High performance liquid chromatography HPLC Dr J Rajesh
























- Slides: 49

High performance liquid chromatography (HPLC) Dr. J. Rajesh Asso. Prof Dept of Chemistry MSEC, Kilakarai

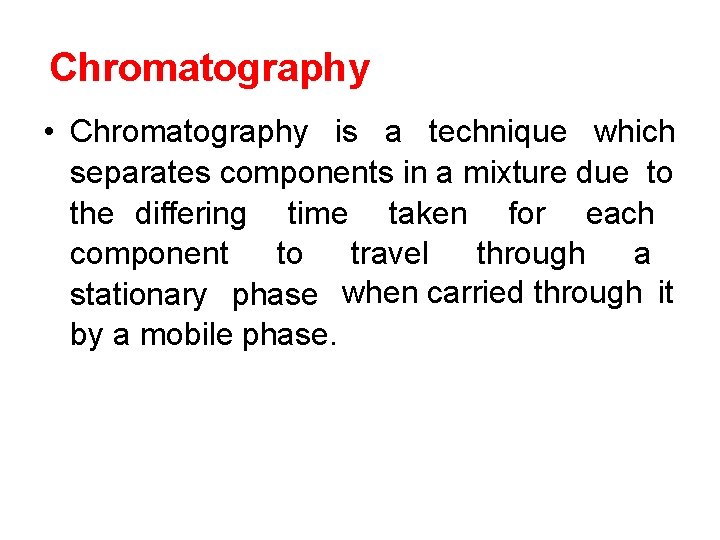
• A form of column chromatography to separate, identify, and quantify the compounds. • Developed in 1970 s. • The most widely used analytical separation technique.

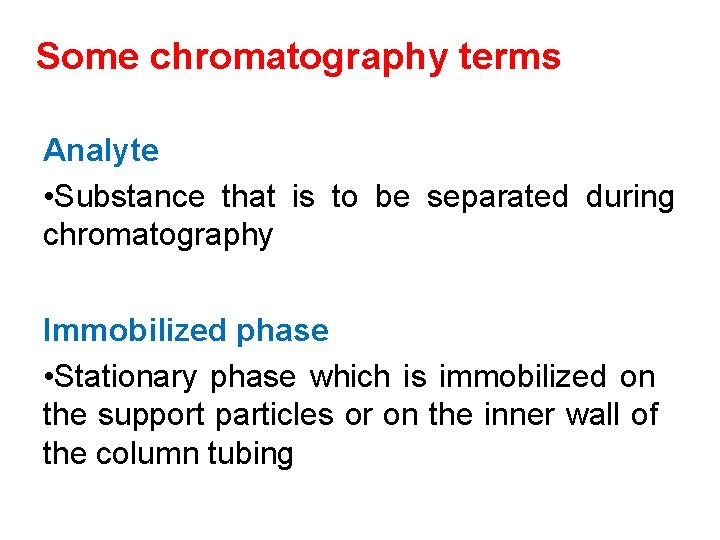
Chromatography • Chromatography is a technique which separates components in a mixture due to the differing time taken for each component to travel through a stationary phase when carried through it by a mobile phase.

Basically, all chromatographic systems consists of two phases. • Mobile phase - liquid or gaseous and flows over or through the stationary phase • Stationary phase - solid, liquid or a solid/liquid mixture which is immobilized

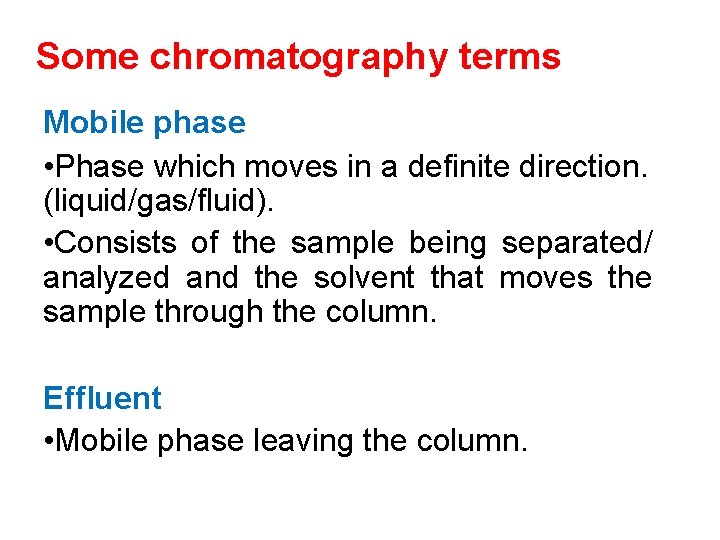
Some chromatography terms Analyte • Substance that is to be separated during chromatography Immobilized phase • Stationary phase which is immobilized on the support particles or on the inner wall of the column tubing

Some chromatography terms Mobile phase • Phase which moves in a definite direction. (liquid/gas/fluid). • Consists of the sample being separated/ analyzed and the solvent that moves the sample through the column. Effluent • Mobile phase leaving the column.

Different types of chromatography methods • Paper chromatography • Liquid chromatography • Gas chromatography • High performance liquid chromatography

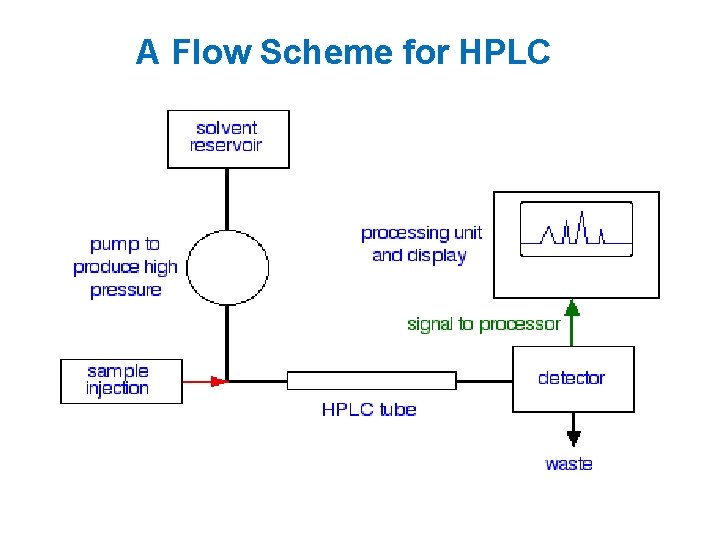
High performance liquid chromatography • HPLC is an extension of conventional liquid chromatography. • Powerful tool in analytical techniques • Columns are tightly packed, and the eluent is forced through the column under high pressure(up to 5, 000 psi) by a pump.

• Allows to use a very smaller particle size for the column packing material which gives a much greater surface area for interactions between the stationary phase and the molecules flowing through it. • Allows a much better separation of the components of the mixture.

HPLC Technique • Utilizes liquid mobile phase to separate the mixture • Analytes are first dissolved in a solvent then through the column under high pressure of up to 400 atm • Mixture is resolved into its components in the column

• The total separation time is often 5 or 10 minutes rather than hours or even days required for some separations by gravity flow with the larger systems.

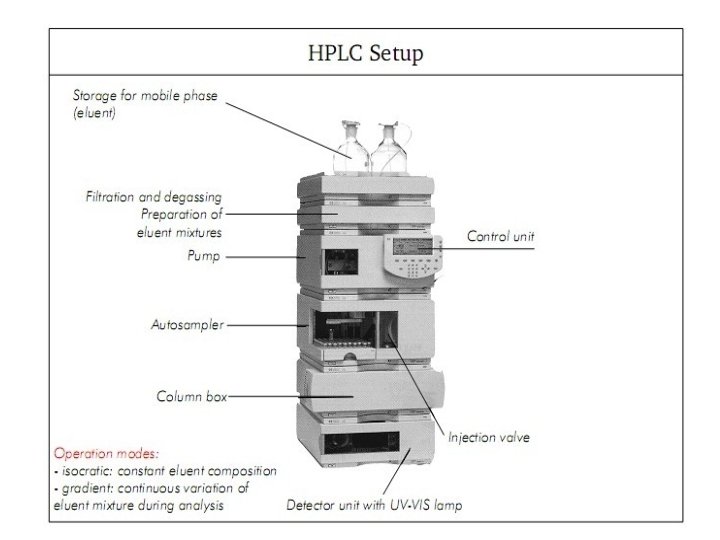
Components of HPLC • • • Pump Injector Column Detector Recorder or data system

A Flow Scheme for HPLC


Pump • A pump forces the mobile phase through the column at a much greater velocity than gravity-flow columns. • The pump can be pneumatic, syringetype, reciprocating, or hydraulic amplifier.

Pump (cont. ) are • Pneumatic pumps preoperative purposes. used for • The most widely used pump today is the multihead pump with two or more reciprocating pistons.

• Pumps are designed in order to maintain a stable flow rate, avoiding pulsations even when the composition of the mobile phase varies • flow range – 0. 01 -10 ml/min

HPLC Pump

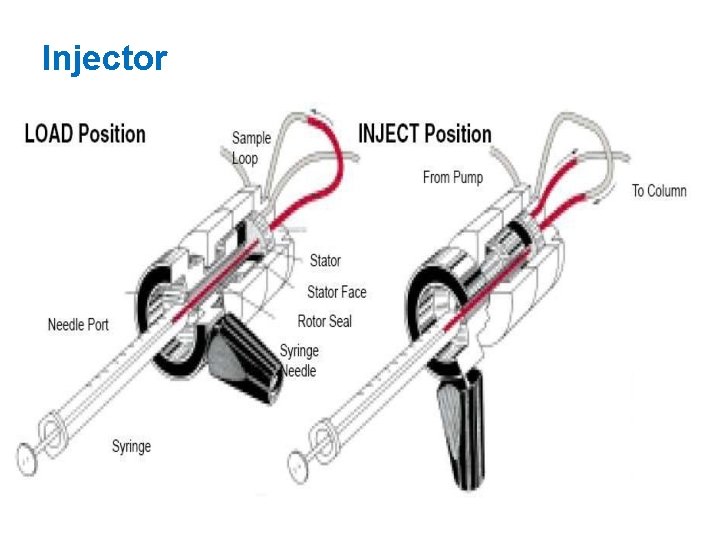
Injectors • Inject the liquid sample within range of 0. 1 - 100 ml of volume under high pressure • Produce minimum band broadening • Produce possible flow disturbances • Volume must be small (0. 1 -500 u. L)

A model injector

Injector

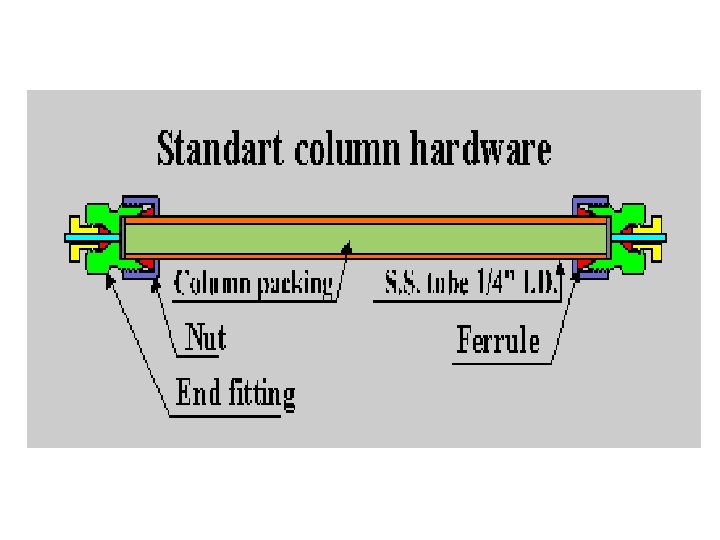
Columns • Smooth-bore stainless walled glass tubing. steel or heavy- • Hundreds of packed columns differing in size and packing are available from manufacture.

Columns • E. g. Column packing vary in size (3 to 20 um) with the smaller particles used mostly for analytical separations and the larger ones for preparative separation. • The most common material used for column packing is silica gel.

HPLC columns


Detector • HPLC detectors monitor the elute as it leaves the column • Produce an electronic signal proportional to the concentration of each separated component

Detector • • • Crucial in trace analysis High sensitivity Fast response Simplifies quantitation Insensitive to changes in type of solvent, flow rate and temp.

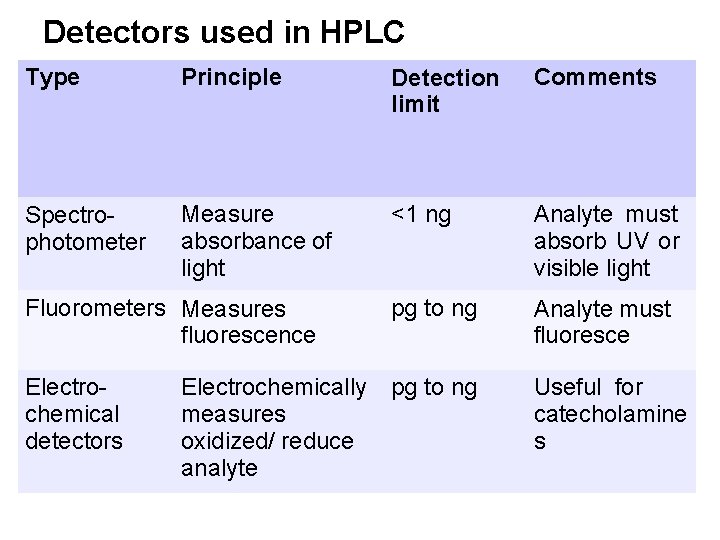
The most widely used detection methods • • • Spectrophotometers Fluorometers Electrochemical detectors Mass spectrometer Refractive index detector

Detectors used in HPLC Type Principle Detection limit Comments Spectrophotometer Measure absorbance of light <1 ng Analyte must absorb UV or visible light Fluorometers Measures fluorescence pg to ng Analyte must fluoresce Electrochemical detectors pg to ng Useful for catecholamine s Electrochemically measures oxidized/ reduce analyte

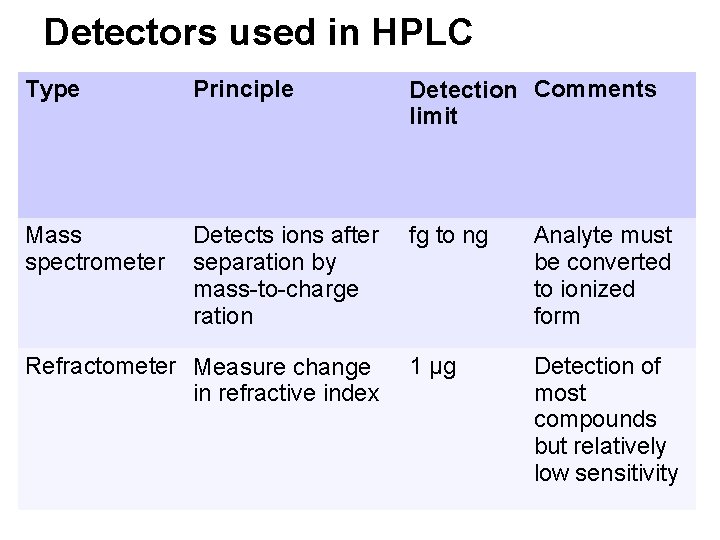
Detectors used in HPLC Type Principle Detection Comments limit Mass spectrometer Detects ions after separation by mass-to-charge ration fg to ng Analyte must be converted to ionized form 1 µg Detection of most compounds but relatively low sensitivity Refractometer Measure change in refractive index

Depending on the relative polarity of the solvent and stationary phase, there are two variants in use in HPLC 1. Normal phase HPLC • Utilize polar adsorbent surface and nonpolar eluent • Polar substance in the mixture sticks to polar adsorbent than non-polar • Non-polar ones will pass more quickly through the column

2. Reversed phase HPLC • Utilize non-polar adsorbent surface and polar eluent • Attraction between non-polar compound in the mixture and non-polar adsorbent

2. Reversed phase HPLC (cont. ) • Polar molecules will travel through the column more quickly because there is strong attraction between polar solvent and polar molecules when pass through the column • Reversed phase HPLC is the most commonly used form of HPLC

Solvents used in mobile phase • hexane, heptane, cyclohexane, carbon tetrachloride, benzene, toluene, diethyl ether, chloroform etc. Adsorbents used in stationary phase • silica gel, alumina, celite, cellulose powder, ion-exchange, cellulose, starch

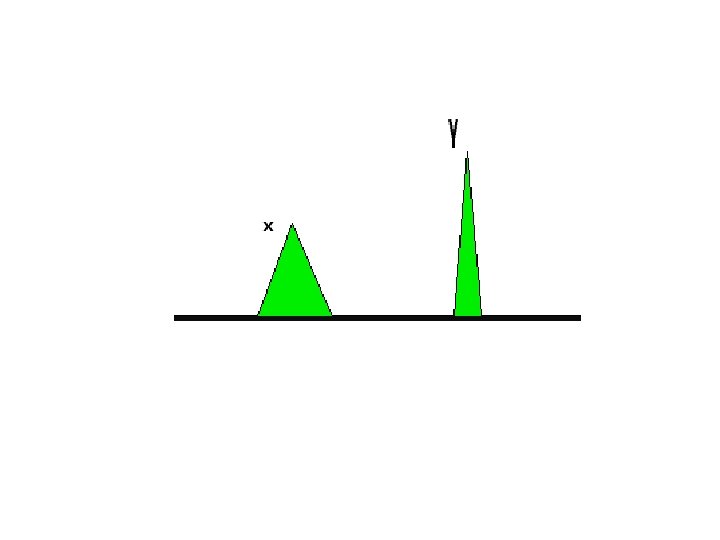
Retention time • The time taken for a particular compound to travel through the column to the detector • From the time at which the sample is injected to the point at which the display shows a maximum peak height for that compound.


Types of chromatic separation • Adsorption Chromatography • Ion- exchange Chromatography • Size Exclusion Chromatography

Adsorption Chromatography • Competition for adsorption sites occurs between the molecules of the mixture to be separated and the molecules of the mobile phase • Mobile phase can be either a single solvent or two or more solvents depend on the analytes to be desorbed • Speed of migration of the component along the column depend on adsorptive affinity

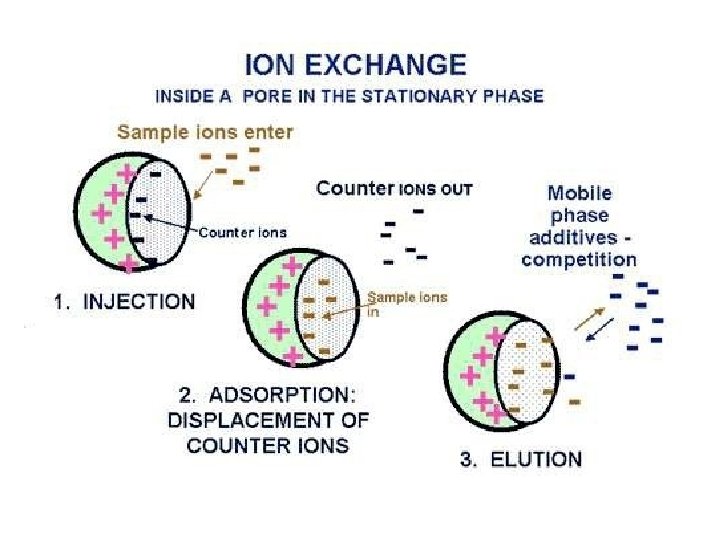
Ion- exchange Chromatography • Molecules can be separated by their ionic charges in a process known as Ionexchange Chromatography. • Ion-exchange resins are used as the column packing materials. • This method is used for separation of ionic species, such as amino acids.


Size Exclusion Chromatography • Known as gel permeation chromatography or gel filtration chromatography. • Packing material with very small pore is used. • Precisely controlled pore size materials in the column • Large molecules, such as polymers are physically prevented from passing through the column

Applications HPLC is used for • Chemistry and biochemistry research analyzing complex mixtures, • Purifying chemical compounds • Quality control to ensure the purity of raw materials • Analyzing air and water pollutants,

Applications (cont. ) • Monitoring materials that may jeopardize occupational safety or health • Monitoring pesticide levels in the environment. • To survey food and drug products, • To identify confiscated narcotics • To determine the amount of such chemical compounds found in new drugs in pharmaceutics

HPLC as compared with the classical technique • Small diameter, reusable stainless steel columns • Column packing with very small particles • Control flow of mobile phase • Precise sample introduction

HPLC as compared with the classical technique (cont. ) • Good pumping system • Special continuous flow detectors- can handle small flow rates and detect very small amounts • Rapid analysis • High resolution

Disadvantages of HPLC • • Cost Complexity Low sensitivity for some compounds Irreversibly adsorbed compounds not detected • Co-elution difficult to detect

Summary • The modern day technique is greatly enhanced in terms of selectivity, resolution, through miniaturization and the use of very elaborate stationary phases. • Therefore HPLC is widely used for separation of molecules in biological, pharmaceutical, food, environmental and industrial process

References: • Harold Varley practical clinical chemistry • http: //scimedia. com • http: //www. forumsci. co. il/HPLC

THANK YOU!