Chapter 4 High Performance Liquid Chromatography HPLC 1952A








































- Slides: 72

Chapter 4 High Performance Liquid Chromatography (HPLC) 高效液相色谱

1952,A. J. Martin and R. L. M. Synge received the Nobel Prize for the discovery of partition chromatography. n In 1941, they wrote: The mobile phase need not be a liquid but may be a vapour. We show below that the efficiency of contact between the phases is far greater in the chromatogram than in ordinary distillation or extraction columns. Very refined separation of volatile substances should therefore be possible in a column in which a permanent gas is made to flow over a gel impregnated with a nonvolatile solvent in which the substances to be separated approximately obey Raoults’ law.

4. 1 Introduction of HPLC n n n Development of HPLC From LC to GC From LC to HPLC Application of HPLC Special characters of HPLC High pressure High Flow High performance Others Types of HPLC Chemical-bond (distribution), Adsorption, Ion exchange*, Exclusion

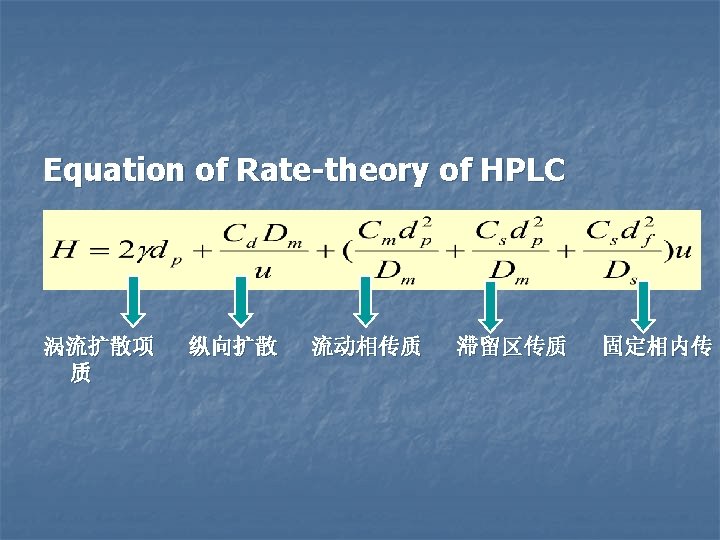
4. 2 Discussion of LC from the Rate Theory n H=A+B/u+Cu n A=2λdp n B→ 0 n C=?

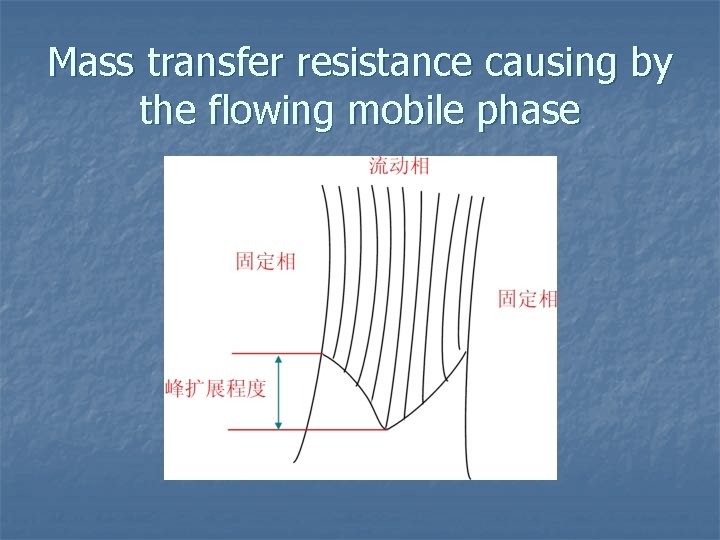
Mass transfer resistance causing by the flowing mobile phase

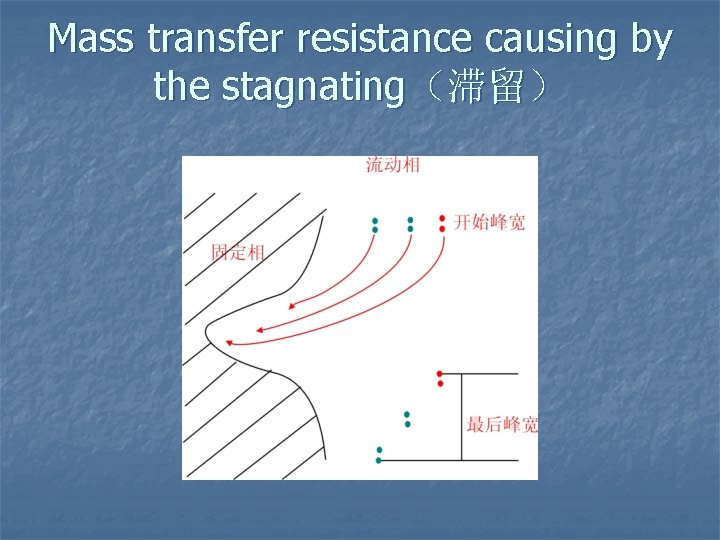
Mass transfer resistance causing by the stagnating(滞留)


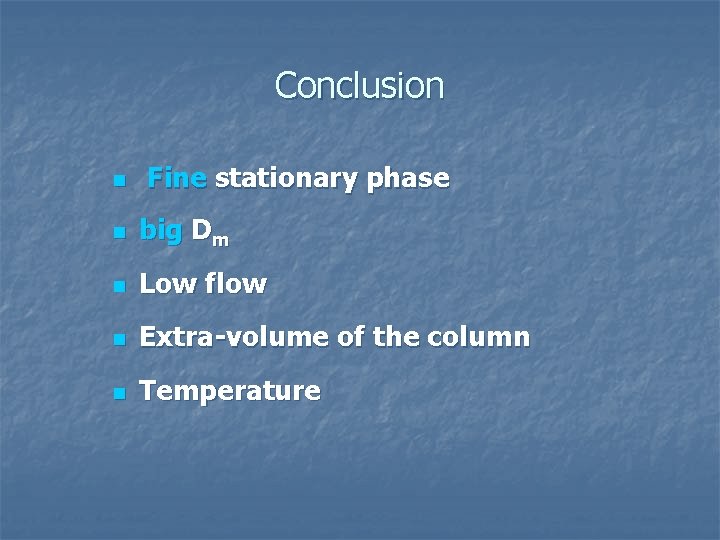
Conclusion n Fine stationary phase n big Dm n Low flow n Extra-volume of the column n Temperature

Glossary n n n HPLC Chemical-bond phase chromatography Ion exchange chromatography Exclusion chromatography Stagnate Isocratic and gradient elution

4. 3 Instrument of HPLC n n n Typical HPLC chromatographs Pumping and Solvent System Sample Injection System Columns Detectors

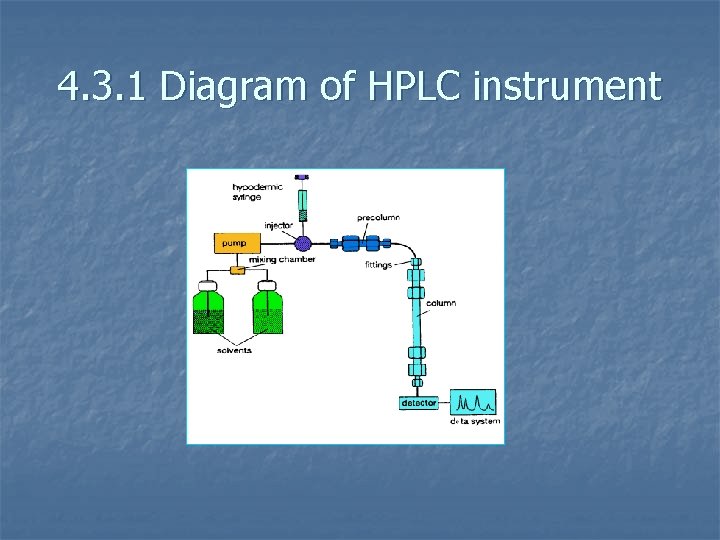
4. 3. 1 Diagram of HPLC instrument

Picture of HPLC instrument

4. 3. 2 Pumping and Solvent System Principle and Picture of reciprocating pump (往复泵)

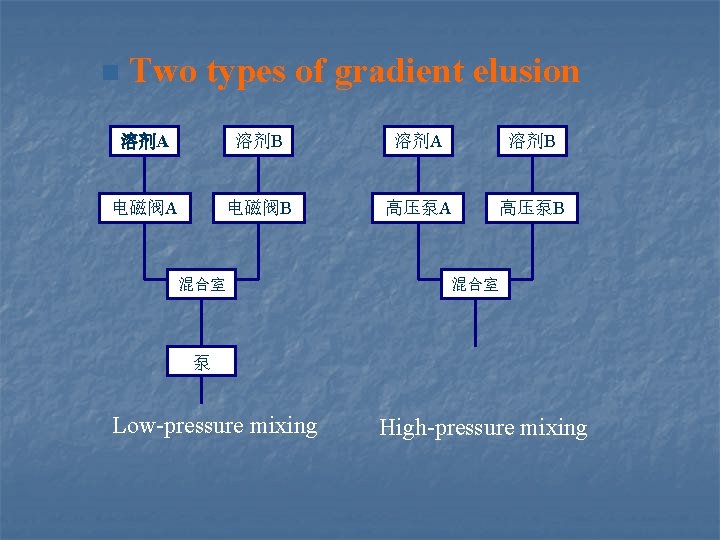
Gradient elusion (剃度洗脱) n Reason for gradient elusion n Compared with GC

n Two types of gradient elusion 溶剂A 溶剂B 电磁阀A 电磁阀B 高压泵A 高压泵B 混合室 泵 Low-pressure mixing High-pressure mixing

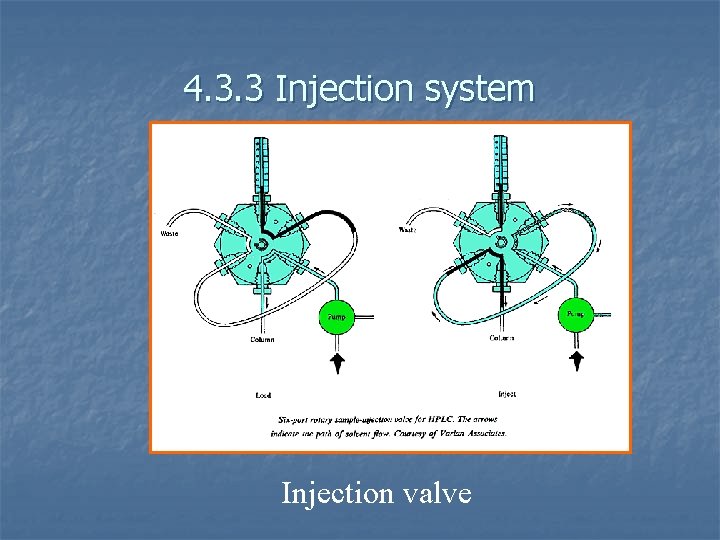
4. 3. 3 Injection system Injection valve

4. 3. 4 Column l Size l Packing materials l Packing technology

4. 3. 5 Detectors n UV-detector (紫外检测器) n Differential refractive index detector RI (示差折光检测器) n Fluorescence Detector (荧光检测器) n Electrical conductivity detector (电导检测器)

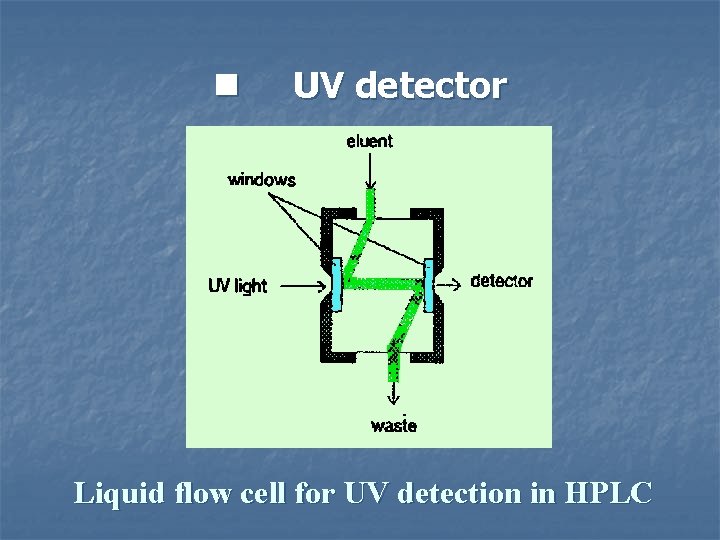
n UV detector Liquid flow cell for UV detection in HPLC

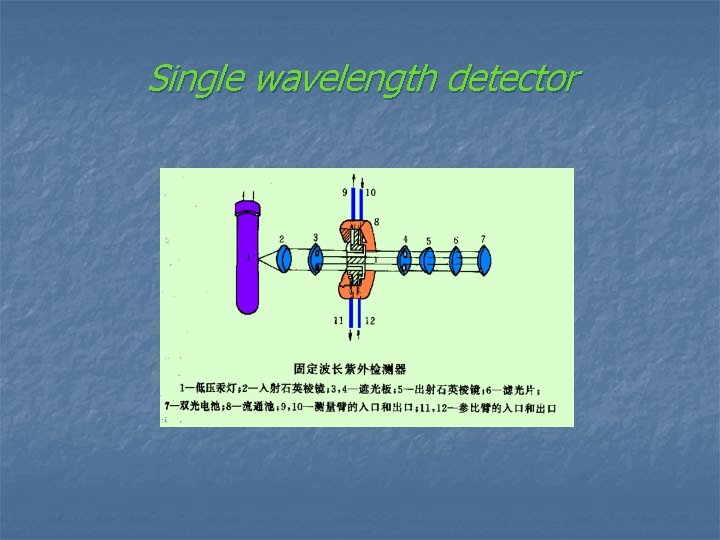
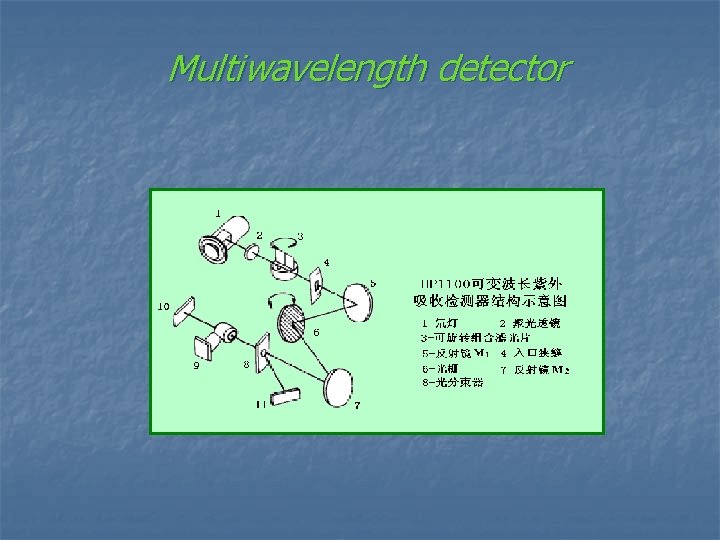
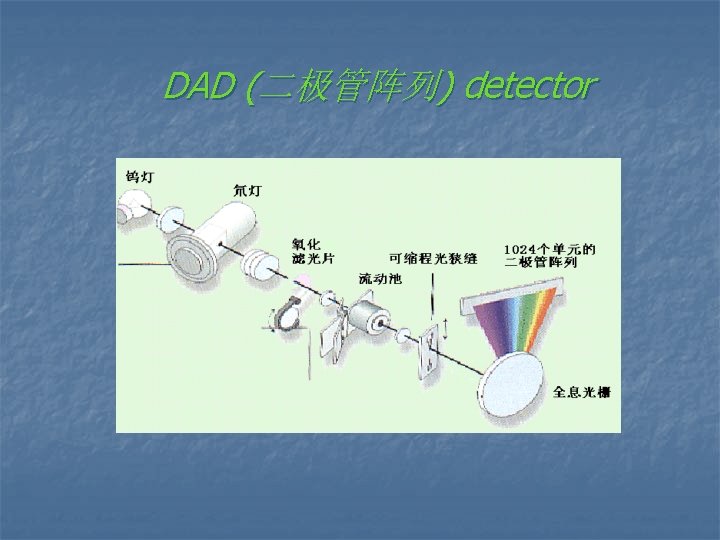
Ø Single wavelength detector ( 固定波长的紫外检测器) Ø Multiwavelength detector ( 可变波长的紫外检测器) Ø Photo-diode array detector ( 光电二极管阵列检测器)

Single wavelength detector

Multiwavelength detector

DAD (二极管阵列) detector

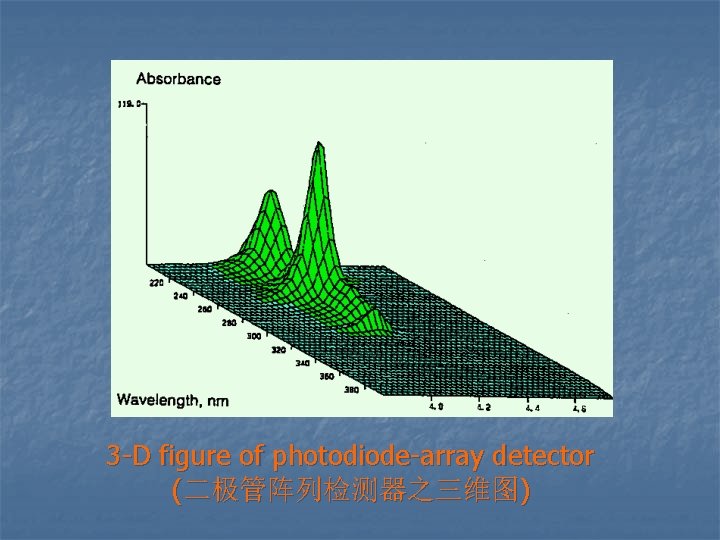
3 -D figure of photodiode-array detector (二极管阵列检测器之三维图)

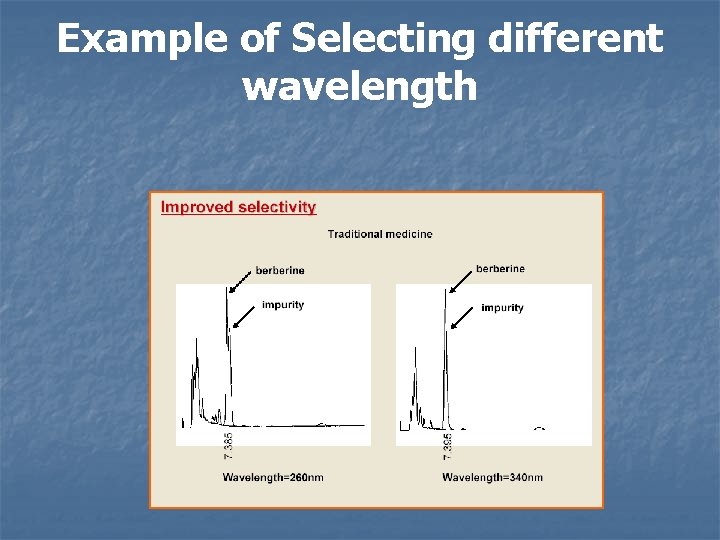
Example of Selecting different wavelength

Characters of UV-detector Ø Sample should be UV-absorption Ø High sensitivity Ø Large linear range Ø Suitable for gradient elution Ø Most widely used

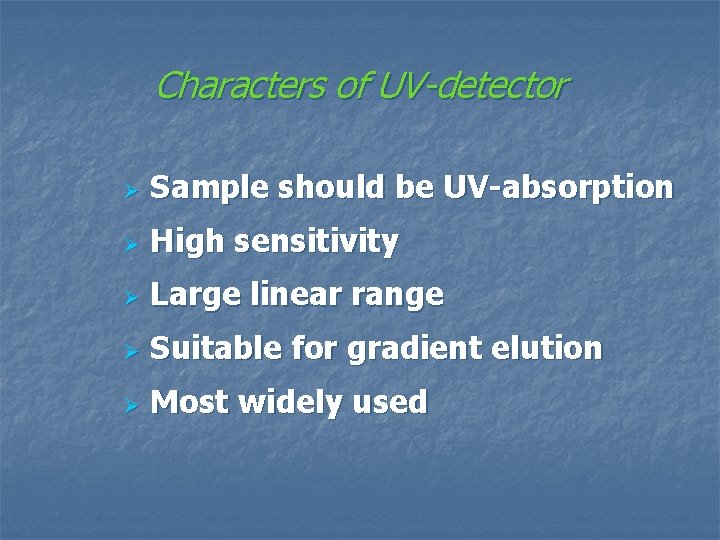
n Refractive index detector (折光检测器)

Properties of RI detector Ø Non-specific Ø Low-sensitivity Ø Easily affected by temperature Ø Unsuitable for gradient elution

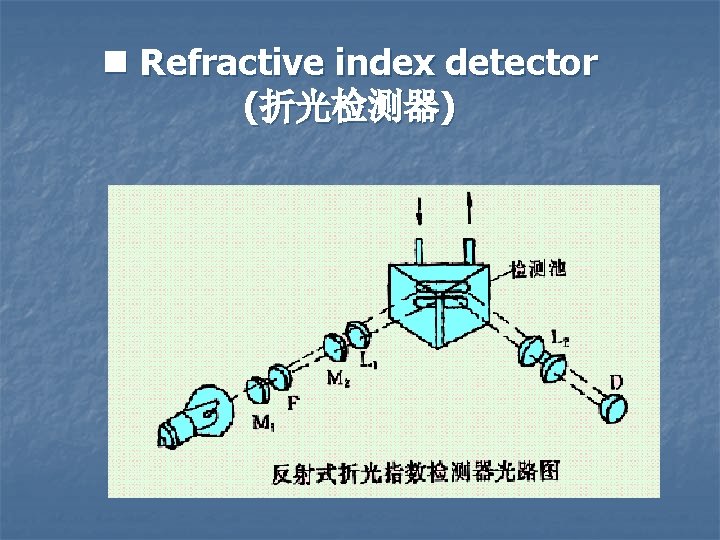
n Fluorescence detector

Properties of Fluorescence detector Ø High selectivity ( for protein, drug) Ø High sensitivity

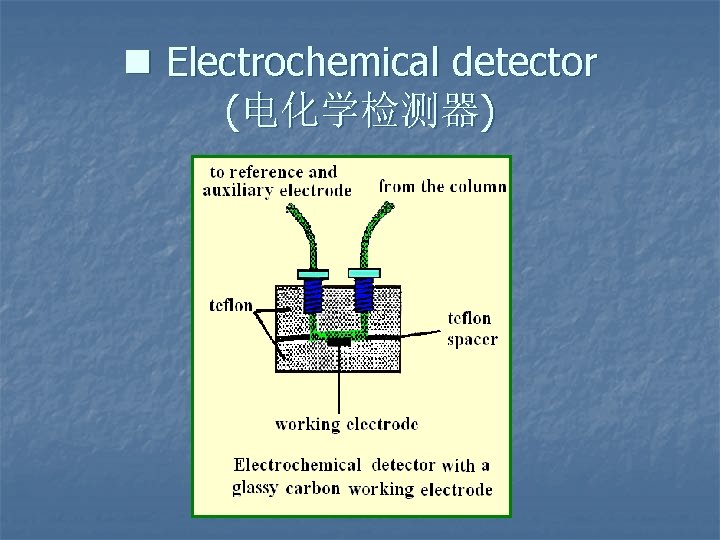
n Electrochemical detector (电化学检测器)

Properties of Electrochemical detector Ø Ø Selective to electro-active subject Easily affected by some surfaceactive substances

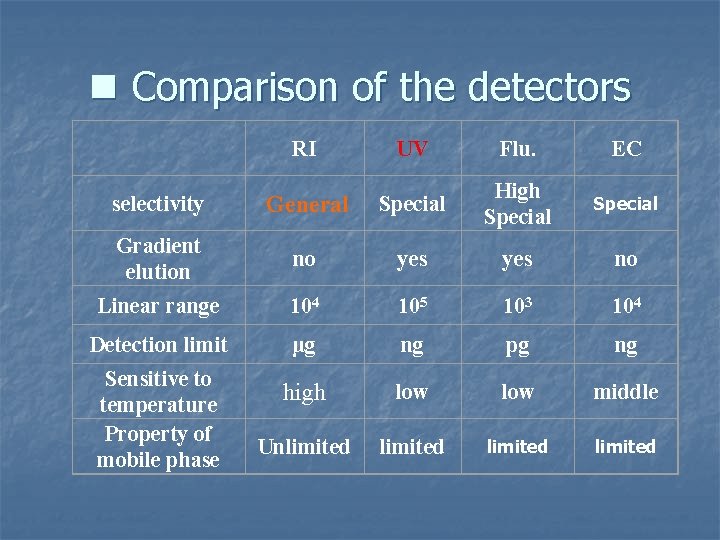
n Comparison of the detectors RI UV Flu. EC selectivity General Special High Special Gradient elution no yes no Linear range 104 105 103 104 Detection limit μg ng pg ng high low middle Unlimited Sensitive to temperature Property of mobile phase

Glossary n n n n Pump Valve Loop Gradient elution Refractive index Fluorescence Cell DAD (photodiode array detector)

4. 4 Types of HPLC (HPLC modules) n Chemically bonded phase HPLC n Adsorption HPLC n Ion pair chromatography n Ion exchange and ion chromatography n Size exclusion chromatography

4. 4. 1 Chemically bonded phase chromatography (化学键合相色谱法) ü Liquid-liquid Partition (液-液分配色谱) chromatography ü Normal phase liquid chromatography: polar stationary phase and less polar or nonpolar mobile phase ü Reverse phase liquid chromatography: Polar mobile phase and less polar or nonpolar stationary phase ü Disadvantage of the partition chromatography

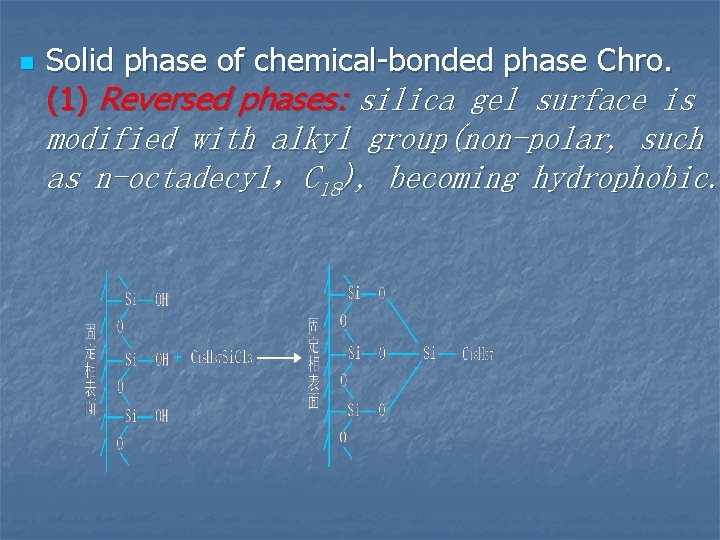
n Solid phase of chemical-bonded phase Chro. (1) Reversed phases: silica gel surface is modified with alkyl group(non-polar, such as n-octadecyl,C 18), becoming hydrophobic.

(2) More about the solid phase of RP-HPLC • Principle of the retaining of the analyte • Alkyl group and other groups • Stability of the solid phase (3) Normal phase: polar functional groups chemically modified on the surface of silica gel The group could be diol, cyano, amino, etc.

n Mobile phase of HPLC ① General rule Mobile phase play an important role on k Suitable k is between 2 and 5 ② Eluotropic series Polarity and polarity index of solvents Polarity of solvent mixtures ③ Elution strength Relationship between polarity of solvent used as mobile phase and solid phase Elution behavior of reversed phase chro. and normal phase chro.

④ Other properties of solvent used as mobile phase: chemical stable compatible to detector low viscosity (粘度) others (boiling point, toxicity and expense) ⑤ Strategy of choosing mobile phase Optimizing k and α through experience or systematic procedure according to some rules (typical solvents trial).

⑥ In reversed-phase Chro. : mixture of the solvents methanol, acetonitrile and tetrahydrofuran with water. In normal-phase chro. :mixture of diethyl ether, methylene chloride and chloroform with n-hexane ⑦ Isocratic elution and Gradient elution Troubles of gradient elusion (column, detector and pump)

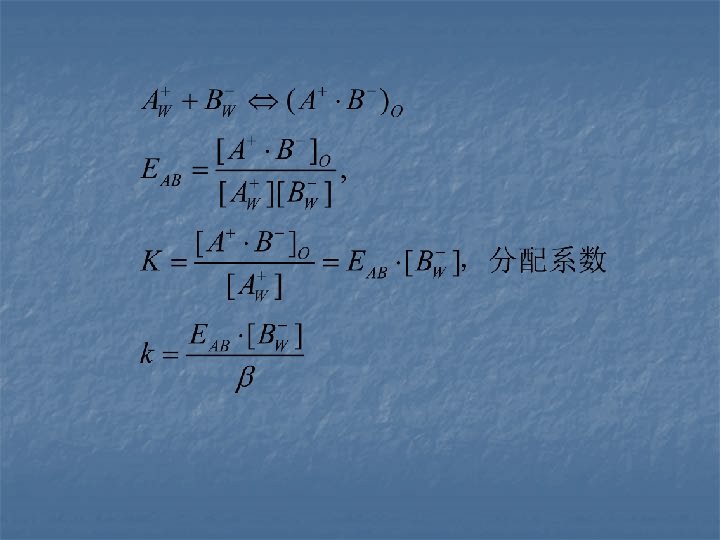
4. 4. 2 Adsorption chromatography of HPLC (liquid-solid chromatography) Mechanism of the separation By using solid absorbent as stationary phase and solvent as mobile phase, the solute of being analyzed could be absorbed by the active centers on the surface of the stationary phase and then desorbed by the solvent. Difference of this procession could cause the separation of different analyte.

Solid phase Polar phase:silica gel,alumina etc. non-polar phase:active carbon, polymer micro-bead

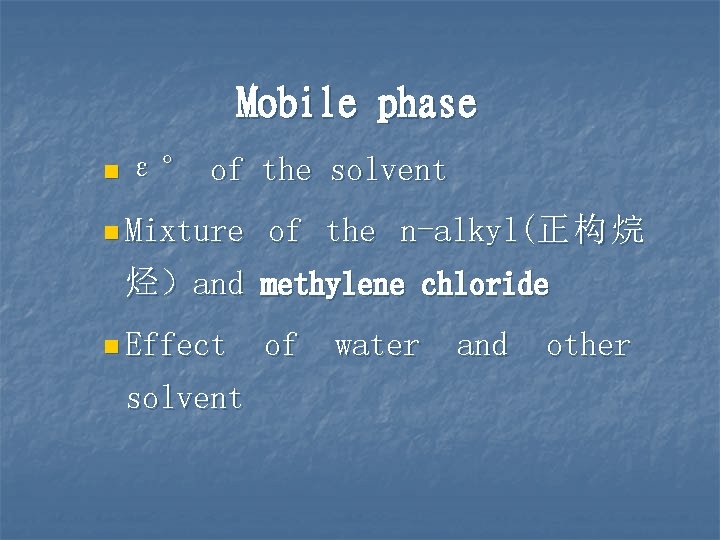
Mobile phase n ε° of the solvent n Mixture of the n-alkyl(正 构 烷 烃)and methylene chloride n Effect solvent of water and other

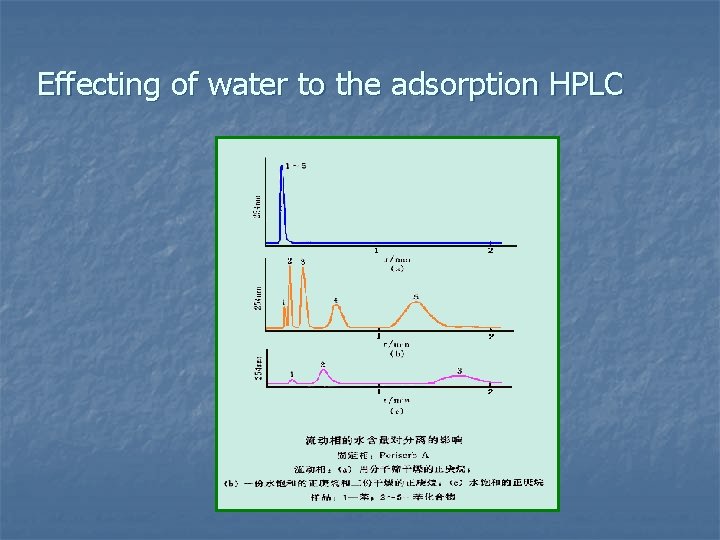
Effecting of water to the adsorption HPLC

(4)Application of adsorption HPLC n Suitable to nonpolar substances which are difficult to dissolve in water, such as fattiness and oil n Positional isomers or stereoisomers

Glossary n n n n n Chemically bonded phase HPLC Reverse Phase HPLC (RP-HPLC) Normal phase HPLC Liquid-liquid Partition HPLC C 18, C 8 , -NH 2, -CN Alkyl group Elution strength Methanol, acetonitrile absorbent

4. 3. 3 Ion pair chromatography (离子对色谱法) (1)Mechanism n If the analyte is ion(A+), which can not be retained by the solid phase, an counter ion (B-) is added into the mobile phase to combine with the ion(A+), forming an neutral molecule (ionpair complex) and then retained by the solid phase.


(2)Solid phase, moble phase and ion-pair reagent n Solid phase:C 18, C 8 n Mobile phase:water solution n Ion-pair reagent: (C 4 H 9)4 N+(四 丁 基 铵 正 离 子 )、 十 六 烷 基 三 甲 基 铵 正 离 子 , Cl. O 4 -,十二烷基磺酸根等

4. 4. 4 Ion chromatography (离子色谱法) and ion exchange chromatography (1) Mechanism n Exchange equilibrium of the ion-exchangers: cation exchange:resin—SO 3 -H++M+ = resin—SO 3 -M ++H + anion exchange:resin—NR 3+Cl-+X- = resin—NR 3+X -+Cl n Retention differences depend on the tendency existing on the resin matrix, while the stronger the tendency the bigger t. R of the ion. -

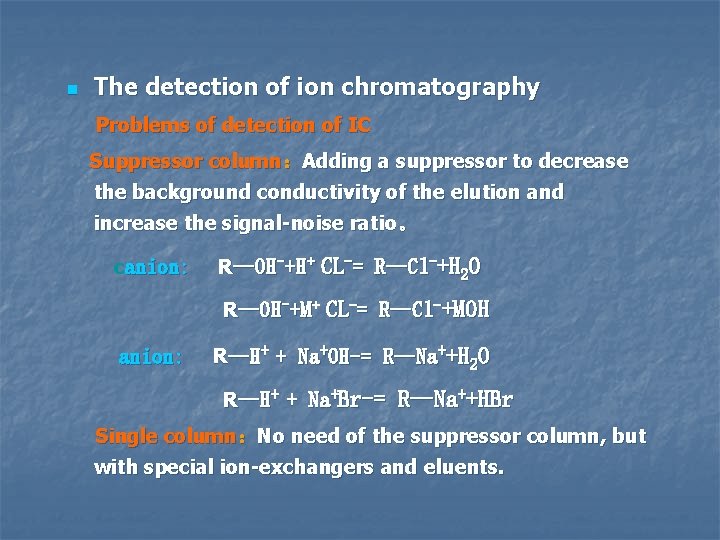
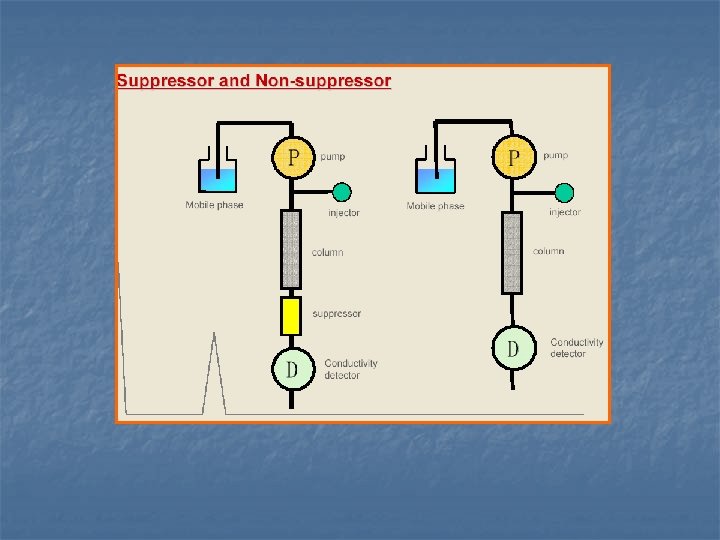
n The detection of ion chromatography Problems of detection of IC Suppressor column:Adding a suppressor to decrease the background conductivity of the elution and increase the signal-noise ratio。 canion: R—OH-+H+ CL-= R—Cl-+H 2 O R—OH-+M+ CL-= R—Cl-+MOH R—H+ + Na+OH-= R—Na++H 2 O R—H+ + Na+Br-= R—Na++HBr Single column:No need of the suppressor column, but with special ion-exchangers and eluents.


(2)Solid Phase ion-exchanger: Coating on supporter or kind of ion-bonded phase, the ion exchangers are divided exchangers (strong/weak). as and cation anion

(3)Mobile phase Ø Suppressor column Cation: HCl,HNO 3 Anion: Na. OH, Na. HCO 3/Na. CO 3。 Ø Single column Cation: HCl,HNO 3(low concentration); Anion: Salt of benzoic acid(苯甲酸), citric acid(柠檬酸) etc.

(4)Application of IC n Inorganic anion n Inorganic cation and organic acid, organic base, sugar etc. n Name of the ion chromatography and ion exchange chromatography

(5)Definition of ion chromatography and ion exchange chromatography

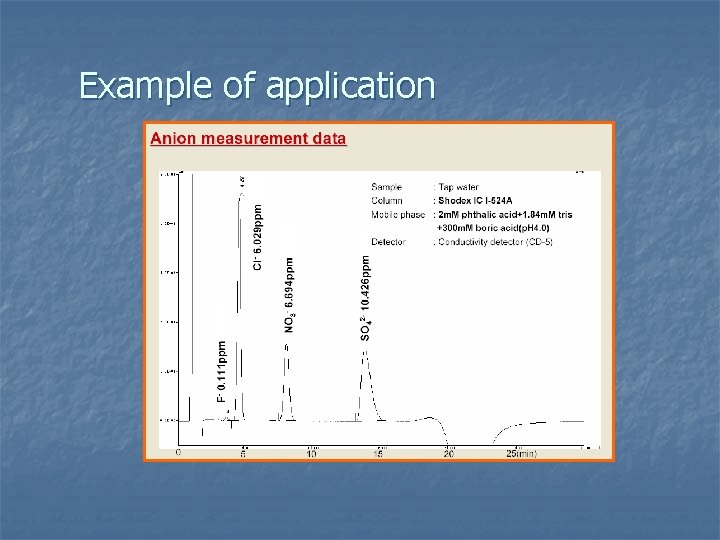
Example of application

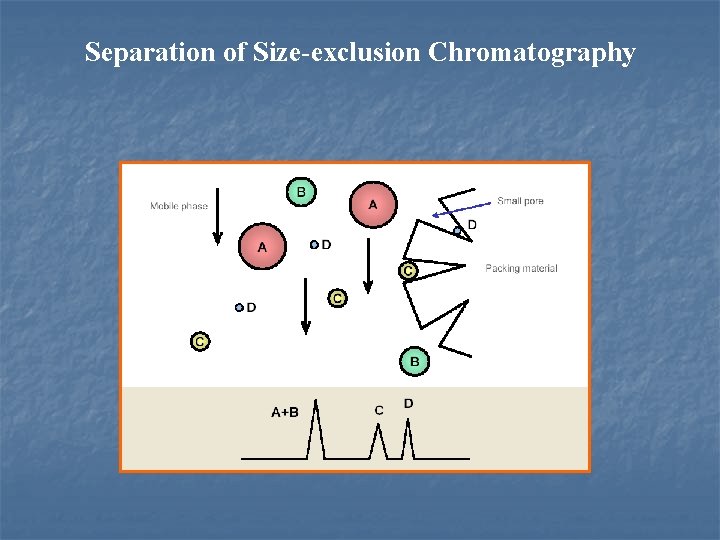
4. 4. 5 Size-exclusion chromatography (体积排阻色谱法)(SEC) (1) Mechanism l Special retention reason: the analyte is trapped into the pore of stationary phase. Separation of molecules is due to their different size --- excluded or retained according to the diameter of gel-pores.

Separation of Size-exclusion Chromatography

l Elution volume: VR VR=V 0+KD·Vp K=0, completely excluded K=1, free permeation 0<KD<1. 0 l Sample with different size (more than 10%).

l Water soluble: Gel filtration chromatography(凝胶过滤色谱法)(GFC), analyzing protein. (多肽、蛋白质、核酸、多糖 ). l Water insoluble: Gel permeation chromatography (GPC) (凝胶渗透色谱法), analyzing Mole Weight of high polymer (高聚 物)(如聚乙烯、聚氯乙烯等).

(2)Solid phase n Porous glass, silica particles, polymers, polysaccharides(多聚糖) n Pore size of packing materials and exclusion limit n Being hydrophobic or hydrophilic n Pressure limit

(3)Mobile phase n Sample-soluble n Solid phase and detector compatible n Buffers of water for GFC Tetrahydrofuran for GPC

(4)Application of SEC n n Separation and Analysis of big molecules Distribution of Molecular Weight of polymer

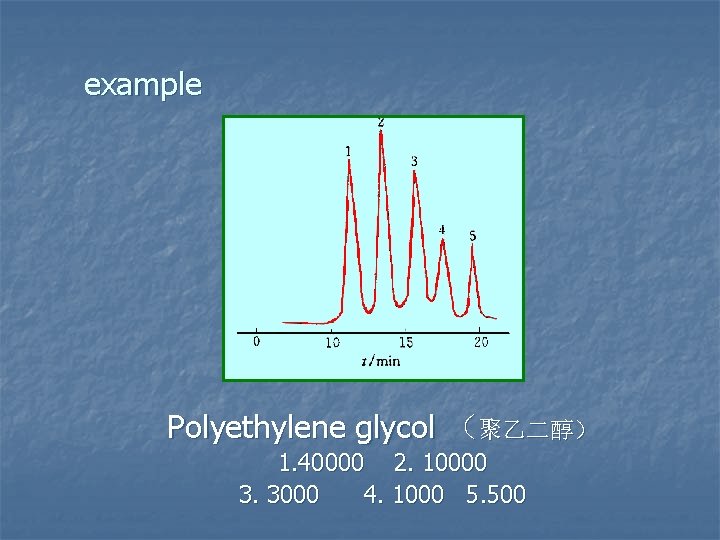
example Polyethylene glycol (聚乙二醇) 1. 40000 2. 10000 3. 3000 4. 1000 5. 500

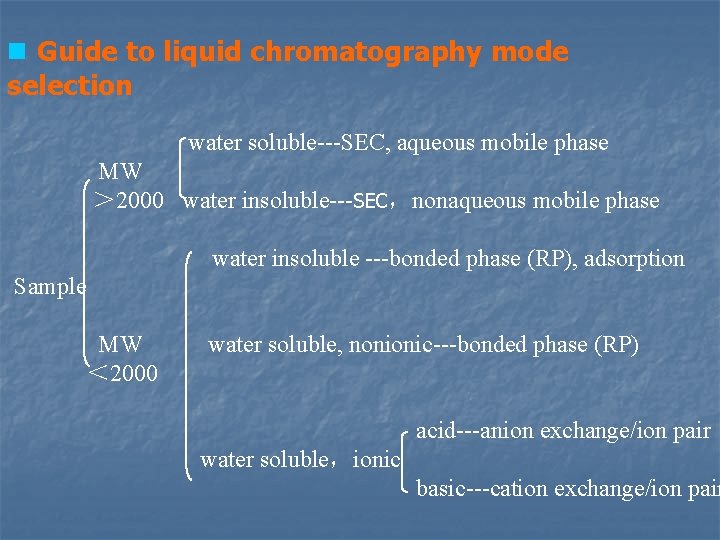
4. 4. 6 Guide to liquid chromatography mode selection n According to the analytical task n Concentration n interference (composition) n Information of the analyte Ø structure Ø molecular weight Ø acidity Ø solubility

n Guide to liquid chromatography mode selection water soluble---SEC, aqueous mobile phase MW > 2000 water insoluble---SEC,nonaqueous mobile phase water insoluble ---bonded phase (RP), adsorption Sample MW water soluble, nonionic---bonded phase (RP) < 2000 acid---anion exchange/ion pair water soluble,ionic basic---cation exchange/ion pair

n HPLC chromatographic conditions n Column n Mobile phase n Detectors and conditions n Preparation of sample

Glossary n n n Ion pair chromatography Counter-ion Ion pair reagent Ion chromatography ion-exchange chromatography Resin Cation Anion Suppressor column Size-exclusion chromatography gel-filtration and gel permeation

n n n n Displacement pump 活塞泵 diaphragm 隔膜 Hydraulic 水力的 液压的 Cartridge 柱 桶 Alkyl 烷基 Elutropic 洗脱的 Chiral 手性 isophthalic acid 异酞酸 Polysaccharid [生化]多醣, 聚糖, 多聚糖 Divinylbenzene [化]二乙烯基苯 Copolymerisate 共聚物 Homolog 同系物 Oligomer 低聚物 dextrane [化][药] 右旋糖苷 Phosphorescence 磷光
