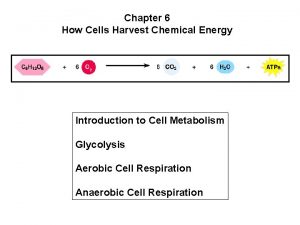
Chapter 6 How Cells Harvest Chemical Energy Power


- Slides: 36

Chapter 6 How Cells Harvest Chemical Energy Power. Point Lectures for Biology: Concepts and Connections, Fifth Edition – Campbell, Reece, Taylor, and Simon Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

How Is a Marathoner Different from a Sprinter? • Human muscles contain two different types of muscle fibers – That perform differently under different conditions Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

• The different types of muscle fibers – Function either aerobically, with oxygen, or anaerobically, without oxygen • Cellular respiration – Is the process by which cells produce energy aerobically Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

INTRODUCTION TO CELLULAR RESPIRATION 6. 1 Photosynthesis and cellular respiration provide energy for life • Cellular respiration makes ATP and consumes O 2 – During the oxidation of glucose to CO 2 and H 2 O Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

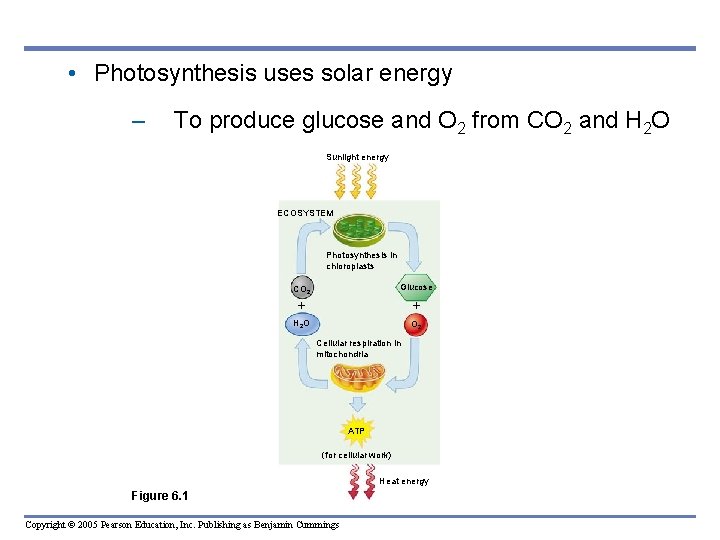
• Photosynthesis uses solar energy – To produce glucose and O 2 from CO 2 and H 2 O Sunlight energy ECOSYSTEM Photosynthesis in chloroplasts CO 2 Glucose H 2 O O 2 Cellular respiration in mitochondria ATP (for cellular work) Heat energy Figure 6. 1 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

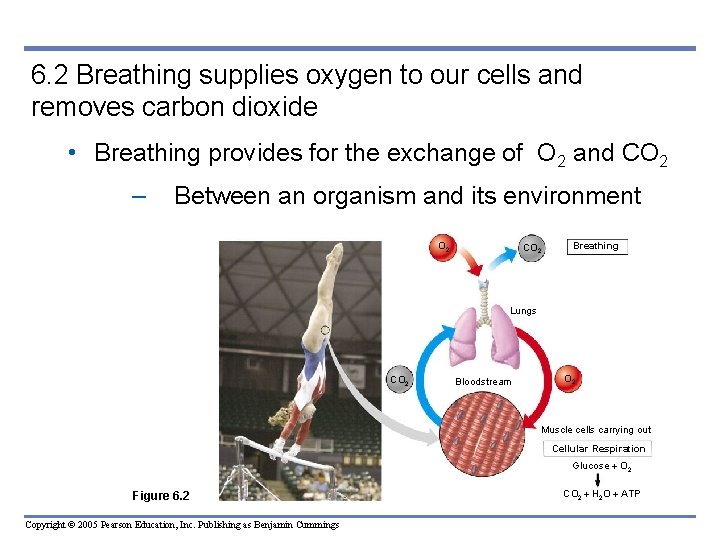
6. 2 Breathing supplies oxygen to our cells and removes carbon dioxide • Breathing provides for the exchange of O 2 and CO 2 – Between an organism and its environment O 2 CO 2 Breathing Lungs CO 2 Bloodstream O 2 Muscle cells carrying out Cellular Respiration Glucose O 2 Figure 6. 2 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings CO 2 H 2 O ATP

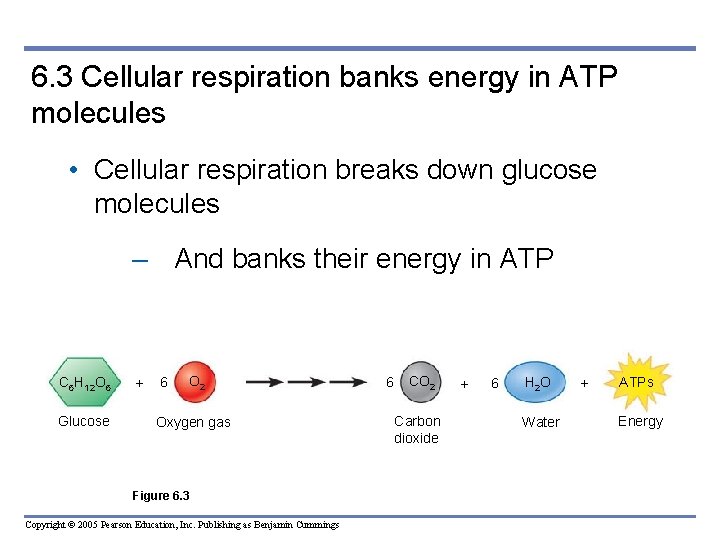
6. 3 Cellular respiration banks energy in ATP molecules • Cellular respiration breaks down glucose molecules – And banks their energy in ATP C 6 H 12 O 6 Glucose + 6 O 2 Oxygen gas Figure 6. 3 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings 6 CO 2 Carbon dioxide + 6 H 2 O Water + ATPs Energy

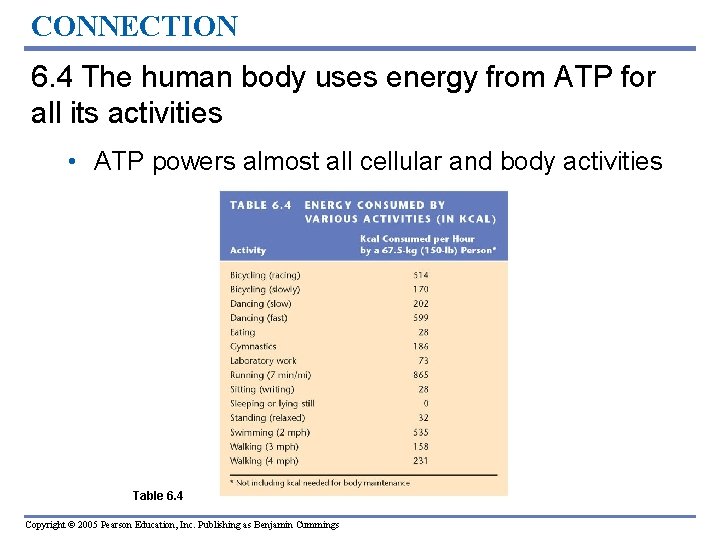
CONNECTION 6. 4 The human body uses energy from ATP for all its activities • ATP powers almost all cellular and body activities Table 6. 4 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

6. 5 Cells tap energy from electrons “falling” from organic fuels to oxygen • Electrons lose potential energy – During their transfer from organic compounds to oxygen Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

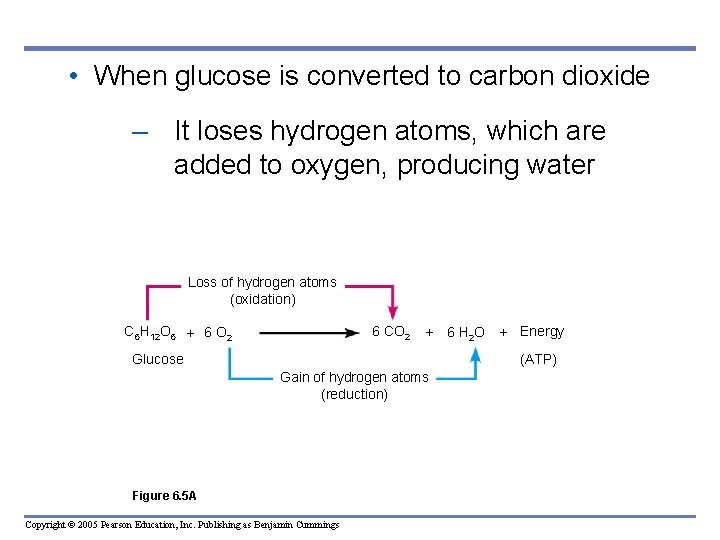
• When glucose is converted to carbon dioxide – It loses hydrogen atoms, which are added to oxygen, producing water Loss of hydrogen atoms (oxidation) C 6 H 12 O 6 + 6 O 2 6 CO 2 + Glucose 6 H 2 O + Energy (ATP) Gain of hydrogen atoms (reduction) Figure 6. 5 A Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

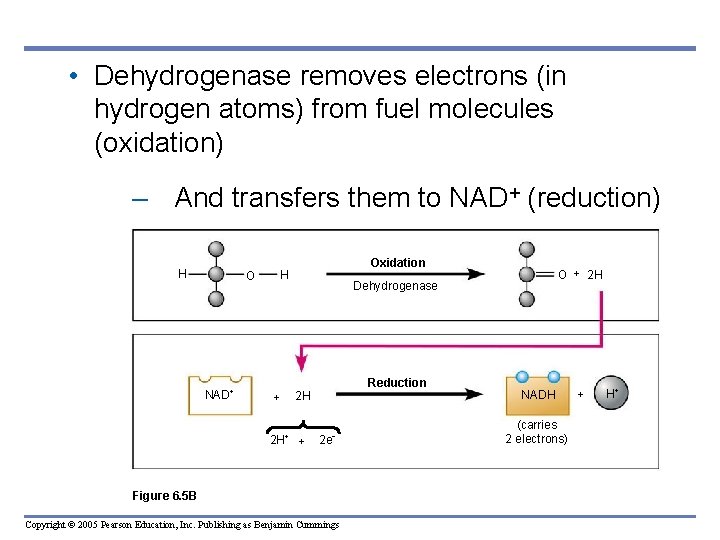
• Dehydrogenase removes electrons (in hydrogen atoms) from fuel molecules (oxidation) – And transfers them to NAD+ (reduction) H O NAD Oxidation H Reduction 2 H + 2 H O + 2 H Dehydrogenase + 2 e Figure 6. 5 B Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings NADH (carries 2 electrons) + H

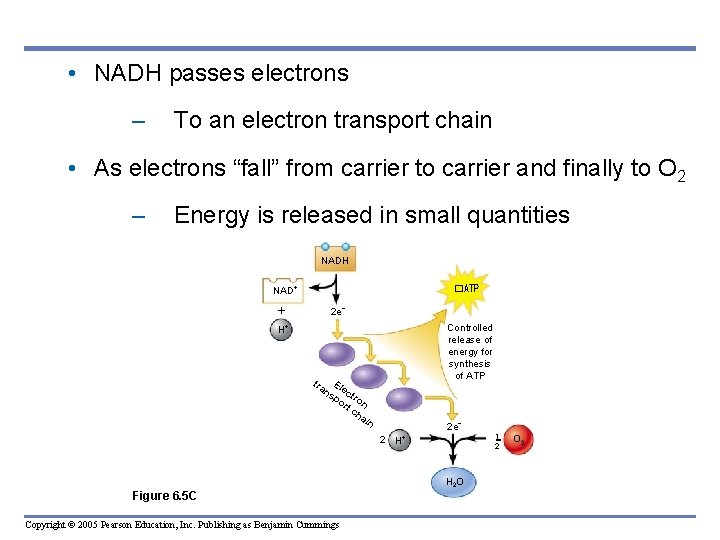
• NADH passes electrons – To an electron transport chain • As electrons “fall” from carrier to carrier and finally to O 2 – Energy is released in small quantities NADH NAD �ATP 2 e H Controlled release of energy for synthesis of ATP tra El ns ect po ro rt n ch ain 2 e 2 H H 2 O Figure 6. 5 C Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings 1 2 O 2

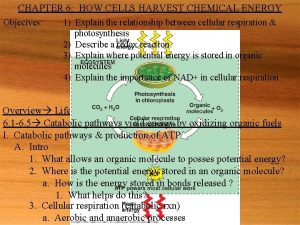
STAGES OF CELLULAR RESPIRATION AND FERMENTATION • 6. 6 Overview: Cellular respiration occurs in three main stages • Cellular respiration – Occurs in three main stages Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

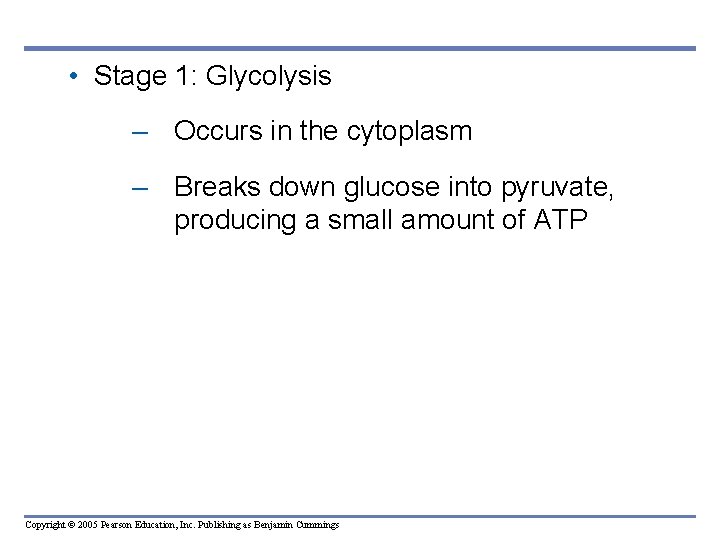
• Stage 1: Glycolysis – Occurs in the cytoplasm – Breaks down glucose into pyruvate, producing a small amount of ATP Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

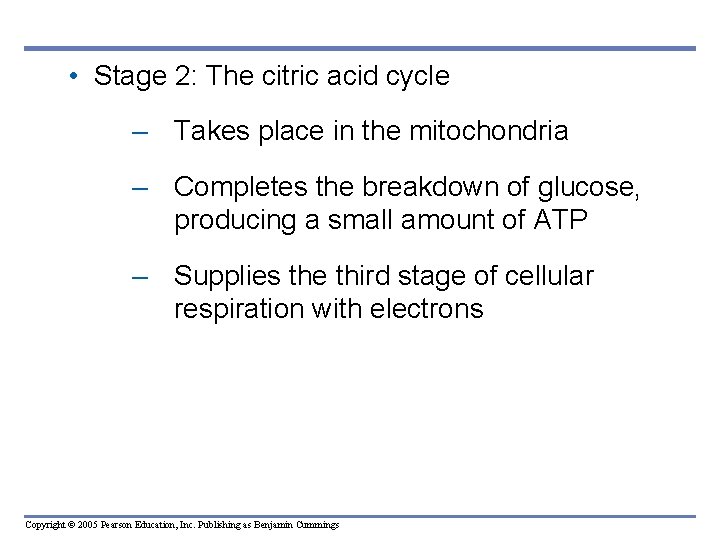
• Stage 2: The citric acid cycle – Takes place in the mitochondria – Completes the breakdown of glucose, producing a small amount of ATP – Supplies the third stage of cellular respiration with electrons Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

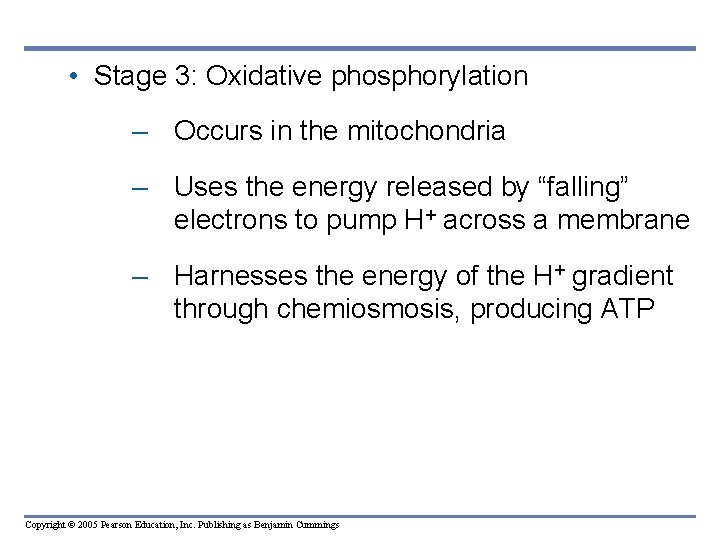
• Stage 3: Oxidative phosphorylation – Occurs in the mitochondria – Uses the energy released by “falling” electrons to pump H+ across a membrane – Harnesses the energy of the H+ gradient through chemiosmosis, producing ATP Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

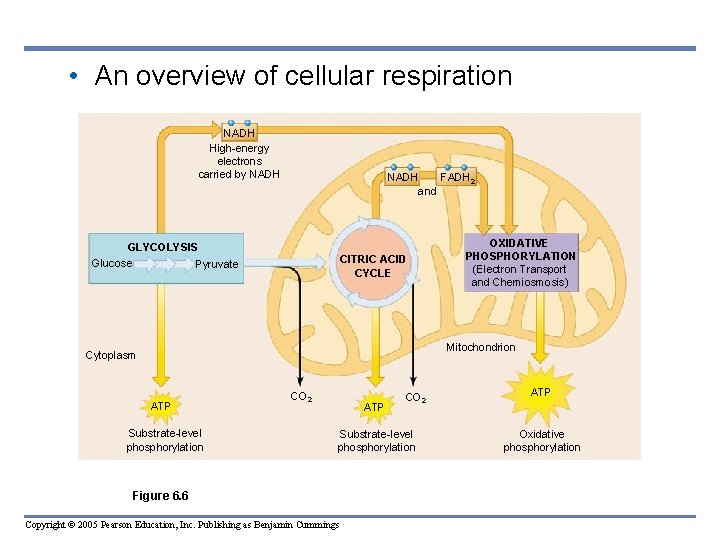
• An overview of cellular respiration NADH High-energy electrons carried by NADH FADH 2 and GLYCOLYSIS Glucose Pyruvate CITRIC ACID CYCLE OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) Mitochondrion Cytoplasm ATP Substrate-level phosphorylation CO 2 ATP CO 2 Substrate-level phosphorylation Figure 6. 6 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings ATP Oxidative phosphorylation

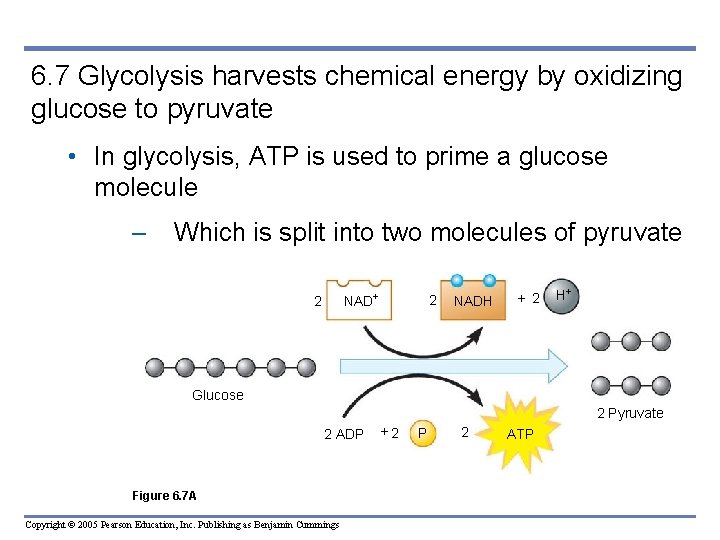
6. 7 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate • In glycolysis, ATP is used to prime a glucose molecule – Which is split into two molecules of pyruvate NAD 2 2 NADH + 2 H Glucose 2 Pyruvate 2 ADP Figure 6. 7 A Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings +2 P 2 ATP

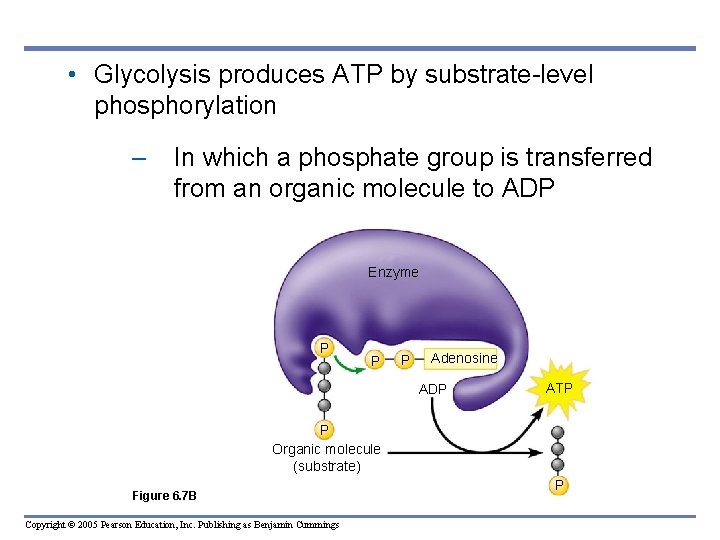
• Glycolysis produces ATP by substrate-level phosphorylation – In which a phosphate group is transferred from an organic molecule to ADP Enzyme P P P Adenosine ADP ATP P Organic molecule (substrate) Figure 6. 7 B Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings P

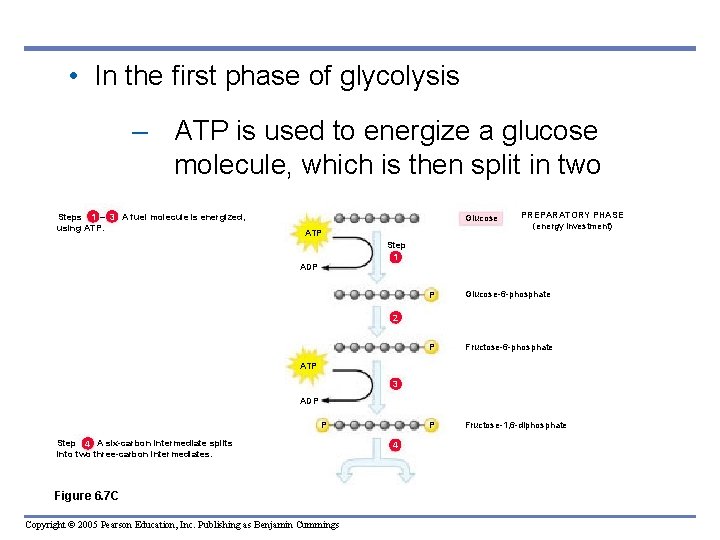
• In the first phase of glycolysis – ATP is used to energize a glucose molecule, which is then split in two 1 3 Steps – A fuel molecule is energized, using ATP. Glucose ATP PREPARATORY PHASE (energy investment) Step 1 ADP P Glucose-6 -phosphate P Fructose-1, 6 -diphosphate 2 ATP 3 ADP P Step A six-carbon intermediate splits 4 into two three-carbon intermediates. Figure 6. 7 C Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings 4

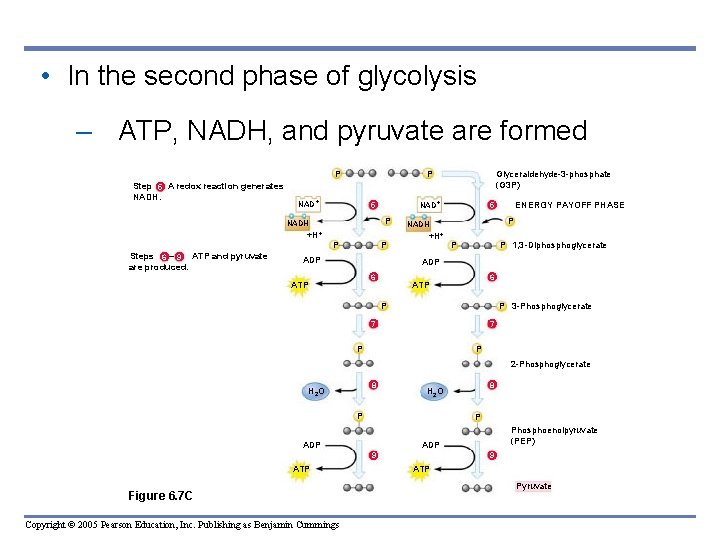
• In the second phase of glycolysis – ATP, NADH, and pyruvate are formed P Step A redox reaction generates 5 6 9 NADH. NAD P 5 +H P ADP P P 1, 3 -Diphosphoglycerate 6 7 6 ATP P P 7 P 8 8 H 2 O 8 2 -Phosphoglycerate 8 H 2 O P P 9 ADP Phosphoenolpyruvate (PEP) 9 ADP 9 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings 7 P 3 -Phosphoglycerate 7 Figure 6. 7 C 6 ADP ATP ENERGY PAYOFF PHASE 5 P 6 NADH +H P P NADH Steps – ATP and pyruvate 6 9 are produced. NAD Glyceraldehyde-3 -phosphate (G 3 P) 9 ATP Pyruvate

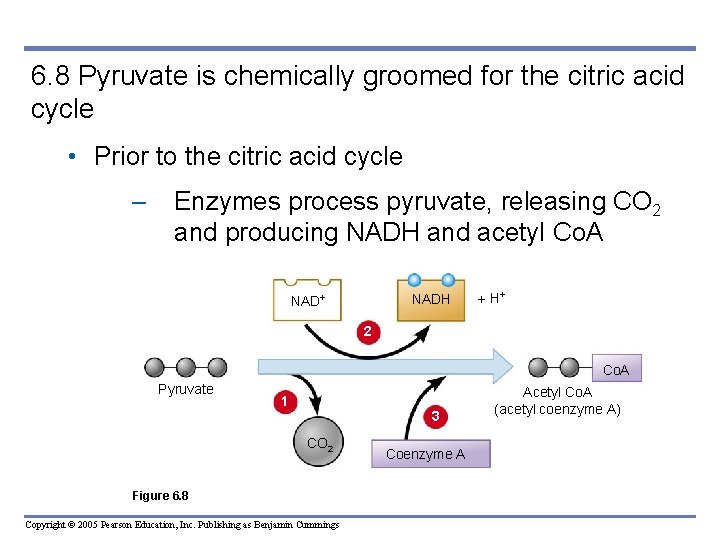
6. 8 Pyruvate is chemically groomed for the citric acid cycle • Prior to the citric acid cycle – Enzymes process pyruvate, releasing CO 2 and producing NADH and acetyl Co. A NADH H 2 Co. A Pyruvate 1 3 CO 2 Figure 6. 8 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings Coenzyme A Acetyl Co. A (acetyl coenzyme A)

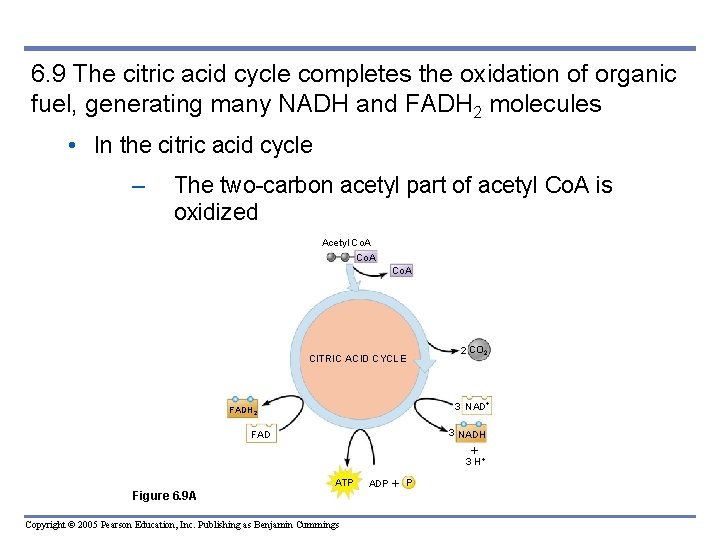
6. 9 The citric acid cycle completes the oxidation of organic fuel, generating many NADH and FADH 2 molecules • In the citric acid cycle – The two-carbon acetyl part of acetyl Co. A is oxidized Acetyl Co. A CITRIC ACID CYCLE 2 CO 2 3 NAD FADH 2 3 NADH FAD 3 H ATP Figure 6. 9 A Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings ADP P

• The two carbons are added to a four-carbon compound, forming citrate – Which is then degraded back to the starting compound Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

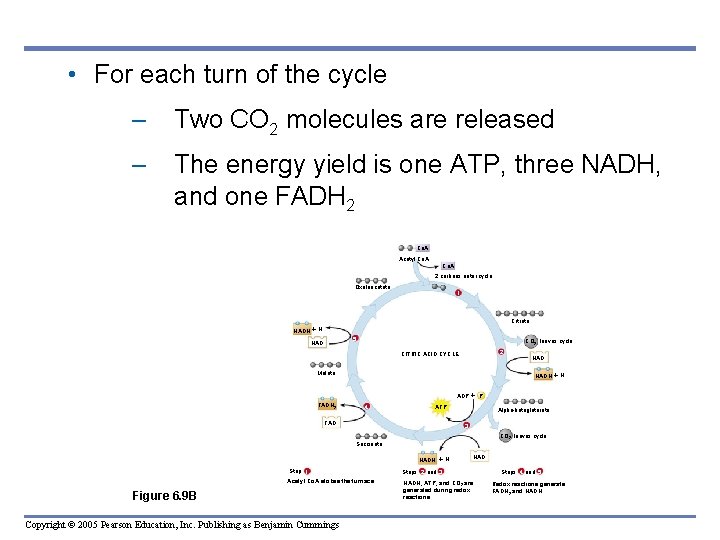
• For each turn of the cycle – Two CO 2 molecules are released – The energy yield is one ATP, three NADH, and one FADH 2 Co. A Acetyl Co. A 2 carbons enter cycle Oxaloacetate NADH 1 Citrate H NAD 5 CO 2 leaves cycle 2 CITRIC ACID CYCLE Malate NADH H ADP P FADH 2 4 ATP FAD Alpha-ketoglutarate 3 CO 2 leaves cycle Succinate NADH Step 1 Acetyl Co. A stokes the furnace. Figure 6. 9 B Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings H NAD Steps 2 and 3 NADH, ATP, and CO 2 are generated during redox reactions. Steps 4 and 5 Redox reactions generate FADH 2 and NADH.

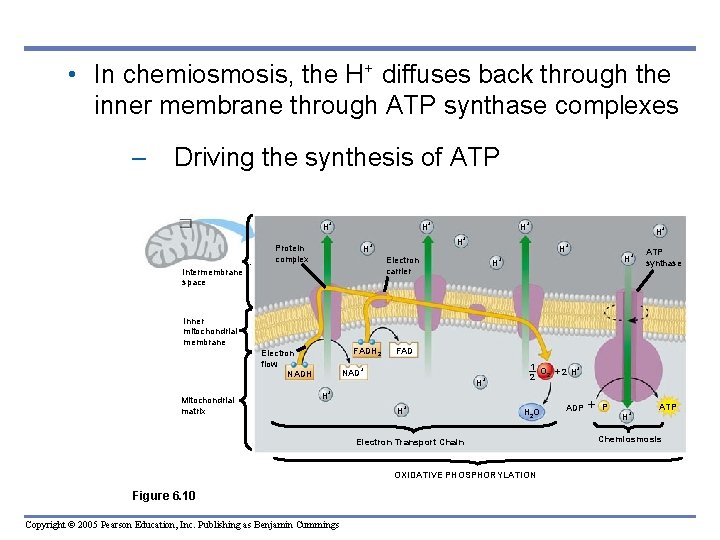
6. 10 Most ATP production occurs by oxidative phosphorylation • Electrons from NADH and FADH 2 – Travel down the electron transport chain to oxygen, which picks up H+ to form water • Energy released by the redox reactions – Is used to pump H+ into the space between the mitochondrial membranes Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

• In chemiosmosis, the H+ diffuses back through the inner membrane through ATP synthase complexes – Driving the synthesis of ATP H+ Intermembrane space . Protein complex H H+ FADH 2 Electron flow NADH H+ + H+ Electron carrier Inner mitochondrial membrane Mitochondrial matrix H+ H+ H H + 1 O + 2 H+ 2 2 H+ H+ H 2 O Electron Transport Chain OXIDATIVE PHOSPHORYLATION Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings ATP synthase FAD NAD+ Figure 6. 10 H+ + ADP P H+ ATP Chemiosmosis

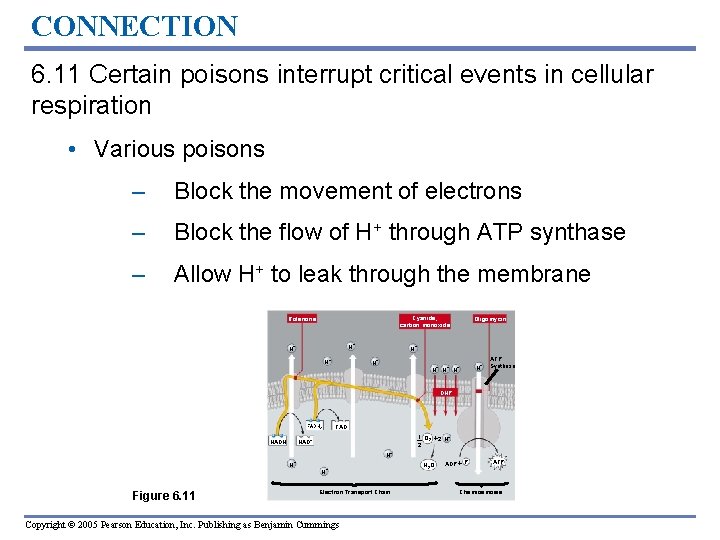
CONNECTION 6. 11 Certain poisons interrupt critical events in cellular respiration • Various poisons – Block the movement of electrons – Block the flow of H+ through ATP synthase – Allow H+ to leak through the membrane Cyanide, carbon monoxide Rotenone H+ H+ H + Oligomycin H+ H + H+ H+ ATP Synthase DNP FADH 2 NADH NAD FAD 1 O 2 2 H+ 2 + H+ H+ Figure 6. 11 H+ Electron Transport Chain Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings H 2 O ADP P ATP Chemiosmosis

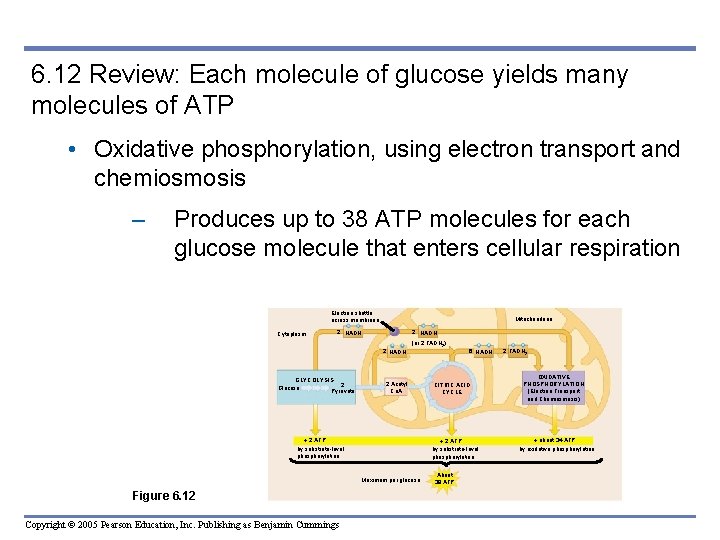
6. 12 Review: Each molecule of glucose yields many molecules of ATP • Oxidative phosphorylation, using electron transport and chemiosmosis – Produces up to 38 ATP molecules for each glucose molecule that enters cellular respiration Electron shuttle across membrane Cytoplasm Mitochondrion 2 NADH (or 2 FADH 2) 2 NADH GLYCOLYSIS 2 Glucose Pyruvate 2 Acetyl Co. A 2 ATP by substrate-level phosphorylation Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings CITRIC ACID CYCLE 2 ATP by substrate-level phosphorylation Maximum per glucose: Figure 6. 12 6 NADH About 38 ATP 2 FADH 2 OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) about 34 ATP by oxidative phosphorylation

6. 13 Fermentation is an anaerobic alternative to cellular respiration • Under anaerobic conditions, many kinds of cells – Can use glycolysis alone to produce small amounts of ATP Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

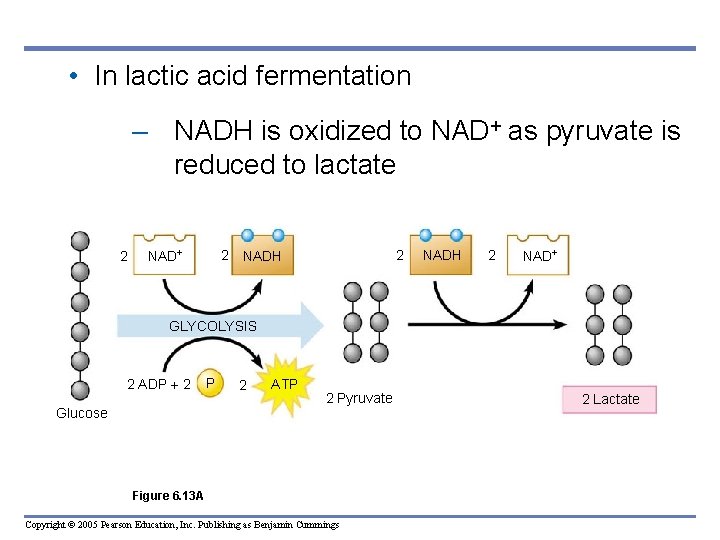
• In lactic acid fermentation – NADH is oxidized to NAD+ as pyruvate is reduced to lactate 2 NAD 2 2 NADH 2 NAD GLYCOLYSIS 2 ADP 2 Glucose P 2 ATP 2 Pyruvate Figure 6. 13 A Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings 2 Lactate

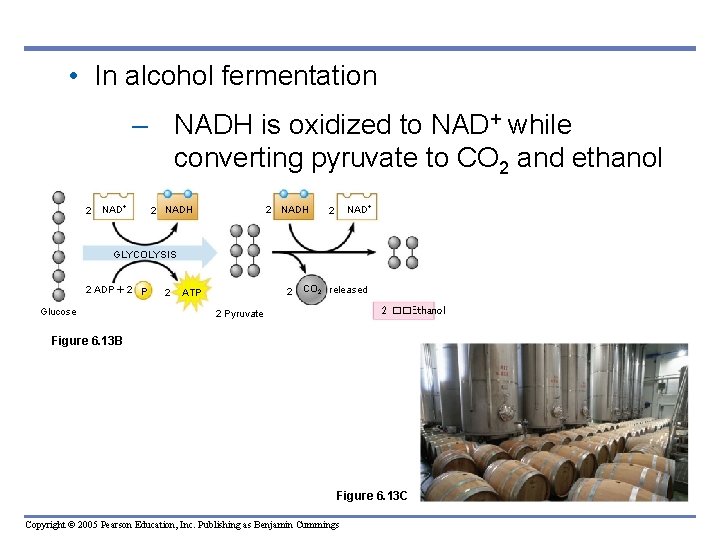
• In alcohol fermentation – NADH is oxidized to NAD+ while converting pyruvate to CO 2 and ethanol 2 NADH NAD 2 GLYCOLYSIS 2 ADP 2 P Glucose 2 2 ATP CO 2 released 2 ��Ethanol 2 Pyruvate Figure 6. 13 B Figure 6. 13 C Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

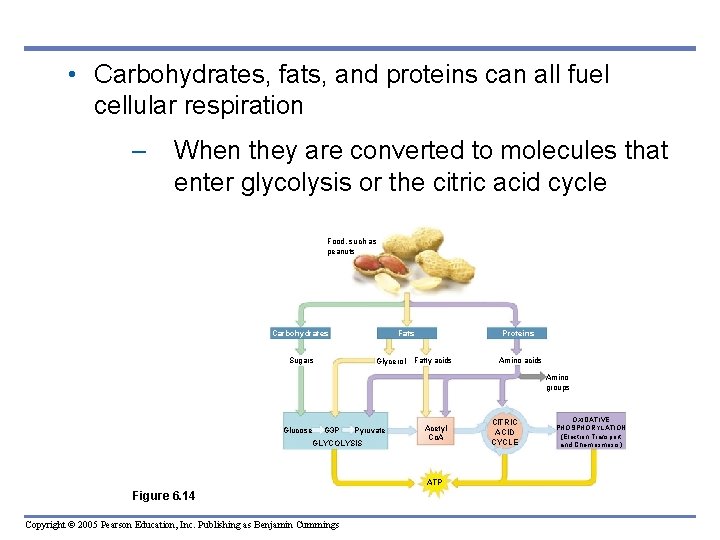
INTERCONNECTIONS BETWEEN MOLECULAR BREAKDOWN AND SYNTHESIS • 6. 14 Cells use many kinds of organic molecules as fuel for cellular respiration Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings

• Carbohydrates, fats, and proteins can all fuel cellular respiration – When they are converted to molecules that enter glycolysis or the citric acid cycle Food, such as peanuts Carbohydrates Fats Sugars Glycerol Proteins Fatty acids Amino groups Glucose G 3 P Pyruvate GLYCOLYSIS Acetyl Co. A ATP Figure 6. 14 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings CITRIC ACID CYCLE OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis)

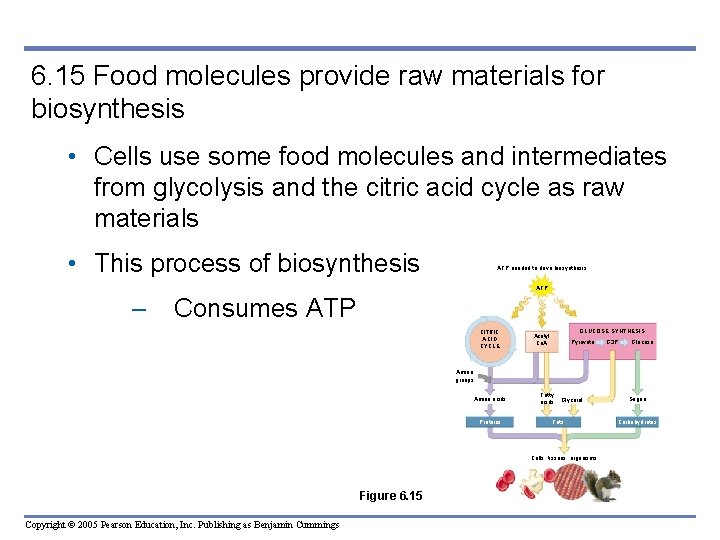
6. 15 Food molecules provide raw materials for biosynthesis • Cells use some food molecules and intermediates from glycolysis and the citric acid cycle as raw materials • This process of biosynthesis ATP needed to drive biosynthesis ATP – Consumes ATP CITRIC ACID CYCLE GLUCOSE SYNTHESIS Acetyl Co. A Pyruvate G 3 P Glucose Amino groups Amino acids Proteins Fatty acids Glycerol Fats Cells, tissues, organisms Figure 6. 15 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings Sugars Carbohydrates

6. 16 The fuel for respiration ultimately comes from photosynthesis • All organisms – Can harvest energy from organic molecules • Plants, but not animals – Can also make these molecules from inorganic sources by the process of photosynthesis Figure 6. 16 Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cummings
 Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy How cells harvest chemical energy chapter 6
How cells harvest chemical energy chapter 6 How cells harvest chemical energy
How cells harvest chemical energy Nondisjunction in meiosis
Nondisjunction in meiosis Energy harvest
Energy harvest Triangle of power
Triangle of power Paranasal sinus development
Paranasal sinus development Tubular secretion
Tubular secretion Parafollicular cells vs follicular cells
Parafollicular cells vs follicular cells Haploid vs diploid venn diagram
Haploid vs diploid venn diagram Why dna is more stable than rna?
Why dna is more stable than rna? Chlorocruorin
Chlorocruorin Prokaryotic v. eukaryotic cells
Prokaryotic v. eukaryotic cells Similarities between plant and animal cells venn diagram
Similarities between plant and animal cells venn diagram Prokaryotic cells
Prokaryotic cells The organelle trail
The organelle trail Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Pseudostratified vs simple columnar
Pseudostratified vs simple columnar 4 types of eukaryotic cells
4 types of eukaryotic cells Prokaryotic vs eukaryotic cells
Prokaryotic vs eukaryotic cells Cells cells they're made of organelles meme
Cells cells they're made of organelles meme As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Elastic potential energy examples
Elastic potential energy examples Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Chapter 4 cells and energy
Chapter 4 cells and energy Empirical formula pogil
Empirical formula pogil Love chemical formula
Love chemical formula Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Water vaporization equation
Water vaporization equation Oxidative phosphorylation enzymes
Oxidative phosphorylation enzymes Chapter 9 cellular respiration harvesting chemical energy
Chapter 9 cellular respiration harvesting chemical energy Chapter 9: cellular respiration: harvesting chemical energy
Chapter 9: cellular respiration: harvesting chemical energy When is harvest
When is harvest The harvest story summary
The harvest story summary