10292020 1 I Introduction II Classification of chromatographic













- Slides: 35

10/29/2020 1

I. Introduction II. Classification of chromatographic methods III. Principle of chromatography IV. High performance liquid chromatography (HPLC) 10/29/2020 V. Gas chromatography (GC) VI. Thin layer chromatography (TLC) 2

Definition: Chromatography is defined as a procedure by which solutes are separated by dynamic differential migration process in a system consisting of two or more phases, one of which moves continuously in a given direction and in which the individual substances exhibit different mobilities by reason of differences in adsorption, partition, solubility, vapor pressure, molecular size, or ionic charge density. 10/29/2020 3

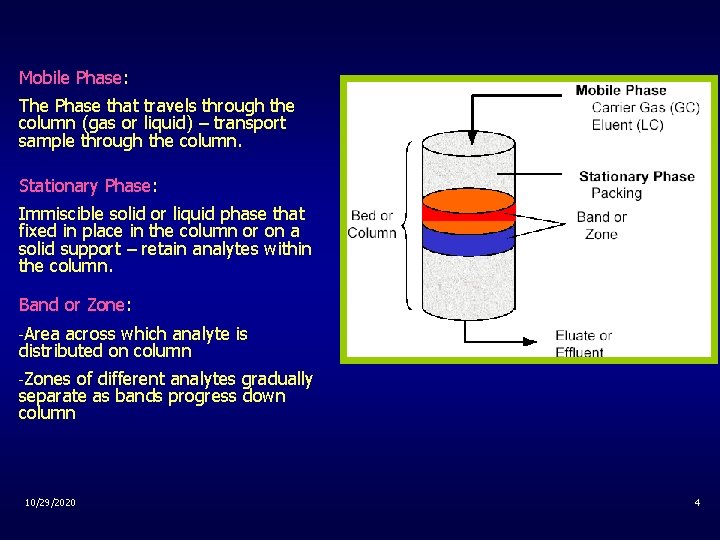
Mobile Phase: The Phase that travels through the column (gas or liquid) – transport sample through the column. Stationary Phase: Immiscible solid or liquid phase that fixed in place in the column or on a solid support – retain analytes within the column. Band or Zone: -Area across which analyte is distributed on column -Zones of different analytes gradually separate as bands progress down column 10/29/2020 4

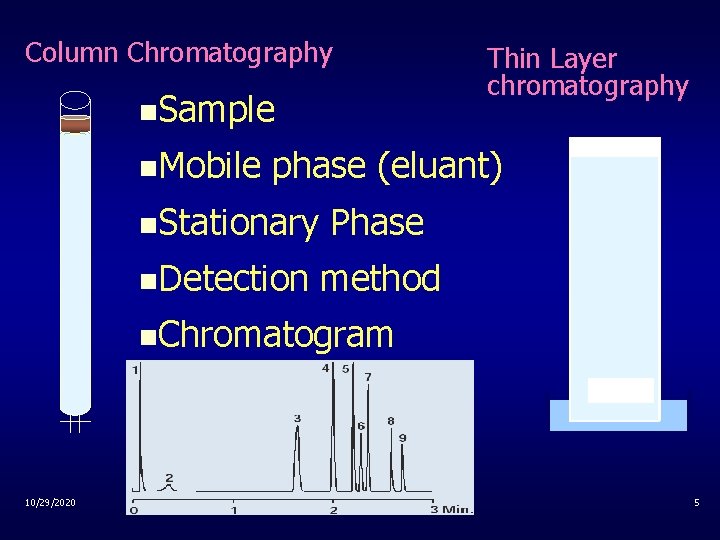
Column Chromatography n. Sample n. Mobile Thin Layer chromatography phase (eluant) n. Stationary n. Detection Phase method n. Chromatogram 10/29/2020 5

n n Method to separate components in a mixture based on different Distribution coefficients between the two phases. Chromatography categorized on the basis of interaction between solute and stationary phase Mobile phase either gas or liquid Stationary phase either liquid or solid – Liq/Liq (Partition) Liquid – Liq/Sol (Adsorption) Chromatography – Gas/Liq (Partition) – Gas/Sol (Adsorption) 10/29/2020 Gas Chromatography 6

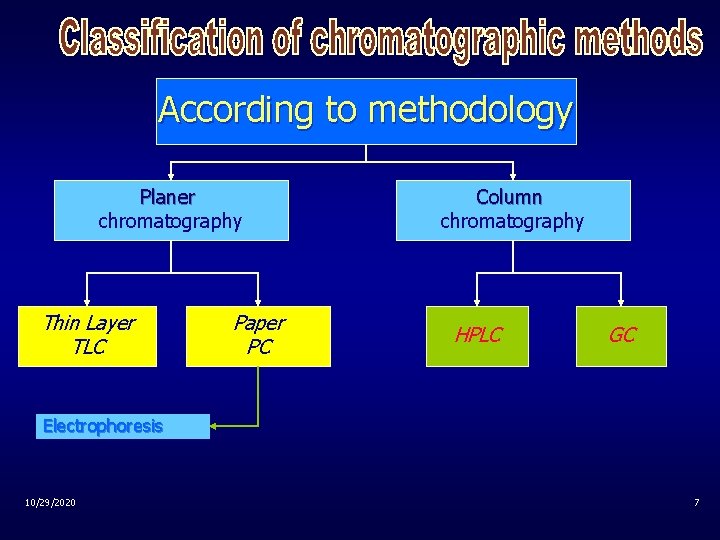
According to methodology Planer chromatography Thin Layer TLC Paper PC Column chromatography HPLC GC Electrophoresis 10/29/2020 7

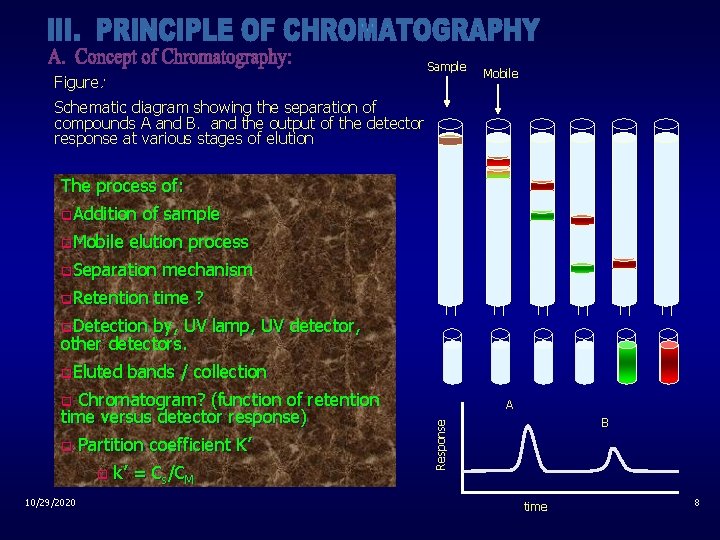
Sample Figure: Mobile Schematic diagram showing the separation of compounds A and B. and the output of the detector response at various stages of elution The process of: q. Addition q. Mobile of sample elution process q. Separation q. Retention mechanism time ? q. Detection by, UV lamp, UV detector, other detectors. q. Eluted bands / collection Chromatogram? (function of retention time versus detector response) q Partition coefficient K’ q 10/29/2020 k’ = Cs/CM B Response q A time 8

10/29/2020 9

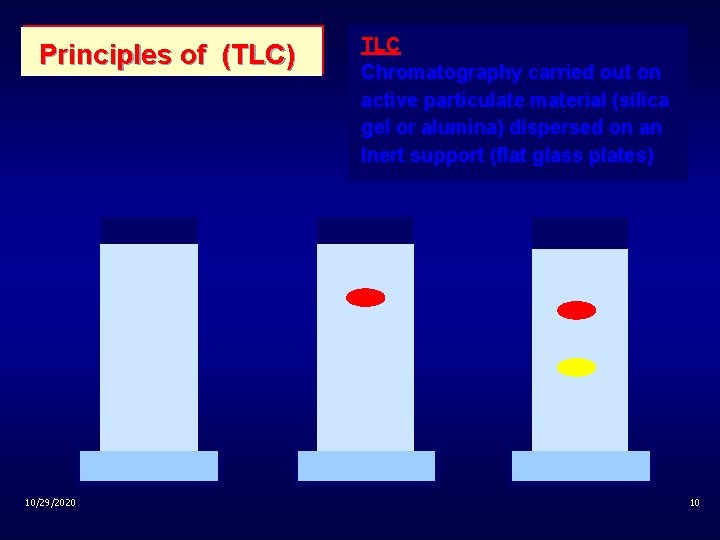
Principles of (TLC) 10/29/2020 TLC Chromatography carried out on active particulate material (silica gel or alumina) dispersed on an Inert support (flat glass plates) 10

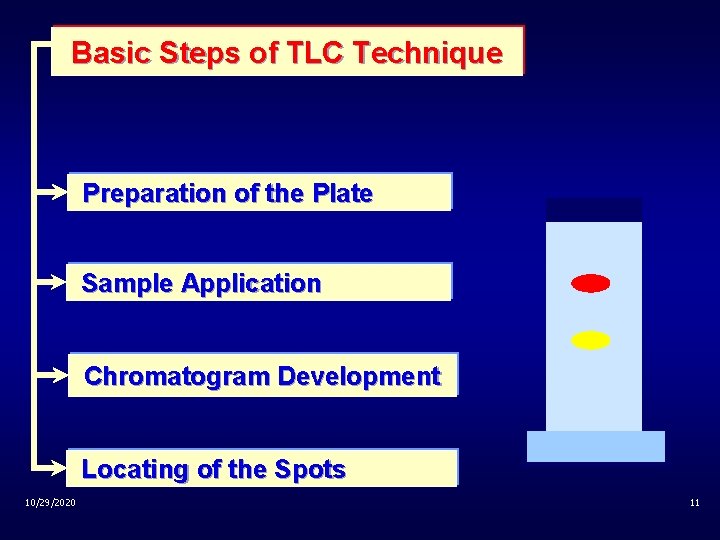
Basic Steps of TLC Technique Preparation of the Plate Sample Application Chromatogram Development Locating of the Spots 10/29/2020 11

Preparation of the Plate § Slurry of the active material is uniformly spread over the plate by means of a commercially available spreader. § Air-drying overnight, or oven-drying at 80 -90 C for about 30 minutes. § Ready to use thin layers (pre-coated plates) are commercially available. 10/29/2020 12

Sample Application 1 -2 cm Base line 2 -2. 5 cm 10/29/2020 13

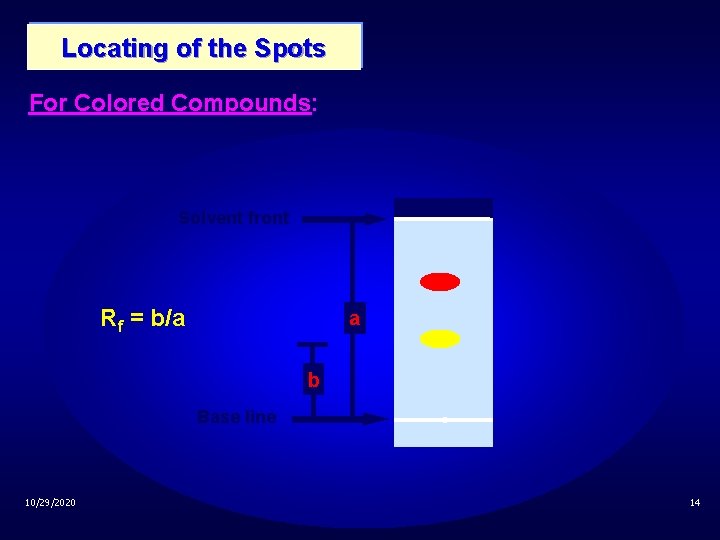
Locating of the Spots For Colored Compounds: Solvent front Rf = b/a a b Base line 10/29/2020 14

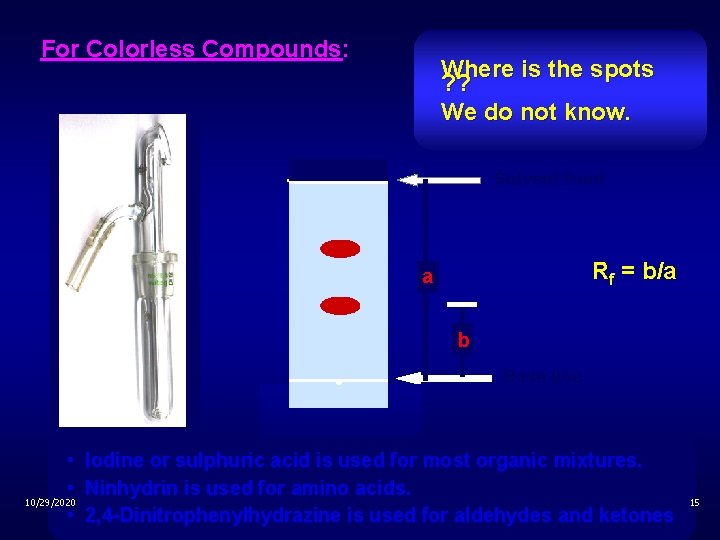
For Colorless Compounds: Where is the spots ? ? We do not know. Solvent front Rf = b/a a b Base line • Iodine or sulphuric acid is used for most organic mixtures. • Ninhydrin is used for amino acids. 10/29/2020 • 2, 4 -Dinitrophenylhydrazine is used for aldehydes and ketones 15

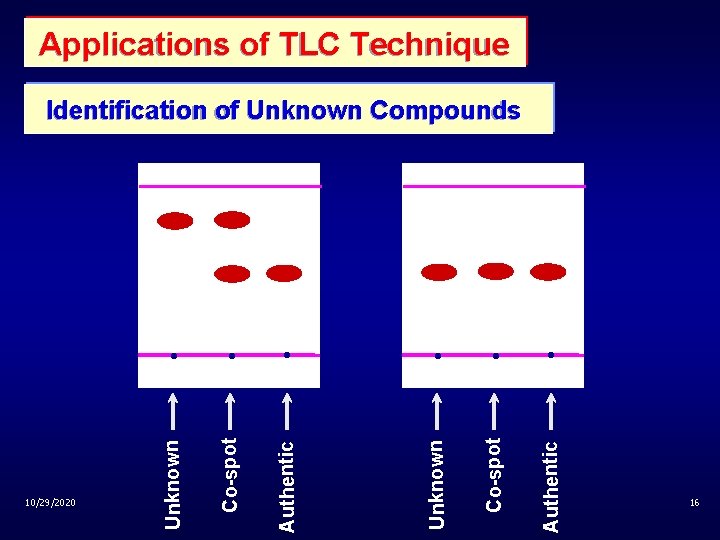
Applications of TLC Technique Co-spo t Authentic Unknown Co-spo t Authentic 10/29/2020 Unknown Identification of Unknown Compounds 16

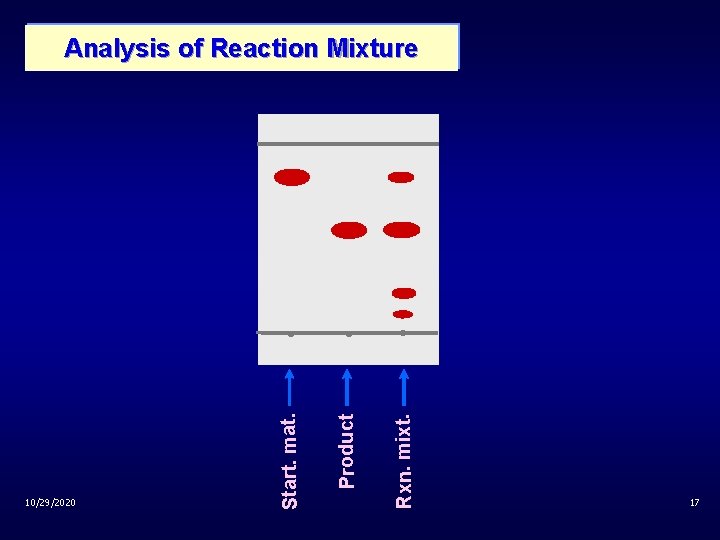
Product Rxn. mixt. 10/29/2020 Start. mat. Analysis of Reaction Mixture 17

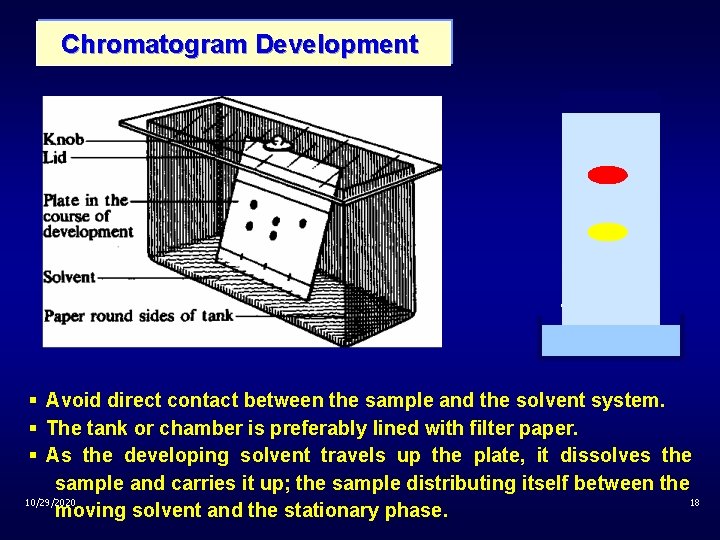
Chromatogram Development § Avoid direct contact between the sample and the solvent system. § The tank or chamber is preferably lined with filter paper. § As the developing solvent travels up the plate, it dissolves the sample and carries it up; the sample distributing itself between the 10/29/2020 18 moving solvent and the stationary phase.

Determination of the Purity of a Product Compound Product compound Impurities 10/29/2020 19

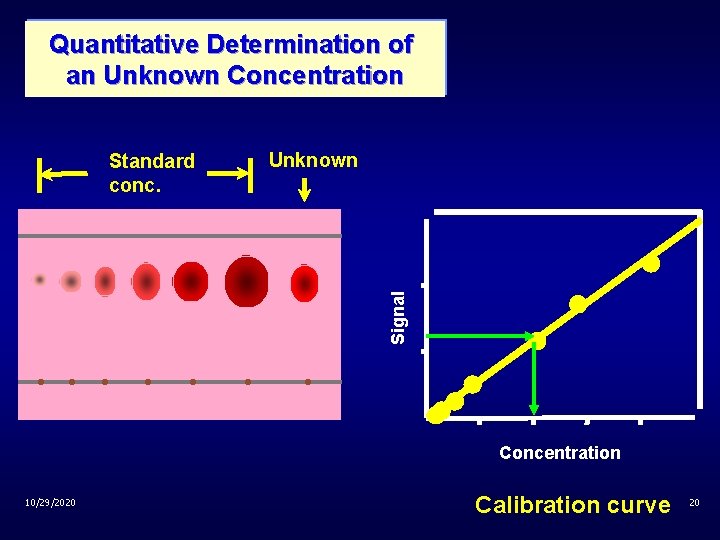
Quantitative Determination of an Unknown Concentration Unknown Signal Standard conc. Concentration 10/29/2020 Calibration curve 20

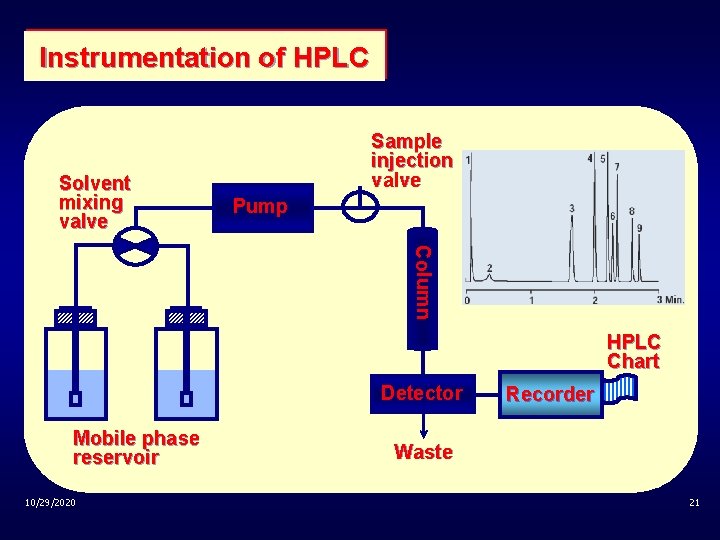
Instrumentation of HPLC Solvent mixing valve Sample injection valve Pump Column HPLC Chart Detector Mobile phase reservoir 10/29/2020 Recorder Waste 21

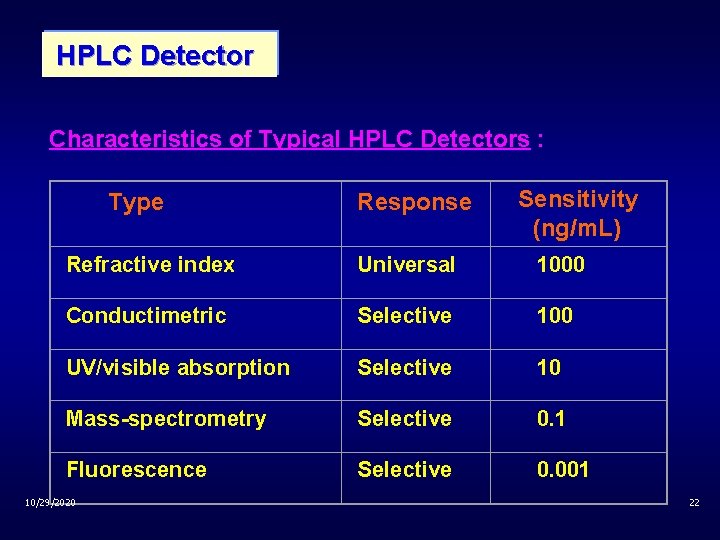
HPLC Detector Characteristics of Typical HPLC Detectors : Type Response Sensitivity (ng/m. L) Refractive index Universal 1000 Conductimetric Selective 100 UV/visible absorption Selective 10 Mass-spectrometry Selective 0. 1 Fluorescence Selective 0. 001 10/29/2020 22

HPLC Recorder Solvent mixing valve injection valve Pump Column Chart Detector Mobile phase reservoir 10/29/2020 Recorder Waste 23

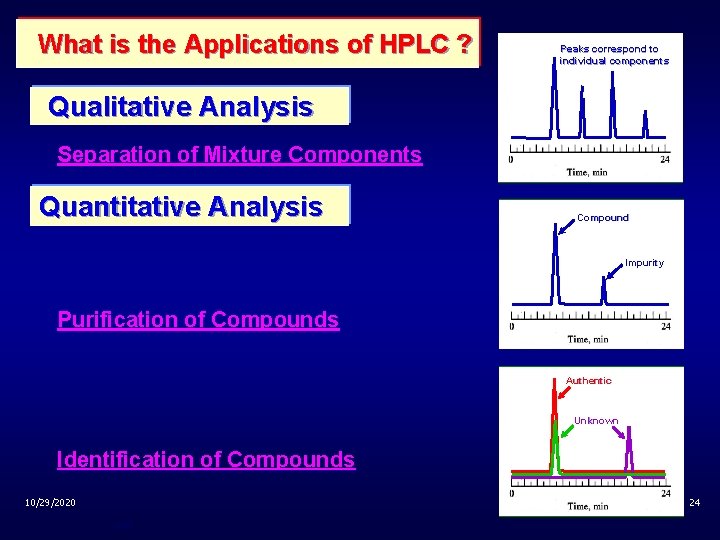
What is the Applications of HPLC ? Peaks correspond to individual components Qualitative Analysis Separation of Mixture Components Quantitative Analysis Compound Impurity Purification of Compounds Authentic Unknown Identification of Compounds 10/29/2020 24

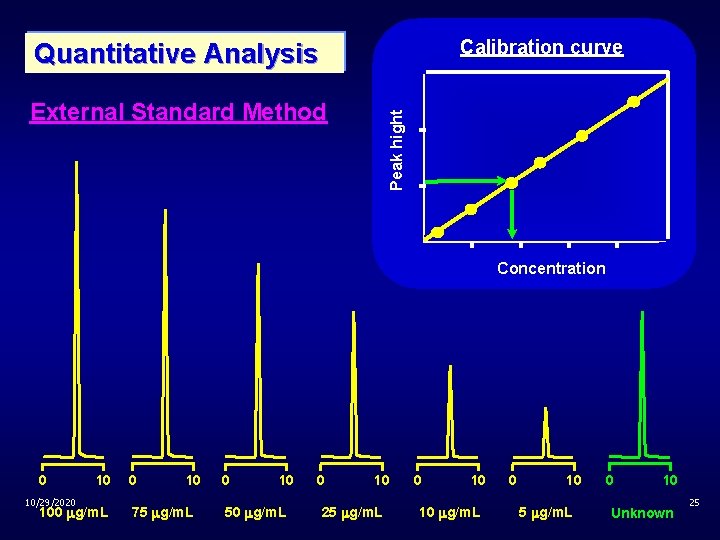
Calibration curve Quantitative Analysis Peak hight External Standard Method Concentration 0 10/29/2020 10 100 g/m. L 0 10 75 g/m. L 0 10 50 g/m. L 0 10 25 g/m. L 0 10 10 g/m. L 0 10 5 g/m. L 0 10 Unknown 25

G C 10/29/2020 26

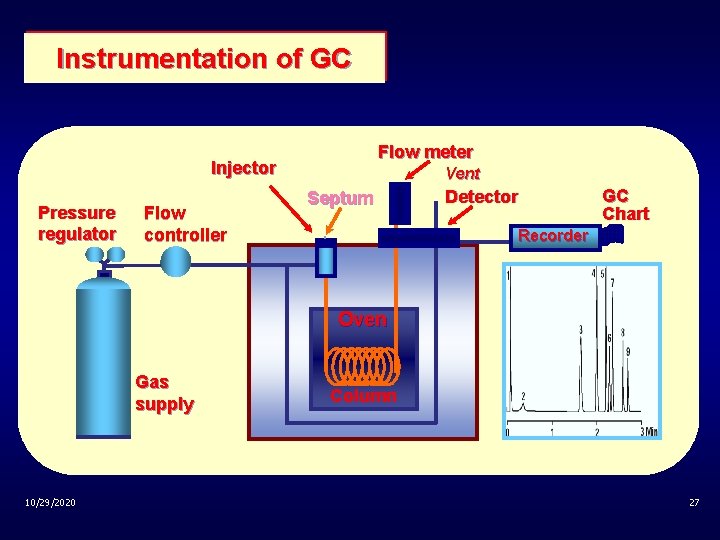
Instrumentation of GC Flow meter Injector Pressure regulator Flow controller Vent Septum Detector GC Chart Recorder Oven Gas supply 10/29/2020 Column 27

GC Column n Packed column * * n ~ 3 -6 mm inner diameter tubing, 1 -5 m long used for preparative separations or to separate gases that are poorly retained lower resolution small, uniform particle size decreases Eddy diffusion (requiring higher pressures) open tubular (more common): * * * 10/29/2020 0. 1 -0. 5 mm inner dia. , 10 -100 m long 0. 1 -5 mm thick sp coated on inner walls higher resolution, shorter analysis times, greater sensitivity compared to packed columns 28

Detectors n Flame Ionization Detector (FID): cathode (collects CHO+ ions) anode air H 2 10/29/2020 column effluent 29

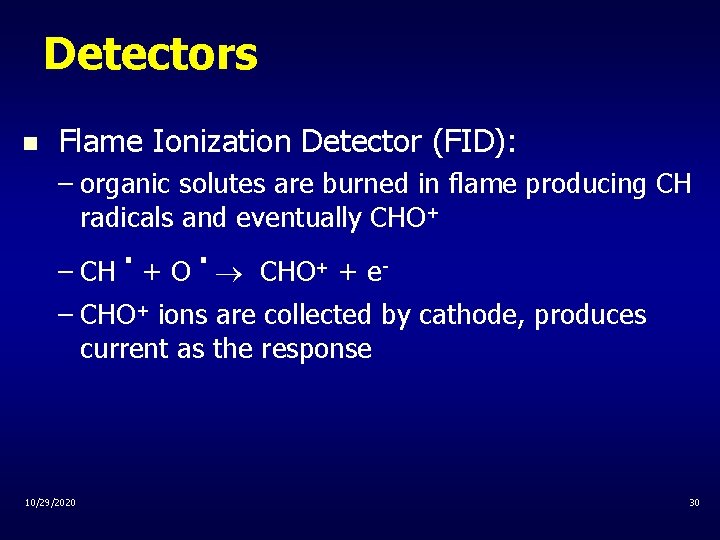
Detectors n Flame Ionization Detector (FID): – organic solutes are burned in flame producing CH radicals and eventually CHO+ . . – CH + O CHO+ + e– CHO+ ions are collected by cathode, produces current as the response 10/29/2020 30

Applications of GC ? Peaks correspond to individual components Qualitative Analysis Separation of Mixture Components: Quantitative Analysis Authentic Identification of Compounds: Unknown Retention time comparsion Pyrolysis gas chromatography It is used for the identification of non-volatile materials (plastics, natural and synthetic polymers, and some microbiological materials. It is based on the fingerprint chromatogram for the sample, which results from its thermal dissociation and fragmentation. 10/29/2020 31

Calibration curve Quantitative Analysis Peak hight External Standard Method Concentration 0 10/29/2020 10 100 ng/m. L 0 10 75 ng/m. L 0 10 50 ng/m. L 0 10 25 ng/m. L 0 10 10 ng/m. L 0 10 5 ng/m. L 0 10 Unknown 32

Aspects of GC Applications: Food Analysis of foods is concerned with confirm the presence and determination the quantities of the analytes (lipids, proteins, carbohydrates, preservatives, flavours, colorants, and also vitamins, steroids, and pesticide residues). Drug Analysis GC is widely applied to identification of the active components, possible impurities as well as the metabolites. 10/29/2020 33

Environmental Analysis The environmental contaminants; e. g. dichlorodiphenyltrichloroethane (DDT) and the polychlorinated biphenyls (PCBs) are present in the environment at very low concentrations and are found among many of other compounds. GC, with its high sensitivity and high separating power, is mostly used in the analysis of environmental samples. Forensic Analysis In forensic cases, very little sample is available, and the concentration of the sample components may be very low. GC is a useful due to its high sensitivity and separation efficiency. 10/29/2020 34

10/29/2020 35
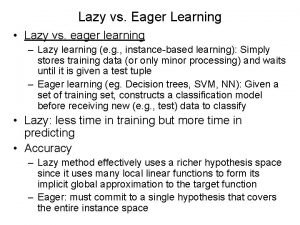
 Lazy and eager learning in machine learning
Lazy and eager learning in machine learning Manifold classification example
Manifold classification example Traditional classification vs modern classification
Traditional classification vs modern classification Introduction of classification of computer
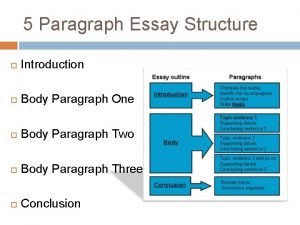
Introduction of classification of computer Body paragraph structure
Body paragraph structure Biofuel classification
Biofuel classification What are the three classes of yeast breads
What are the three classes of yeast breads Tortoise classification
Tortoise classification Lion
Lion Ax 86s
Ax 86s Wound type
Wound type Distal humerous
Distal humerous What is wind energy conversion system
What is wind energy conversion system Can i come over
Can i come over Group dances having special distinctive features
Group dances having special distinctive features Basic english grammar topics
Basic english grammar topics Caesalpiniaceae classification
Caesalpiniaceae classification Apg system of classification
Apg system of classification Front and back vowels are classified according to
Front and back vowels are classified according to Classification of vowel
Classification of vowel Baltimore classification
Baltimore classification What is verb
What is verb Strongly flavored vegetables
Strongly flavored vegetables Classification of vegetables
Classification of vegetables Sphenoid paranasal sinus
Sphenoid paranasal sinus Lathe classification
Lathe classification Chicago classification of achalasia
Chicago classification of achalasia Hci classification
Hci classification Munitions history program answers
Munitions history program answers What is the classification of sucrose
What is the classification of sucrose Classification lab answer key
Classification lab answer key Classification of primates
Classification of primates Difference between primitive and non primitive data types
Difference between primitive and non primitive data types Pug mill diagram
Pug mill diagram Uscs classification flow chart
Uscs classification flow chart Which kingdoms include only prokaryotes
Which kingdoms include only prokaryotes