylation (electron transport and chemiosmosis) Substrate. ATP Oxidative level ATP phosphorylation

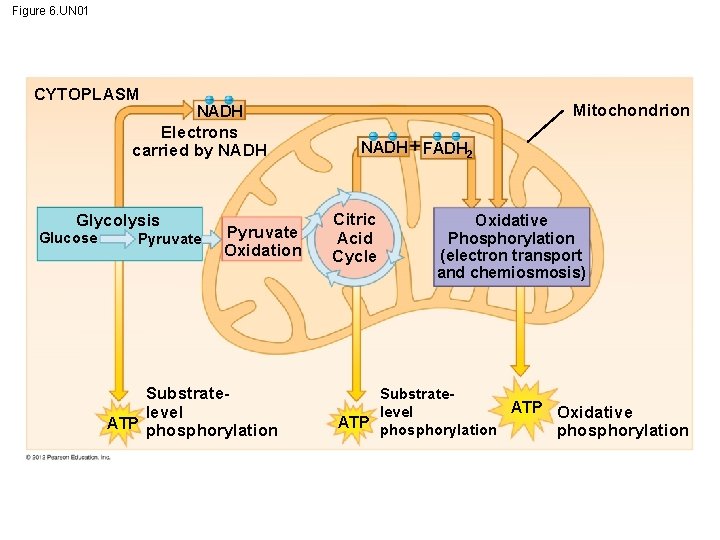
Figure 6. UN 02 Cellular respiration has three stages generates oxidizes uses produce some produces many energy for glucose and organic fuels (a) (b) (d) to pull electrons down (c) cellular work (f) by a process called chemiosmosis uses (g) H diffuse through ATP synthase (e) uses pumps H to create H gradient to

Figure 6. UN 03 Color intensity 0. 3 0. 2 0. 3 0. 1 0. 2 0. 1 a. Time 0. 2 0. 1 b. 0. 3 Time c. Time
 Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy How cells harvest chemical energy
How cells harvest chemical energy Chapter 8 cellular reproduction cells from cells
Chapter 8 cellular reproduction cells from cells Energy harvest
Energy harvest Real power formula
Real power formula Paranasal sinus at birth
Paranasal sinus at birth Regulation of tubular reabsorption
Regulation of tubular reabsorption Thyroid parafollicular cells
Thyroid parafollicular cells Somatic cells vs gametes
Somatic cells vs gametes Somatic vs germ cells
Somatic vs germ cells Red blood cells and white blood cells difference
Red blood cells and white blood cells difference Prokaryotic vs eukaryotic cells worksheet
Prokaryotic vs eukaryotic cells worksheet Animal cell venn diagram
Animal cell venn diagram Prokaryotic vs eukaryotic cells venn diagram
Prokaryotic vs eukaryotic cells venn diagram Organelle trail
Organelle trail Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Label
Label 4 types of eukaryotic cells
4 types of eukaryotic cells Which organisms are prokaryotes
Which organisms are prokaryotes Cells and life lesson 1 answer key
Cells and life lesson 1 answer key As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Chemical energy
Chemical energy Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Chapter 4 cells and energy
Chapter 4 cells and energy Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Chapter 7 chemical formulas and chemical compounds
Chapter 7 chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Water vaporization equation
Water vaporization equation Oxidative phosphorylation enzymes
Oxidative phosphorylation enzymes Chapter 9 cellular respiration harvesting chemical energy
Chapter 9 cellular respiration harvesting chemical energy Chapter 9: cellular respiration: harvesting chemical energy
Chapter 9: cellular respiration: harvesting chemical energy What is harvest
What is harvest The harvest plot
The harvest plot Abbott nutrition enteral formulas
Abbott nutrition enteral formulas Zarzuela is a drama play shown after the harvest season
Zarzuela is a drama play shown after the harvest season Ca harvest software change manager
Ca harvest software change manager Immfa
Immfa Supernatural harvest
Supernatural harvest Law of the harvest
Law of the harvest Kingdom harvest alliance
Kingdom harvest alliance What is the feast of first fruits
What is the feast of first fruits Ipc personal goals
Ipc personal goals Harvest evangelism inc
Harvest evangelism inc The harvest is plentiful but the laborers are few meaning
The harvest is plentiful but the laborers are few meaning Graft biology
Graft biology Send forth prayer
Send forth prayer Harvest festival scuola primaria
Harvest festival scuola primaria Ckanext-harvest
Ckanext-harvest The harvest is ready
The harvest is ready Record peach harvest—price lowest in a decade
Record peach harvest—price lowest in a decade Egg plant harvest
Egg plant harvest Freetutorical.com harvest land
Freetutorical.com harvest land Haemopro
Haemopro Harvest genomics
Harvest genomics A harvest of death gettysburg pennsylvania
A harvest of death gettysburg pennsylvania Post harvest management definition
Post harvest management definition Post harvest management of sugarcane
Post harvest management of sugarcane Post harvest handling of cut flowers and greens
Post harvest handling of cut flowers and greens Harvest plus
Harvest plus Subjective maturity indices
Subjective maturity indices Bountiful harvest poe
Bountiful harvest poe Ni-nta affinity chromatography
Ni-nta affinity chromatography Good harvest
Good harvest Cell harvest
Cell harvest Jch harvest
Jch harvest Monsanto's harvest of fear summary
Monsanto's harvest of fear summary How do cells obtain energy
How do cells obtain energy Why does a cell need energy
Why does a cell need energy How do cells obtain energy
How do cells obtain energy Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chapter 9 chemical names and formulas chapter quiz answers
Chapter 9 chemical names and formulas chapter quiz answers Power chemical corporation
Power chemical corporation Power chemical corporation
Power chemical corporation Power chemical corporation
Power chemical corporation Section 1 work and machines section 2 describing energy
Section 1 work and machines section 2 describing energy