Practical HPLC In This Section We Will Discuss





- Slides: 31

Practical HPLC

In This Section, We Will Discuss: How to set up an HPLC System for a sample injection including: · Solvent Handling · Mobile Phase preparation · Priming the HPLC · Column Handling - Equilibration · System Performance Checks 2

Solvent Handling Solvent Characteristics (Specifications): · · · · Purity Viscosity Refractive index Boiling Point Toxicity UV Transparency/UV-Cutoff Solubility 3

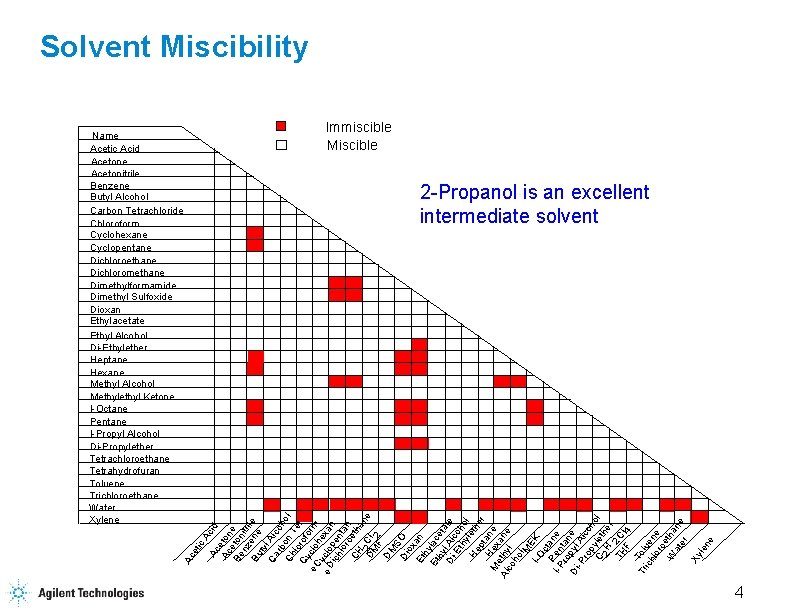
Solvent Miscibility Immiscible Miscible ic A Ac cid Ac eton e e Be ton nz itri Bu ene le ty Ca l Alc rb oh Ch on T ol lo e Cy rofo t c e. C lo rm y he e D clo xan ich pen lor ta o n CH eth a 2 Cl ne DM F 2 DM S Di O ox Et an hy Et lac hy eta Di l Alc te -E o th ho y l He leth er pt a M Hex ne Al ethy ane co l ho l. M E I-O K c P ta I-P en ne ro tan Di pyl e A -P ro lco py h C leth ol 2 H er TH 2 Cl F 4 Tr Tolu ich e lor ne oe W tha at er ne Xy len e 2 -Propanol is an excellent intermediate solvent Ac et Name Acetic Acid Acetone Acetonitrile Benzene Butyl Alcohol Carbon Tetrachloride Chloroform Cyclohexane Cyclopentane Dichloroethane Dichloromethane Dimethylformamide Dimethyl Sulfoxide Dioxan Ethylacetate Ethyl Alcohol Di-Ethylether Heptane Hexane Methyl Alcohol Methyl Ketone I-Octane Pentane I-Propyl Alcohol Di-Propylether Tetrachloroethane Tetrahydrofuran Toluene Trichloroethane Water Xylene 4

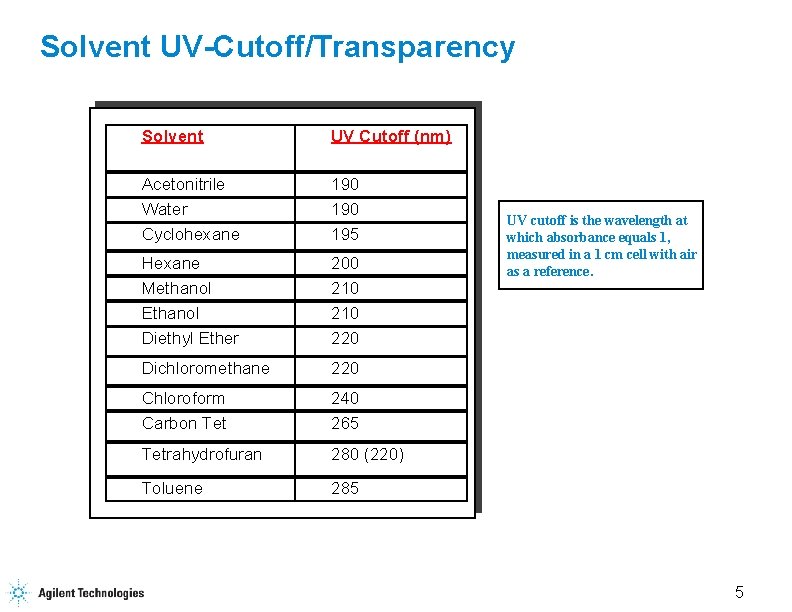
Solvent UV-Cutoff/Transparency Solvent UV Cutoff (nm) Acetonitrile Water Cyclohexane 190 195 Hexane Methanol Ethanol Diethyl Ether 200 210 220 Dichloromethane 220 Chloroform Carbon Tet 240 265 Tetrahydrofuran 280 (220) Toluene 285 UV cutoff is the wavelength at which absorbance equals 1, measured in a 1 cm cell with air as a reference. 5

Mobile Phase Preparation Major Steps: · · · Measure appropriate volume of each solvent Mix solvents Add buffers and additives* Filter mobile phase Degas mobile phase 6

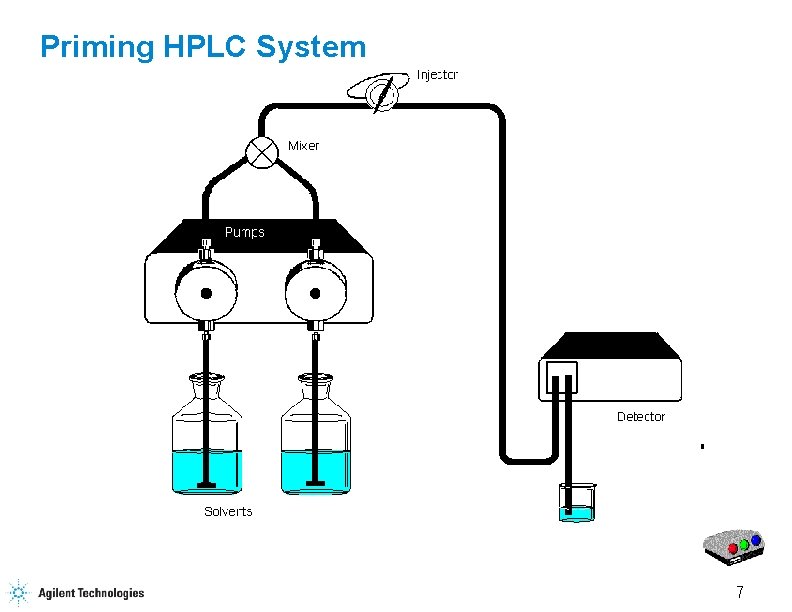
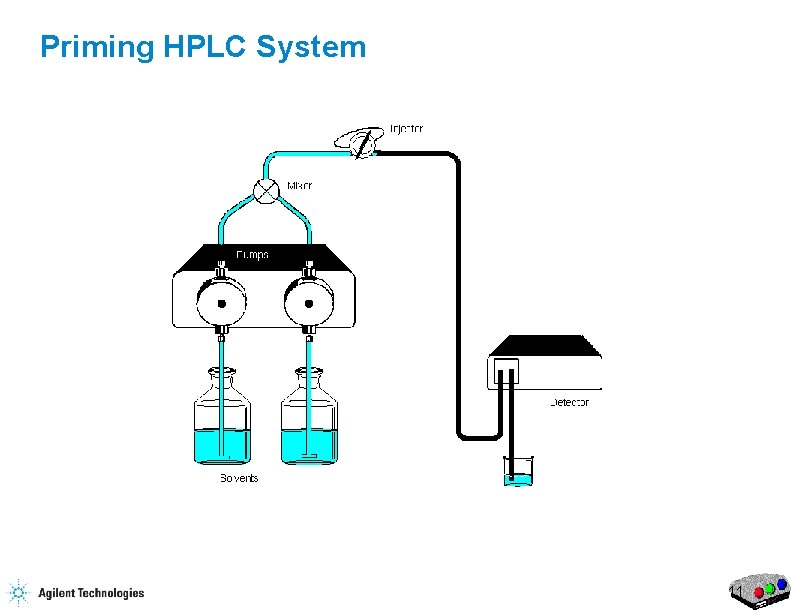
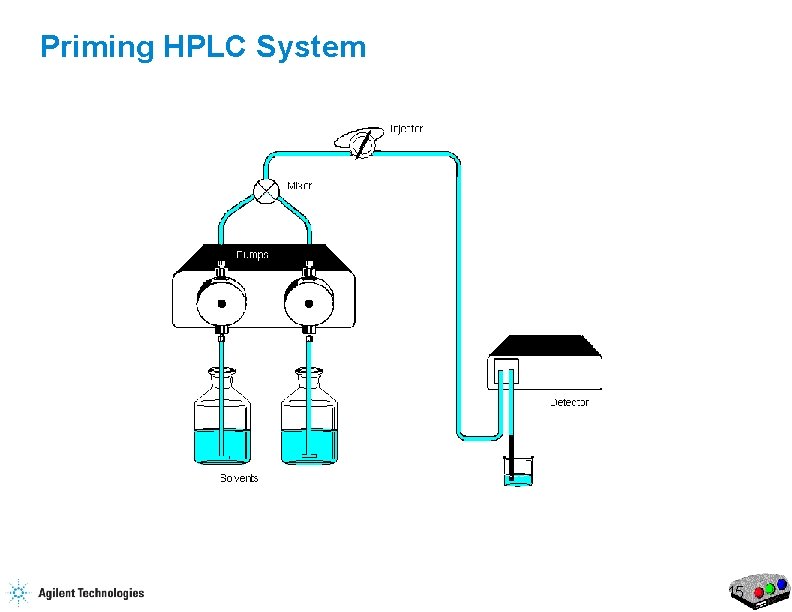
Priming HPLC System 7

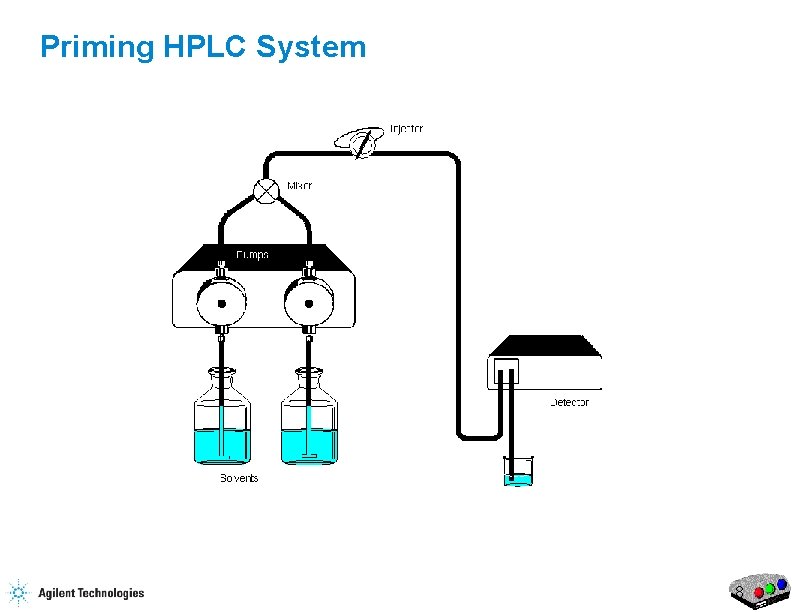
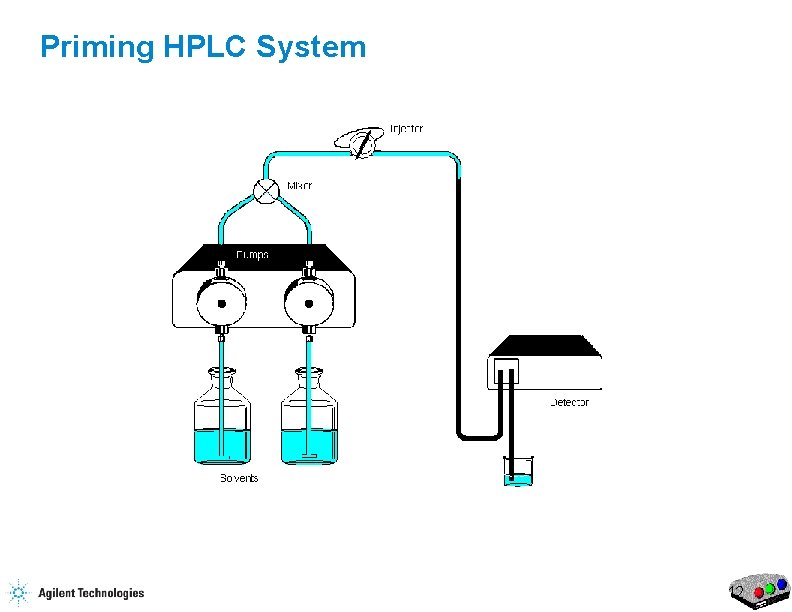
Priming HPLC System 8

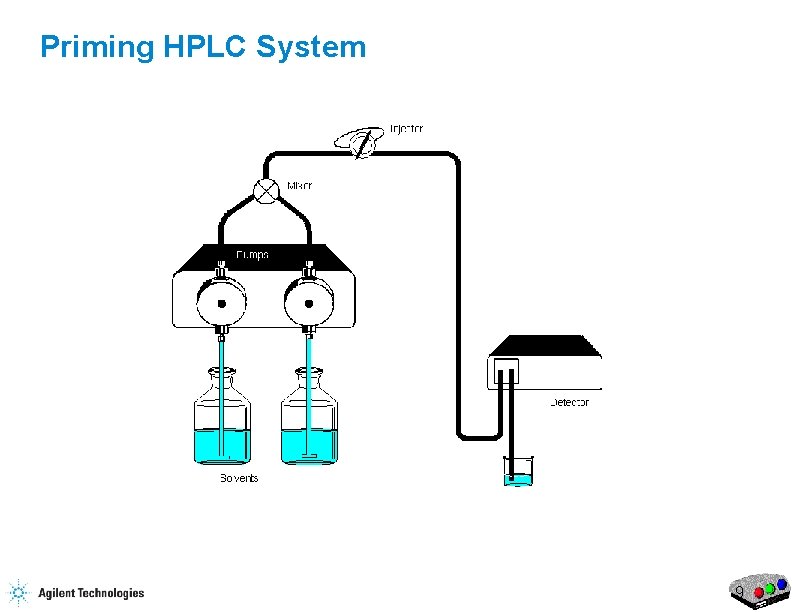
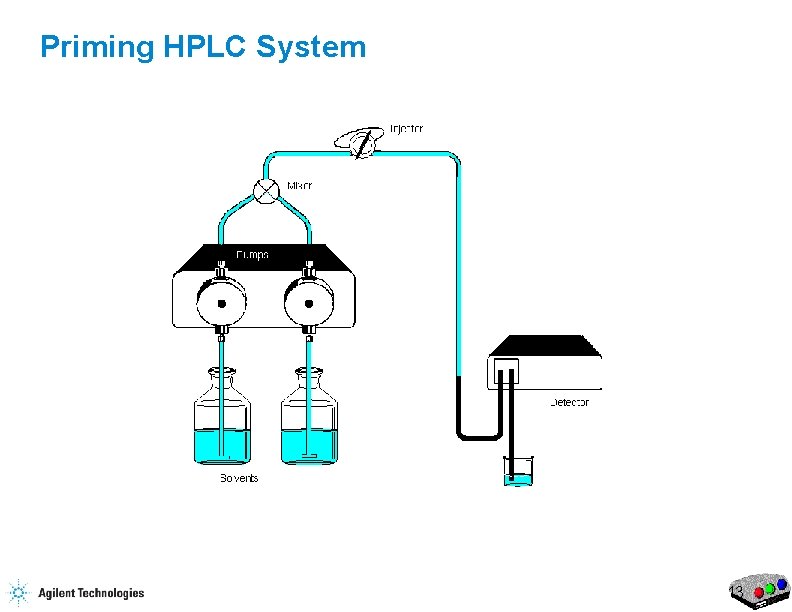
Priming HPLC System 9

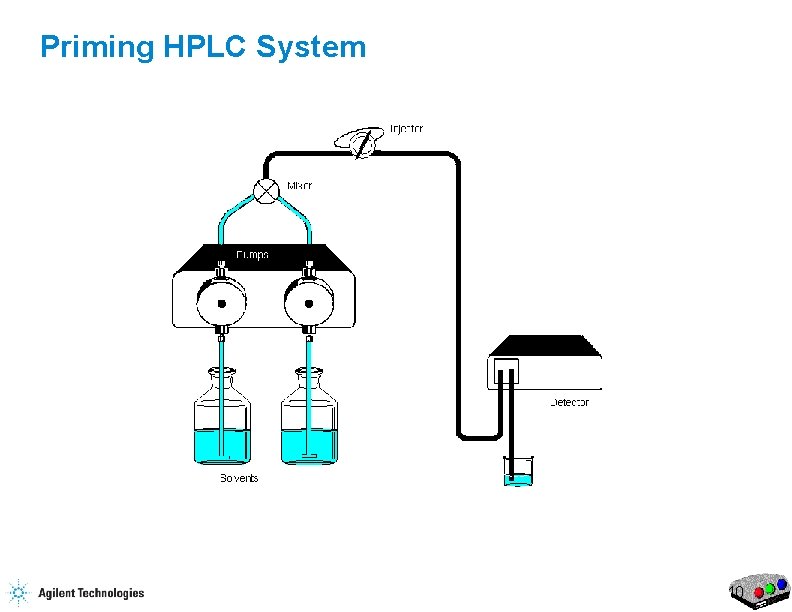
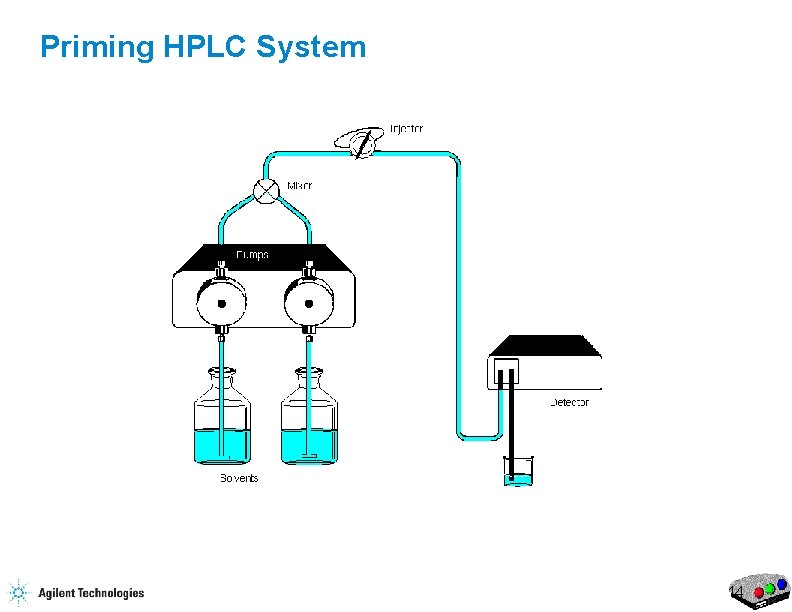
Priming HPLC System 10

Priming HPLC System 11

Priming HPLC System 12

Priming HPLC System 13

Priming HPLC System 14

Priming HPLC System 15

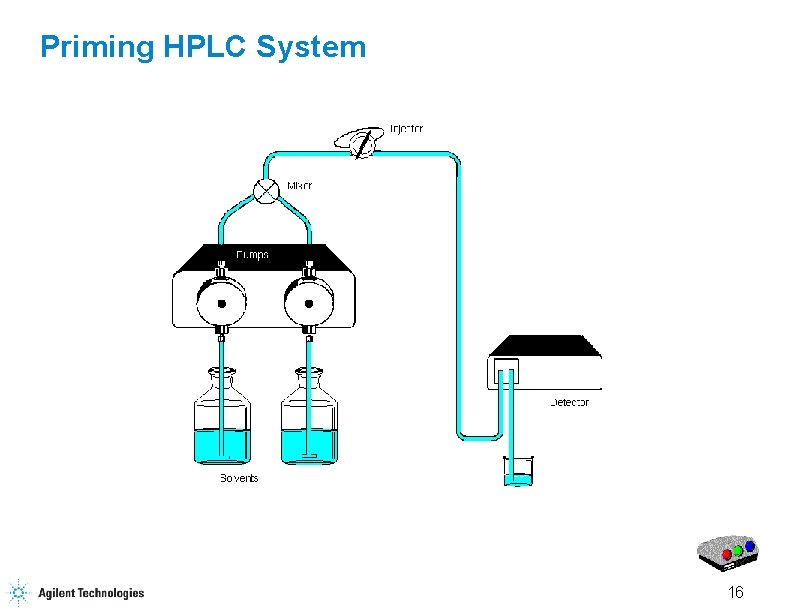
Priming HPLC System 16

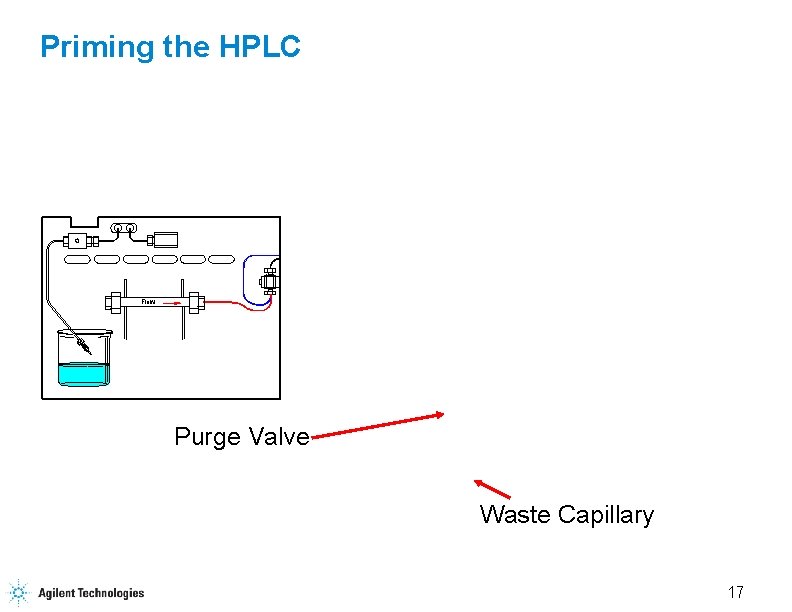
Priming the HPLC Flow Purge Valve Waste Capillary 17

Sample Preparation At a minimum - filter samples: · Nylon - hydrophilic nature works with aqueous and solvent based samples, autoclavable to 121ºC, p. H range 3 -12, no concentrated acids. · PTFE- a hydrophobic membrane which is highly resistant to solvents, acids, and alkalis. This filter is generally used for nonaqueous samples. p. H range 1 -14. · Cellulose Acetate- good filter for aqueous biological samples with very low protein retention. p. H range 4 -8. · PVDF- highly resistant to most solvents, exhibits low protein binding. p. H 2 -12. · Ultrafilter Membranes- molecular weight cut-off filters for biological samples. · Nitrocellulose- exhibits high protein retention. · Solid Phase Extraction. 18

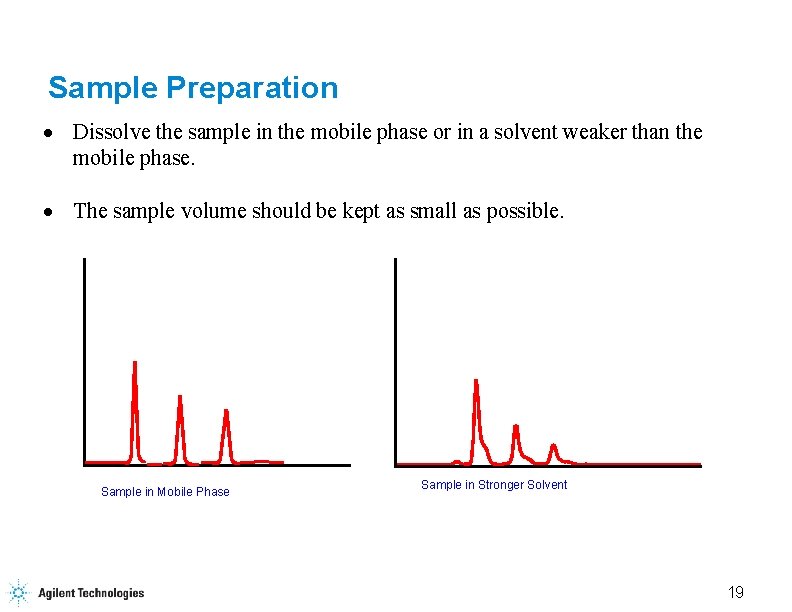
Sample Preparation · Dissolve the sample in the mobile phase or in a solvent weaker than the mobile phase. · The sample volume should be kept as small as possible. Sample in Mobile Phase Sample in Stronger Solvent 19

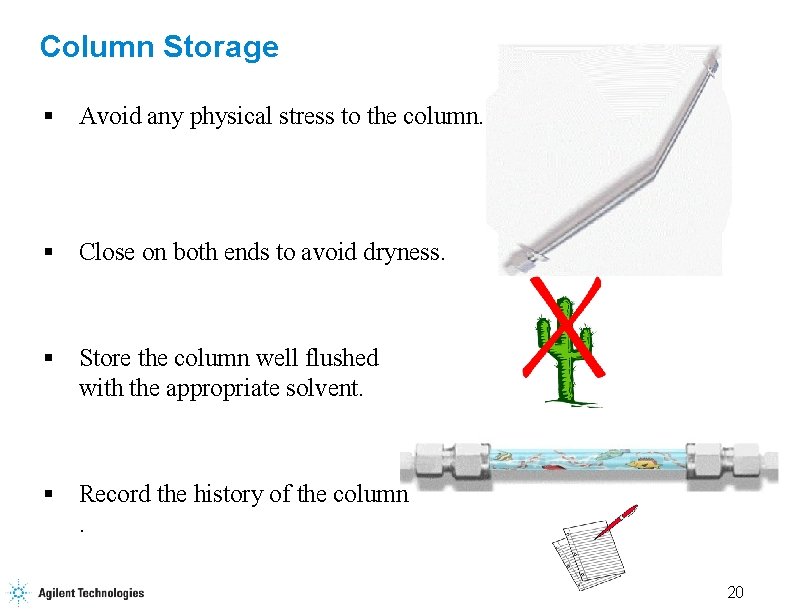
Column Storage § Avoid any physical stress to the column. § Close on both ends to avoid dryness. § Store the column well flushed with the appropriate solvent. § Record the history of the column. 20

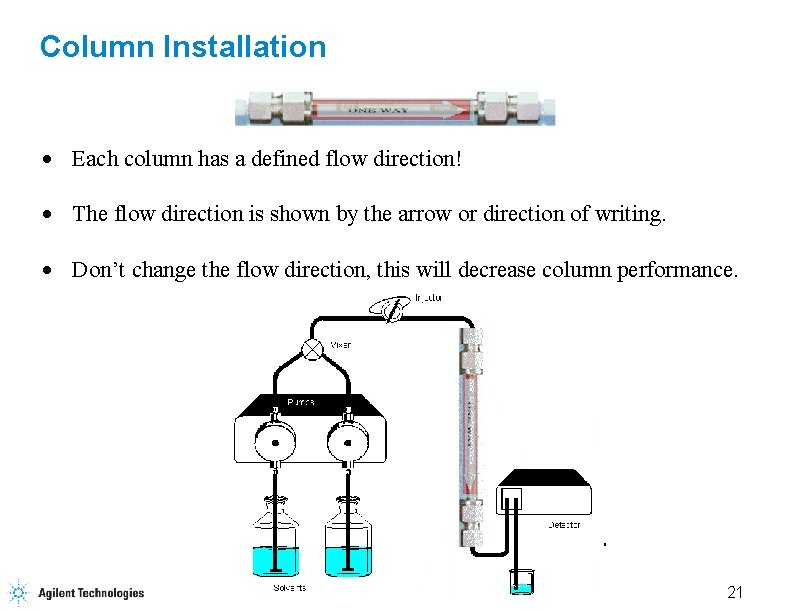
Column Installation · Each column has a defined flow direction! · The flow direction is shown by the arrow or direction of writing. · Don’t change the flow direction, this will decrease column performance. 21

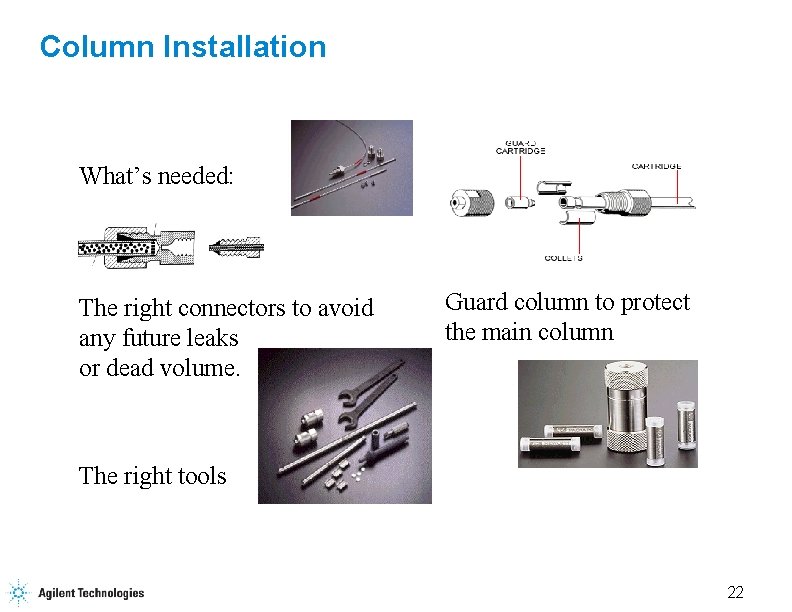
Column Installation What’s needed: The right connectors to avoid any future leaks or dead volume. Guard column to protect the main column The right tools 22

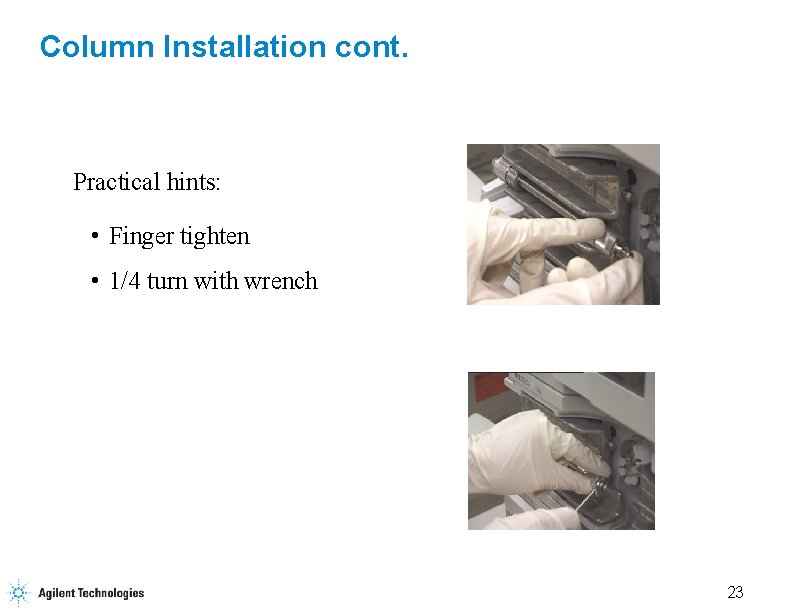
Column Installation cont. Practical hints: • Finger tighten • 1/4 turn with wrench 23

Column Equilibration Equilibrate with mobile phase • Do not pressure shock the column. • 5 -10 column volumes for reversed-phase equilibration. • Assures reproducible results. 24

Column Check · New columns should be delivered with a performance certificate. · Each additional use should be documented including: – – – Back pressure Mobile Phase Temperature Sample type Storage condition (Solvent) Based on that history the column can be checked with defined compound mixture. 25

Column Care and Handling · Wash the column after use with selected solvents; flush highly retained sample components from the column, eliminate buffers. · Do not store a column in 100% water. Microbes may grow and clog the column. · Do not store the column in 100% Acetonitrile. · Don’t open the column and repack the material if you want to maintain performance. · Use the column at its optimal flow rate - avoid high flow rates. · Do not operate silica or bonded phases for extended periods at high temperature. · Keep the p. H of the mobile phase in an appropriate range for the column. 26

System Check - Routinely Principle: The HPLC system (including the column) can be checked out using a defined test sample and method. Use at least three replicates. Preparations for a system check: • HPLC system is primed with mobile phase. • Column is equilibrated. • Detector shows a stable response. • There are no leaks. • System is ready for injection 27

System Check - routinely cont. Test sample requirements: • Sample is well characterized. • Detector response is known. • Sample contains multiple components. Test design: The test sample is analyzed using a defined test method. The results are compared with the expected results. If the results are in the defined range, than the system is ready for use. This is not comparable to an OQ test or PV test!! 28

Summary • Prepare mobile phase • Prime the HPLC system • Install the column • Turn on the detector (warm-up at least 20 minutes for UV) • Equilibrate the column • Prepare the samples • Record the detector response - stable response • Perform a system check using a test sample and test method • Compare the results with the expectations (limits) • Document the results (Control Chart) • Record any failures/errors if appropriate • If system check is OK, then 29

Review 1. You are running a routine analysis when you notice a periodic perturbation in the baseline. The pressure reading is fluctuating up and down. What is the problem? How would you correct it? 30

Review 2. You decide to run a reversed-phase analysis on an instrument in lab. The previous operator does not indicate the solvents last used on the instrument. You place water in channel A and turn on the pump. You cannot get a stable baseline. Suggest a possible reason for this dilemma. 31