Cellular Respiration Harvesting Chemical Energy Cells harvest chemical















- Slides: 22

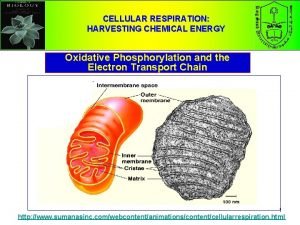
Cellular Respiration: Harvesting Chemical Energy

Cells harvest chemical energy stored in molecules and use this to generate ATP. Organic compounds store energy in their arrangements of atoms.

Catabolic (releases energy) Exergonic (- G, products store less energy than reactants) Occurs mostly in the mitochondria (but first step is in cytosol) C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy (ATP and heat)

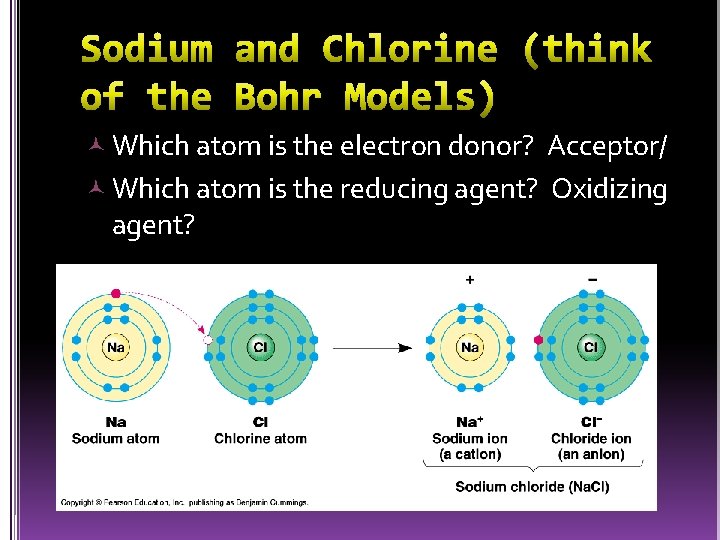
Involves the transfer of electrons l LEO (loss of electrons) oxidation (less -) l GER (gain of electrons) reduction (more -) Electron donor (reducing agent) the atom that becomes less negative (positive) l Electron acceptor (oxidizing agent) the atom that becomes more negative l

Which atom is the electron donor? Acceptor/ Which atom is the reducing agent? Oxidizing agent?


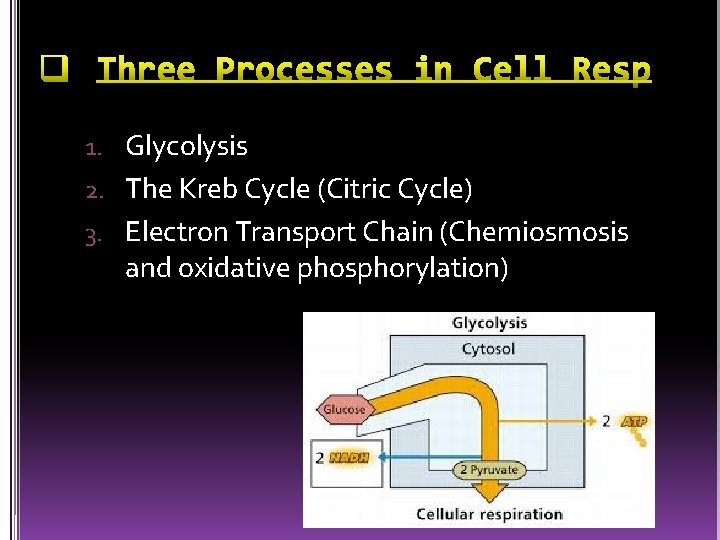
1. Glycolysis 2. The Kreb Cycle (Citric Cycle) 3. Electron Transport Chain (Chemiosmosis and oxidative phosphorylation)

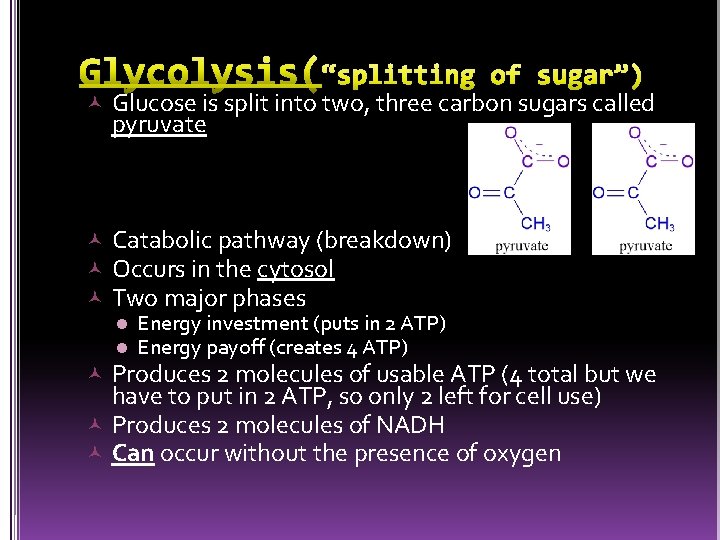
Glucose is split into two, three carbon sugars called pyruvate Catabolic pathway (breakdown) Occurs in the cytosol Two major phases l Energy investment (puts in 2 ATP) l Energy payoff (creates 4 ATP) Produces 2 molecules of usable ATP (4 total but we have to put in 2 ATP, so only 2 left for cell use) Produces 2 molecules of NADH Can occur without the presence of oxygen


glycolysis

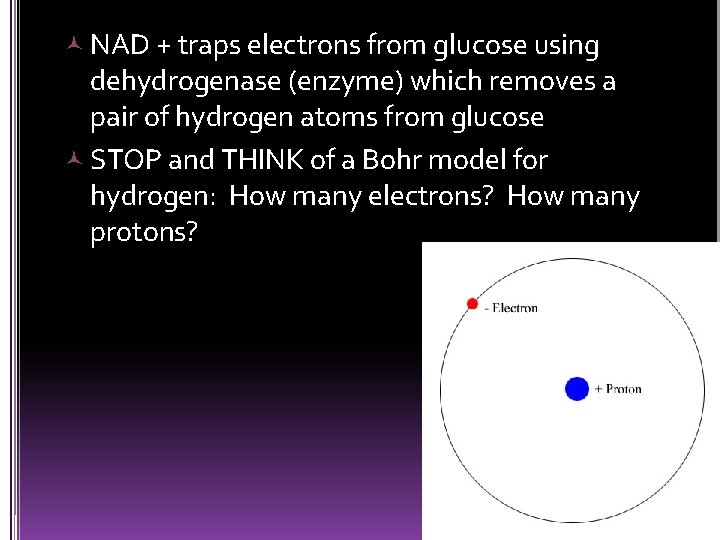
The oxidation of glucose allows energy to be taken out of storage and make energy available to make ATP. Glucose is broken down gradually in a series of steps catalyzed by an enzyme: dehydrogenase Hydrogen atoms are stripped from glucose and passed to a coenzyme: NAD + (oxidizing agent)

The dehydrogenase removes two hydrogen atoms from the glucose (2 p+ and 2 e-) NAD+ traps 2 e- and 1 p+ from glucose break down = NADH stores energy ready and will pass on electrons to make ATP when e- complete their journey to oxygen.

Step by step process that releases energy along the way (does not release all energy at once) Hydrogen atoms are stripped away from glucose Hydrogen atoms ultimate destination is the oxygen molecule First they are transferred to a coenzyme NAD+ • NAD + wants to gain electrons (to be neutral)


NAD + traps electrons from glucose using dehydrogenase (enzyme) which removes a pair of hydrogen atoms from glucose STOP and THINK of a Bohr model for hydrogen: How many electrons? How many protons?

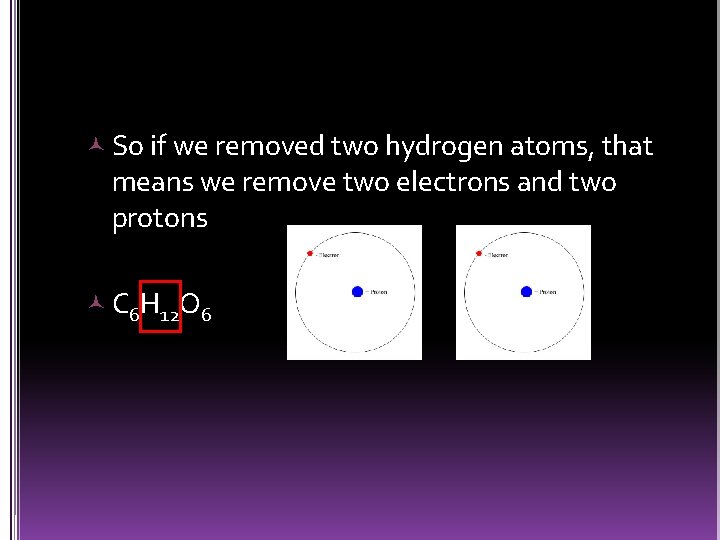
So if we removed two hydrogen atoms, that means we remove two electrons and two protons C 6 H 12 O 6

Enzyme gives two electrons and one proton to its coenzyme NAD + forming NADH The other proton (left over) is released into the surrounding environment Is NAD+ an electron acceptor or donor? Is NAD+ an oxidizing agent or a reducing agent? **NAD+ is the most versatile electron acceptor in cell resp.


Little potential energy is lost when e- are transferred from glucose (food) to NAD+ NADH molecules represent stored energy that can be used to make ATP when ecomplete their “fall” to oxygen An electron transport chain is used to break the fall of electrons to oxygen l Produces several energy releasing steps NOT one big explosion of energy


Electrons (from glucose in the form of hydrogen atoms) move to NAD+ NADH oxygen (final electron acceptor)

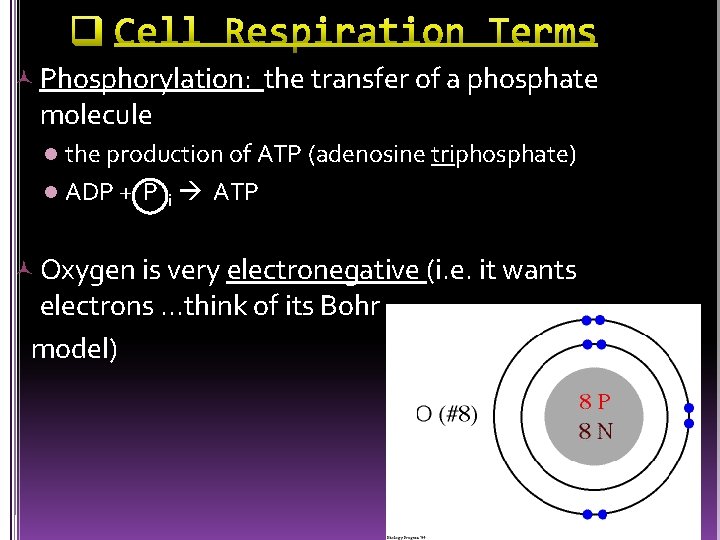
Phosphorylation: the transfer of a phosphate molecule the production of ATP (adenosine triphosphate) l ADP + P i ATP l Oxygen is very electronegative (i. e. it wants electrons. . . think of its Bohr model)
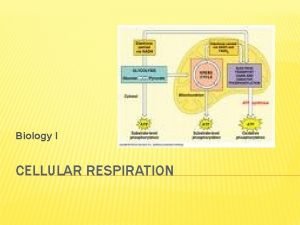
 The stages of cellular respiration
The stages of cellular respiration Explain how amp stimulates cellular respiration
Explain how amp stimulates cellular respiration Chapter 9: cellular respiration: harvesting chemical energy
Chapter 9: cellular respiration: harvesting chemical energy Phosphorelation
Phosphorelation How cells harvest chemical energy
How cells harvest chemical energy Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy Chapter 8 cellular reproduction cells from cells
Chapter 8 cellular reproduction cells from cells Cellular respiration chemical equation
Cellular respiration chemical equation Summary equation for cellular respiration
Summary equation for cellular respiration Cellular respiration chemical formula
Cellular respiration chemical formula Energy flow in cellular respiration
Energy flow in cellular respiration Consumer
Consumer Cellular respiration obtaining energy from food
Cellular respiration obtaining energy from food Cellular respiration obtaining energy from food
Cellular respiration obtaining energy from food Section 1 how organisms obtain energy
Section 1 how organisms obtain energy Redox reaction in cellular respiration
Redox reaction in cellular respiration The gray-brown haze often found over large cities is called
The gray-brown haze often found over large cities is called Chemiosmosis steps
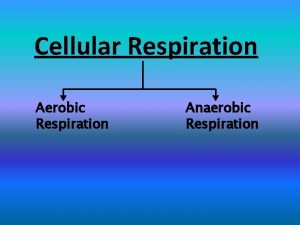
Chemiosmosis steps Aerobic vs anaerobic
Aerobic vs anaerobic Lactic acid fermentation steps
Lactic acid fermentation steps Why is cellular respiration important
Why is cellular respiration important Complimentary processes
Complimentary processes