Chapter 17 Rearrangement of DNA 17 1 Introduction







- Slides: 50

Chapter 17 Rearrangement of DNA

17. 1 Introduction 17. 2 The mating pathway is triggered by pheromone-receptor interactions 17. 3 The mating response activates a G protein 17. 4 Yeast can switch silent and active loci for mating type 17. 5 The MAT locus codes for regulator proteins 17. 6 Silent cassettes at HML and HMR are repressed 17. 7 Unidirectional transposition is initiated by the recipient MAT locus 17. 8 Regulation of HO expression 17. 9 Trypanosomes switch the VSG frequently during infection 17. 10 New VSG sequences are generated by gene switching 17. 11 VSG genes have an unusual structure 17. 12 The bacterial Ti plasmid causes crown gall disease in plants 17. 13 T-DNA carries genes required for infection 17. 14 Transfer of T-DNA resembles bacterial conjugation 17. 15 Selection of amplified genomic sequences 17. 16 Transfection introduces exogenous DNA into cells 17. 17 Genes can be injected into animal eggs 17. 18 ES cells can be incorporated into embryonic mice 17. 19 Gene targeting allows genes to be replaced or knocked out

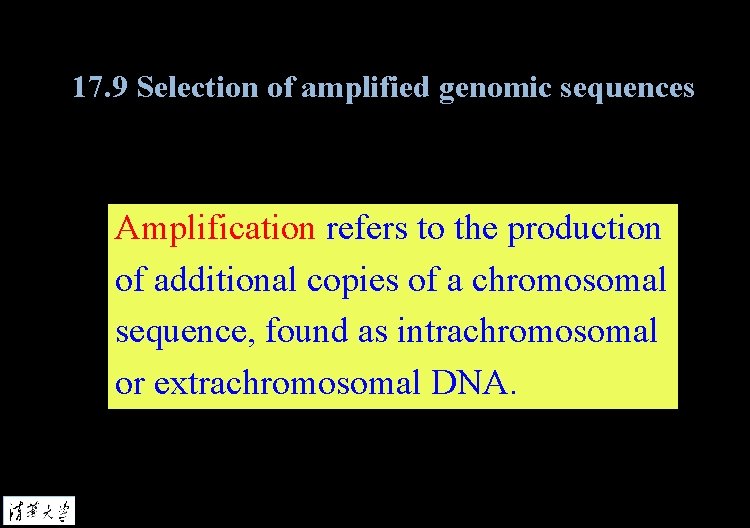
17. 1 Introduction Amplification refers to the production of additional copies of a chromosomal sequence, found as intrachromosomal or extrachromosomal DNA. Transgenic animals are created by introducing new DNA sequences into the germline via addition to the egg.

17. 1 Introduction Amplification refers to the production of additional copies of a chromosomal sequence, found as intrachromosomal or extrachromosomal DNA. Transgenic animals are created by introducing new DNA sequences into the germline via addition to the egg.

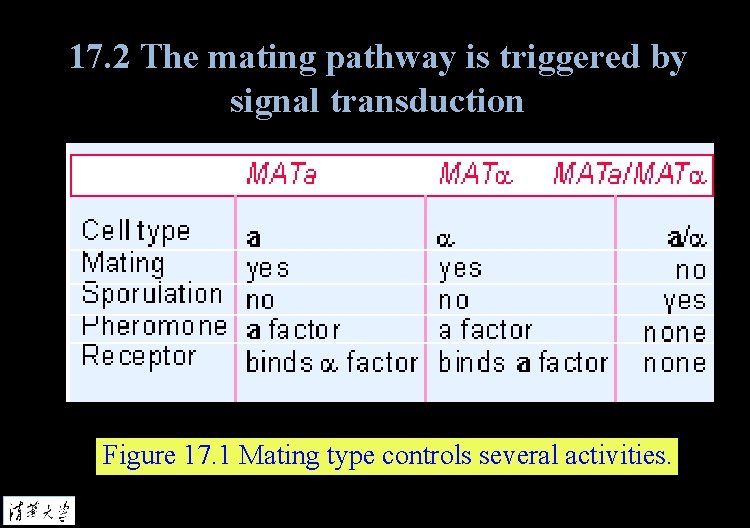
17. 2 The mating pathway is triggered by signal transduction Figure 17. 1 Mating type controls several activities.

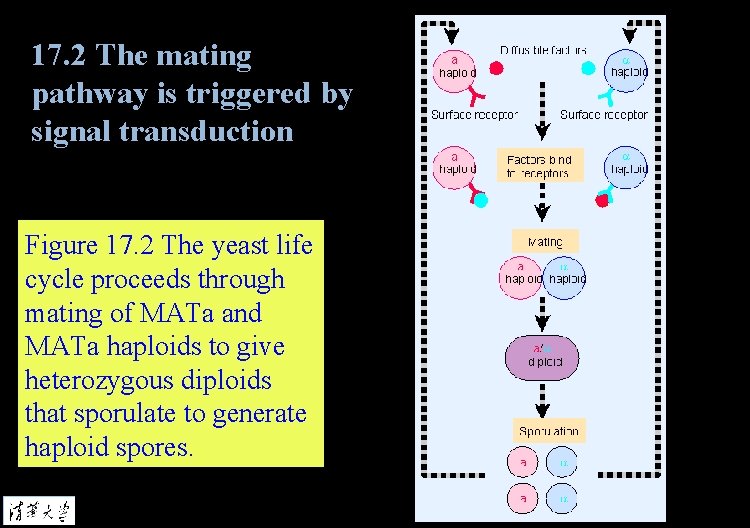
17. 2 The mating pathway is triggered by signal transduction Figure 17. 2 The yeast life cycle proceeds through mating of MATa and MATa haploids to give heterozygous diploids that sporulate to generate haploid spores.

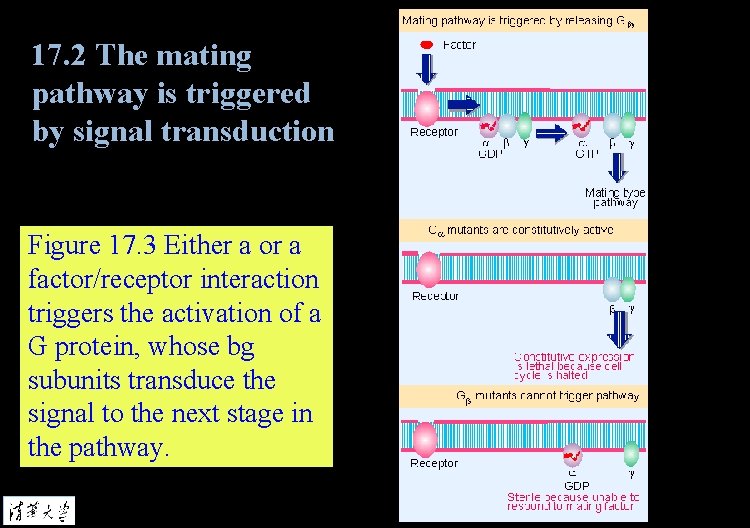
17. 2 The mating pathway is triggered by signal transduction Figure 17. 3 Either a or a factor/receptor interaction triggers the activation of a G protein, whose bg subunits transduce the signal to the next stage in the pathway.

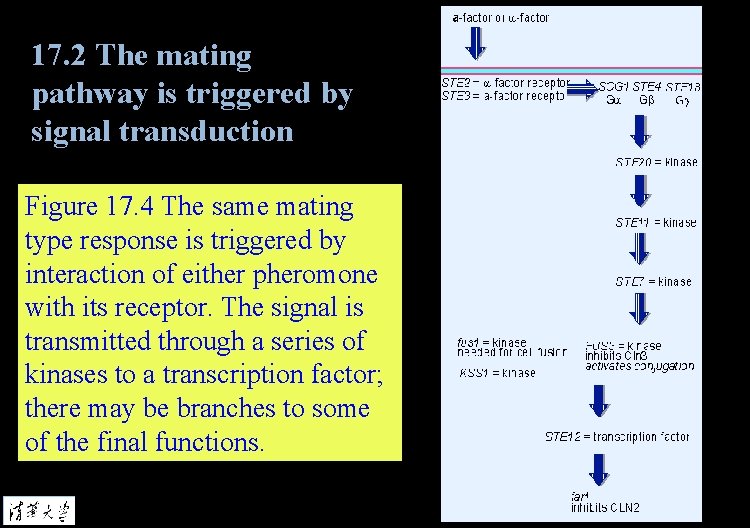
17. 2 The mating pathway is triggered by signal transduction Figure 17. 4 The same mating type response is triggered by interaction of either pheromone with its receptor. The signal is transmitted through a series of kinases to a transcription factor; there may be branches to some of the final functions.

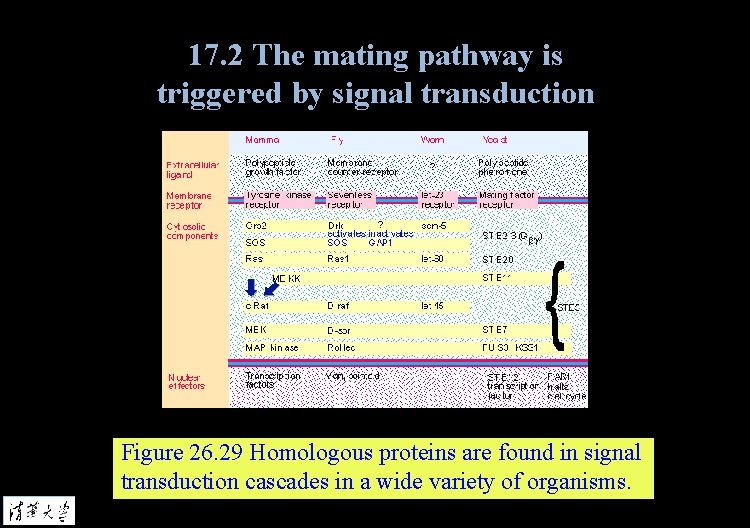
17. 2 The mating pathway is triggered by signal transduction Figure 26. 29 Homologous proteins are found in signal transduction cascades in a wide variety of organisms.

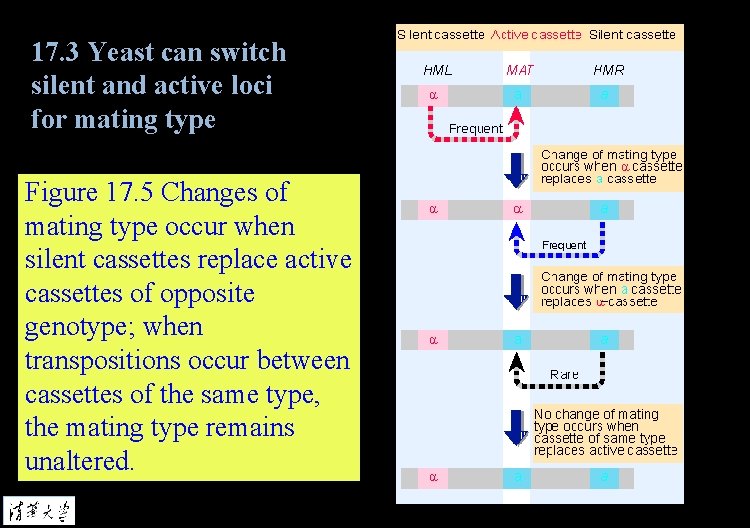
17. 3 Yeast can switch silent and active loci for mating type Figure 17. 5 Changes of mating type occur when silent cassettes replace active cassettes of opposite genotype; when transpositions occur between cassettes of the same type, the mating type remains unaltered.

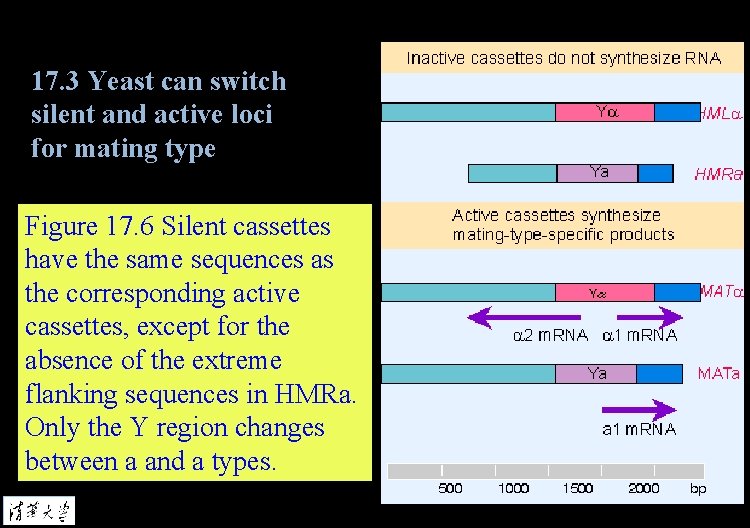
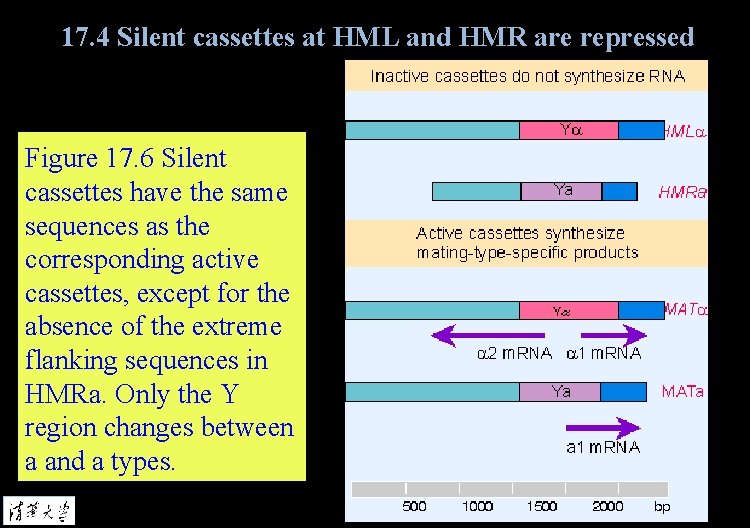
17. 3 Yeast can switch silent and active loci for mating type Figure 17. 6 Silent cassettes have the same sequences as the corresponding active cassettes, except for the absence of the extreme flanking sequences in HMRa. Only the Y region changes between a and a types.

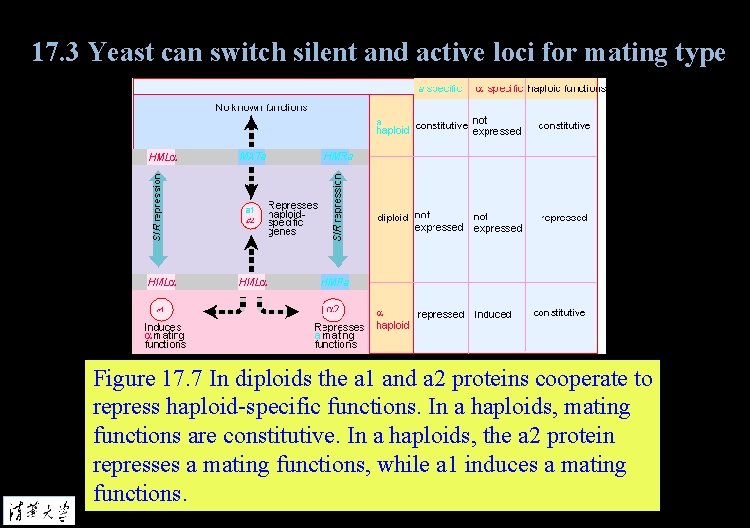
17. 3 Yeast can switch silent and active loci for mating type Figure 17. 7 In diploids the a 1 and a 2 proteins cooperate to repress haploid-specific functions. In a haploids, mating functions are constitutive. In a haploids, the a 2 protein represses a mating functions, while a 1 induces a mating functions.

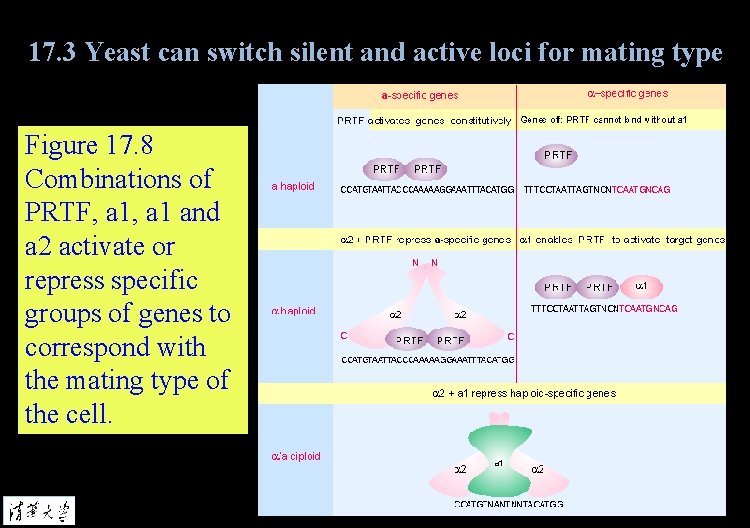
17. 3 Yeast can switch silent and active loci for mating type Figure 17. 8 Combinations of PRTF, a 1 and a 2 activate or repress specific groups of genes to correspond with the mating type of the cell.

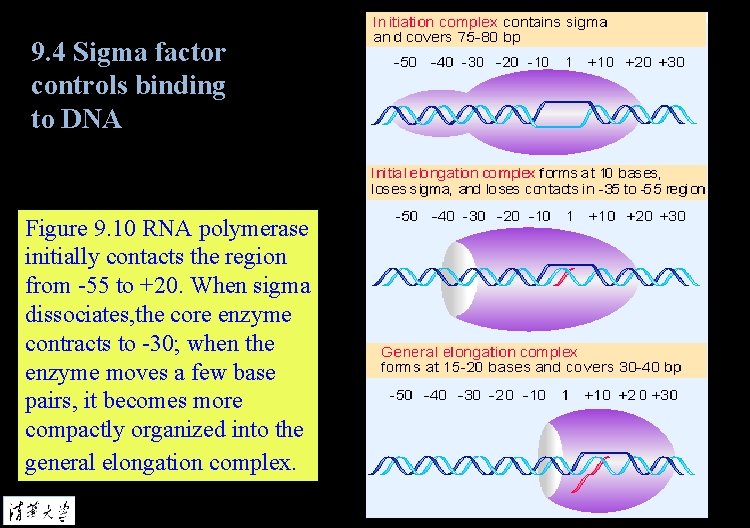
9. 4 Sigma factor controls binding to DNA Figure 9. 10 RNA polymerase initially contacts the region from -55 to +20. When sigma dissociates, the core enzyme contracts to -30; when the enzyme moves a few base pairs, it becomes more compactly organized into the general elongation complex.

17. 4 Silent cassettes at HML and HMR are repressed Figure 17. 6 Silent cassettes have the same sequences as the corresponding active cassettes, except for the absence of the extreme flanking sequences in HMRa. Only the Y region changes between a and a types.

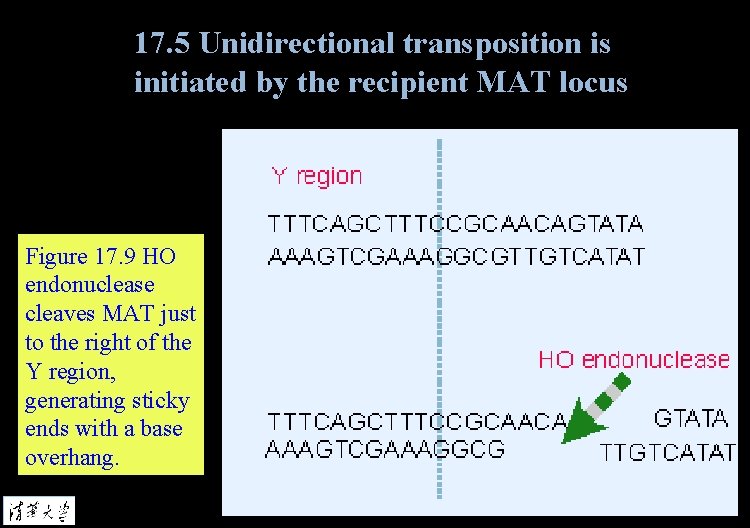
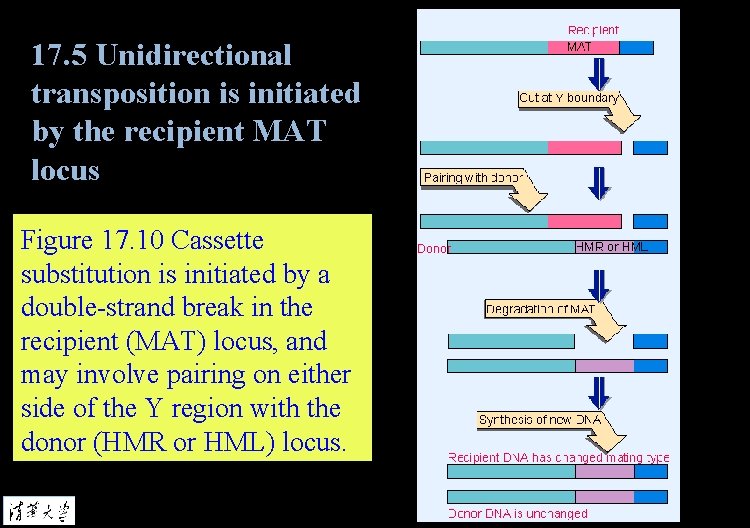
17. 5 Unidirectional transposition is initiated by the recipient MAT locus Figure 17. 9 HO endonuclease cleaves MAT just to the right of the Y region, generating sticky ends with a base overhang.

17. 5 Unidirectional transposition is initiated by the recipient MAT locus Figure 17. 10 Cassette substitution is initiated by a double-strand break in the recipient (MAT) locus, and may involve pairing on either side of the Y region with the donor (HMR or HML) locus.

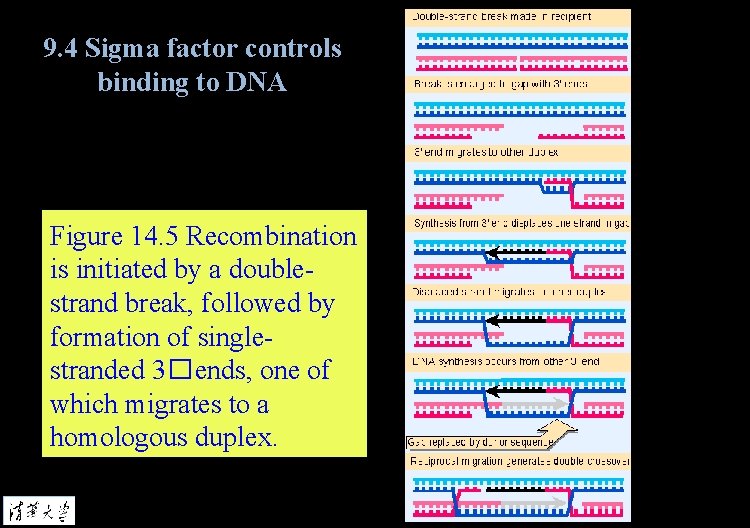
9. 4 Sigma factor controls binding to DNA Figure 14. 5 Recombination is initiated by a doublestrand break, followed by formation of singlestranded 3�ends, one of which migrates to a homologous duplex.

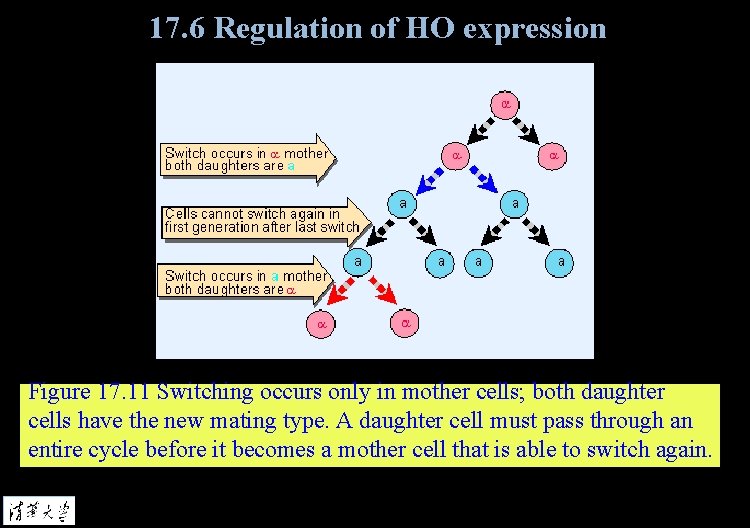
17. 6 Regulation of HO expression Figure 17. 11 Switching occurs only in mother cells; both daughter cells have the new mating type. A daughter cell must pass through an entire cycle before it becomes a mother cell that is able to switch again.

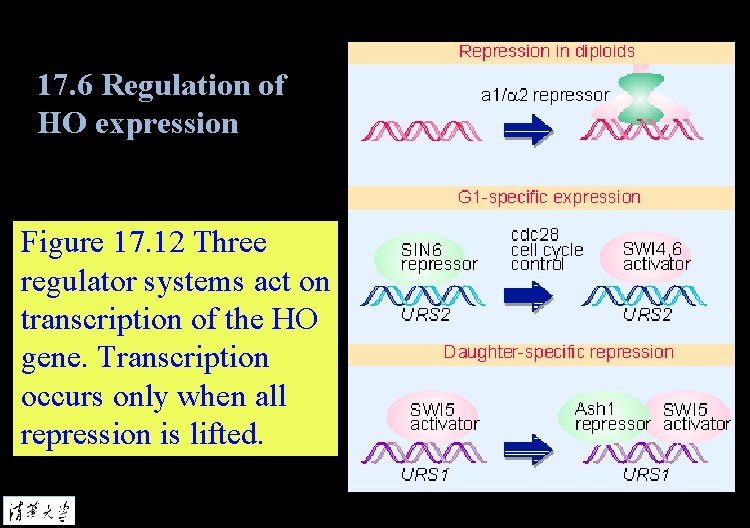
17. 6 Regulation of HO expression Figure 17. 12 Three regulator systems act on transcription of the HO gene. Transcription occurs only when all repression is lifted.

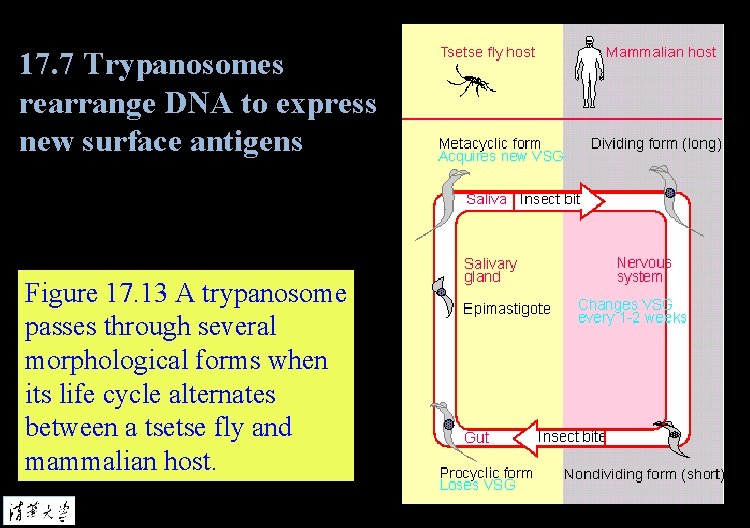
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 13 A trypanosome passes through several morphological forms when its life cycle alternates between a tsetse fly and mammalian host.

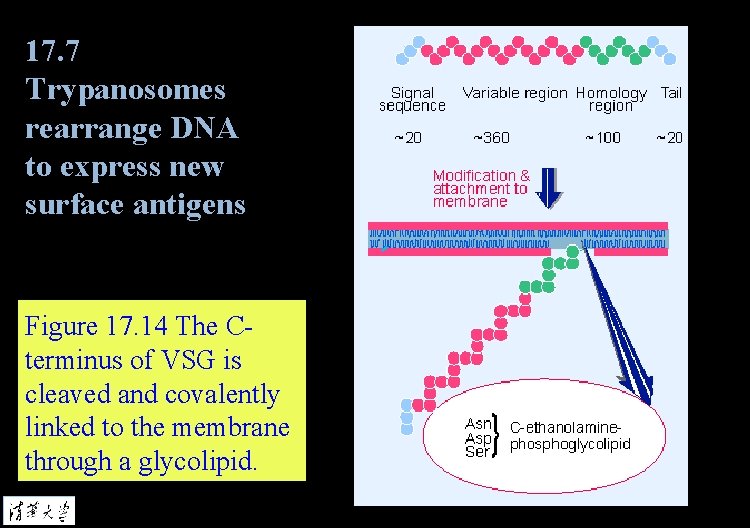
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 14 The Cterminus of VSG is cleaved and covalently linked to the membrane through a glycolipid.

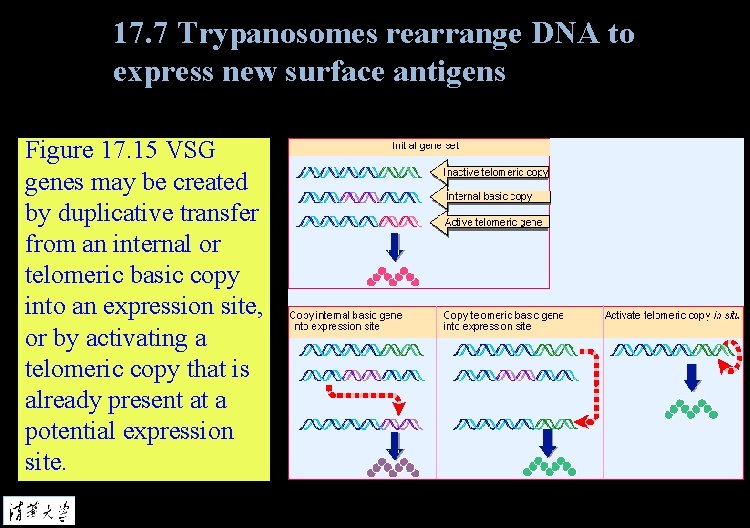
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 15 VSG genes may be created by duplicative transfer from an internal or telomeric basic copy into an expression site, or by activating a telomeric copy that is already present at a potential expression site.

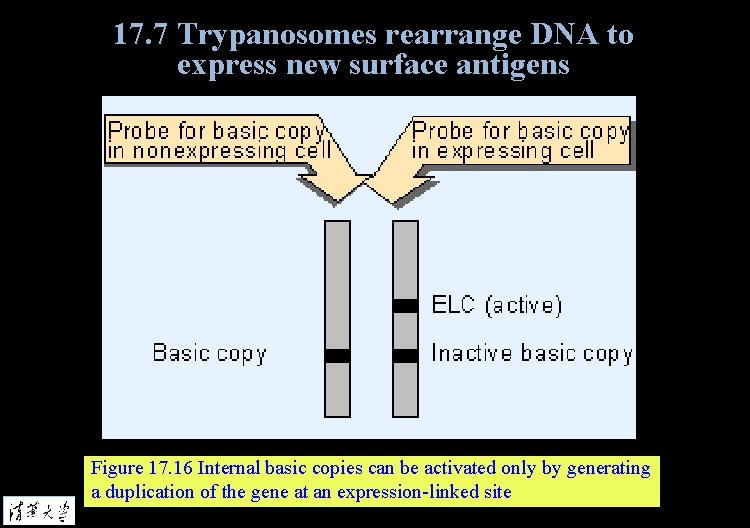
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 16 Internal basic copies can be activated only by generating a duplication of the gene at an expression-linked site

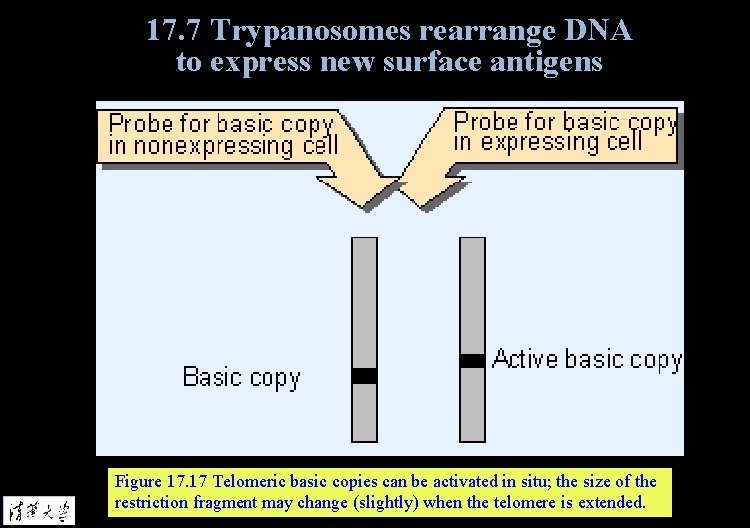
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 17 Telomeric basic copies can be activated in situ; the size of the restriction fragment may change (slightly) when the telomere is extended.

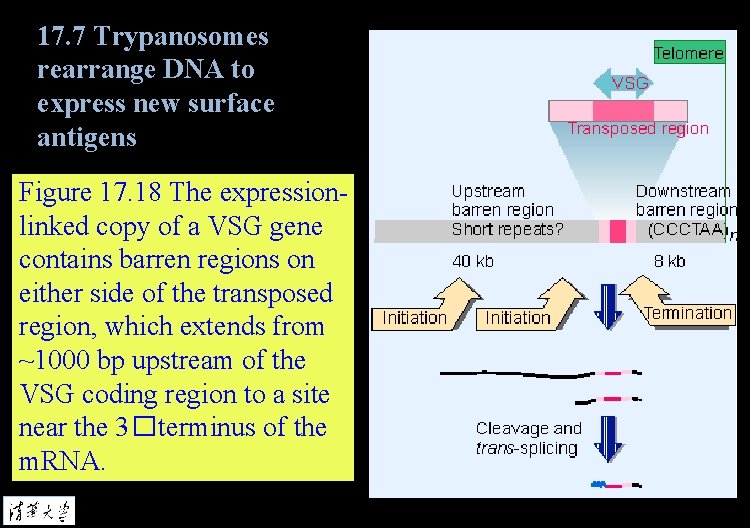
17. 7 Trypanosomes rearrange DNA to express new surface antigens Figure 17. 18 The expressionlinked copy of a VSG gene contains barren regions on either side of the transposed region, which extends from ~1000 bp upstream of the VSG coding region to a site near the 3�terminus of the m. RNA.

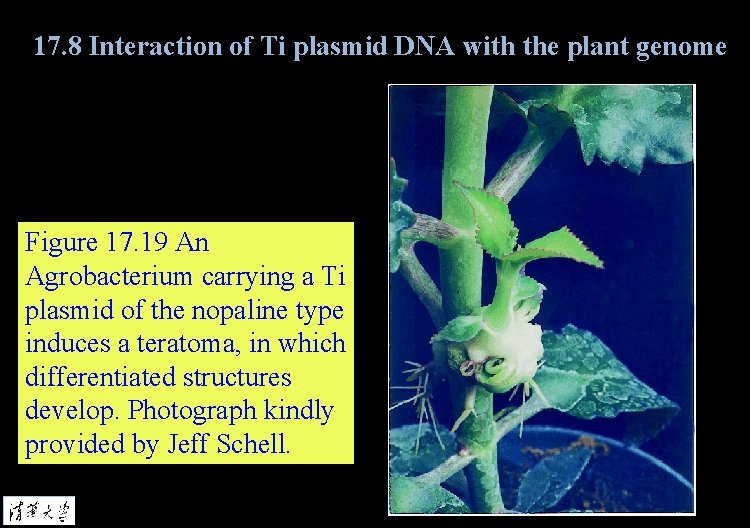
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 19 An Agrobacterium carrying a Ti plasmid of the nopaline type induces a teratoma, in which differentiated structures develop. Photograph kindly provided by Jeff Schell.

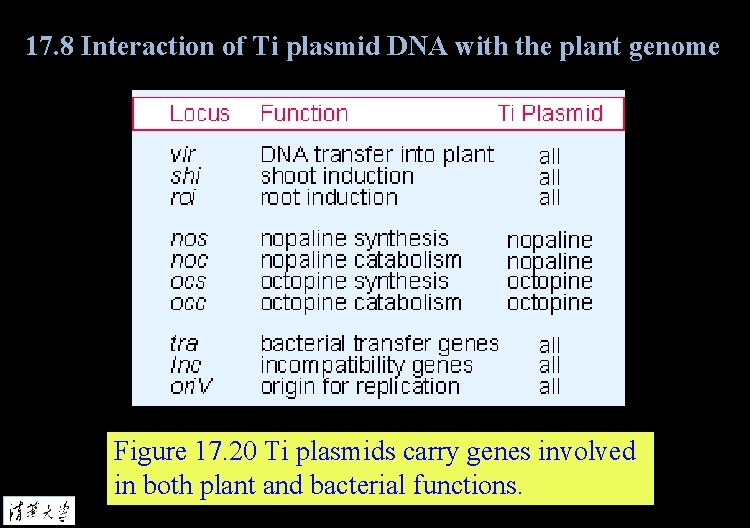
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 20 Ti plasmids carry genes involved in both plant and bacterial functions.

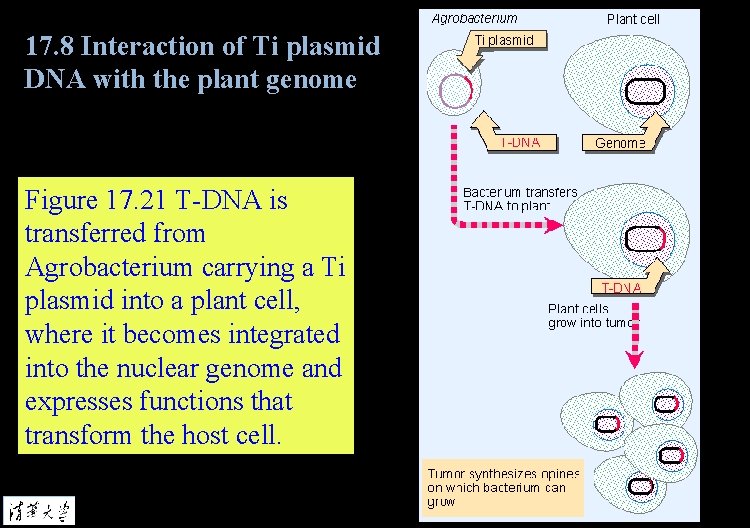
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 21 T-DNA is transferred from Agrobacterium carrying a Ti plasmid into a plant cell, where it becomes integrated into the nuclear genome and expresses functions that transform the host cell.

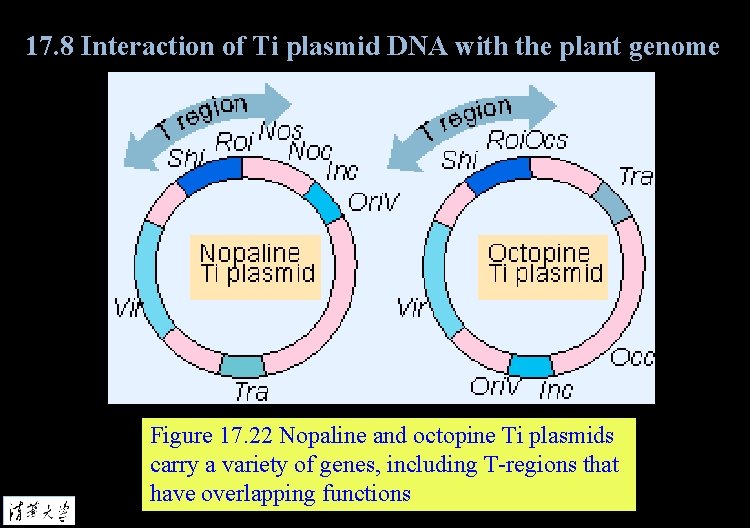
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 22 Nopaline and octopine Ti plasmids carry a variety of genes, including T-regions that have overlapping functions

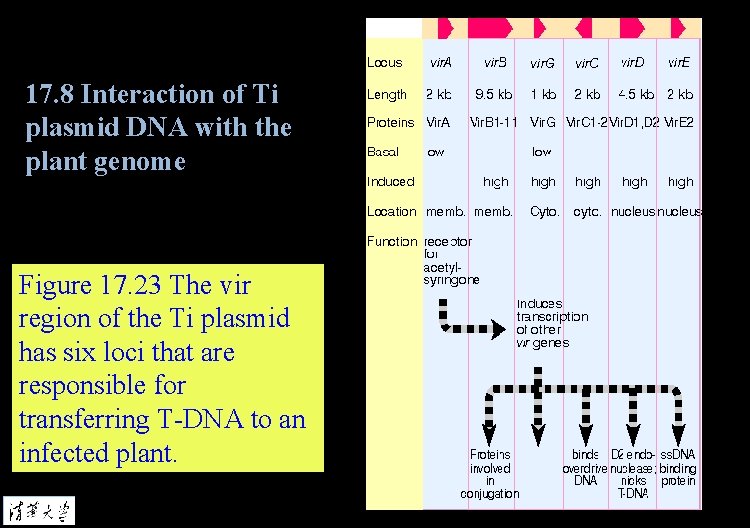
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 23 The vir region of the Ti plasmid has six loci that are responsible for transferring T-DNA to an infected plant.

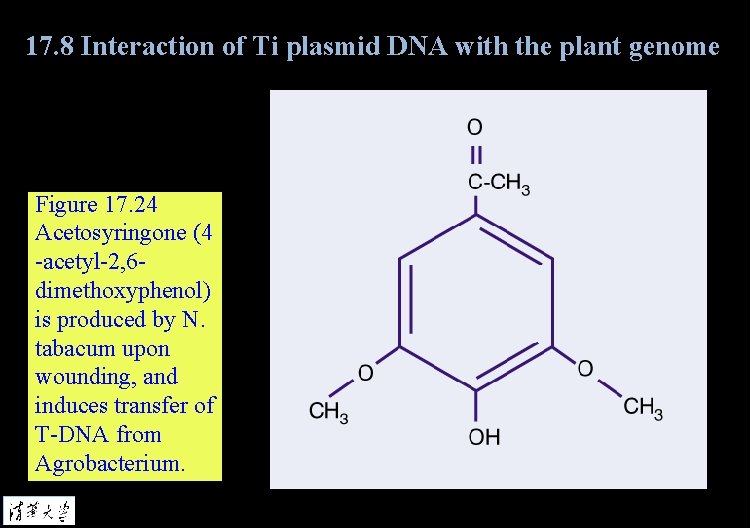
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 24 Acetosyringone (4 -acetyl-2, 6 dimethoxyphenol) is produced by N. tabacum upon wounding, and induces transfer of T-DNA from Agrobacterium.

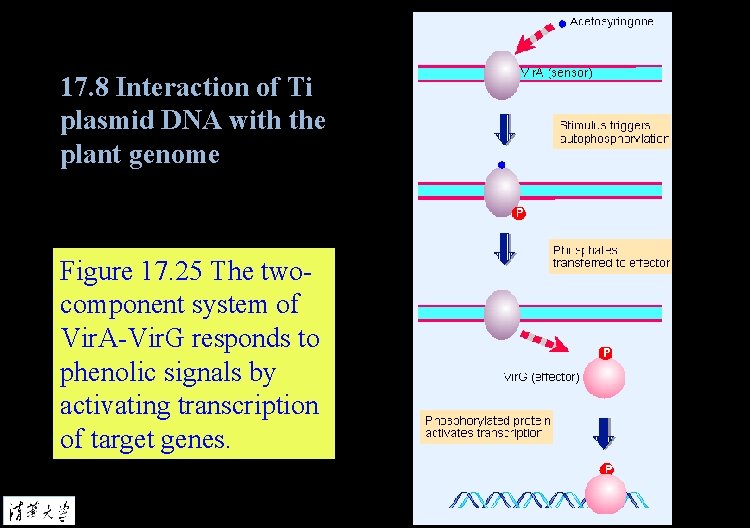
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 25 The twocomponent system of Vir. A-Vir. G responds to phenolic signals by activating transcription of target genes.

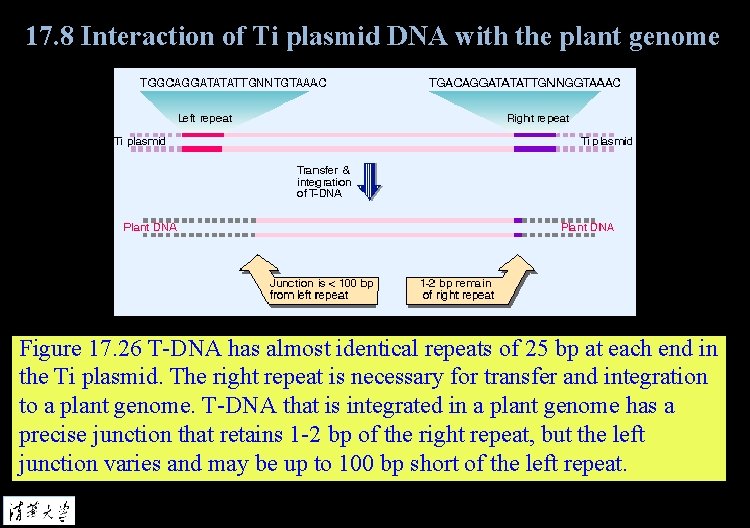
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 26 T-DNA has almost identical repeats of 25 bp at each end in the Ti plasmid. The right repeat is necessary for transfer and integration to a plant genome. T-DNA that is integrated in a plant genome has a precise junction that retains 1 -2 bp of the right repeat, but the left junction varies and may be up to 100 bp short of the left repeat.

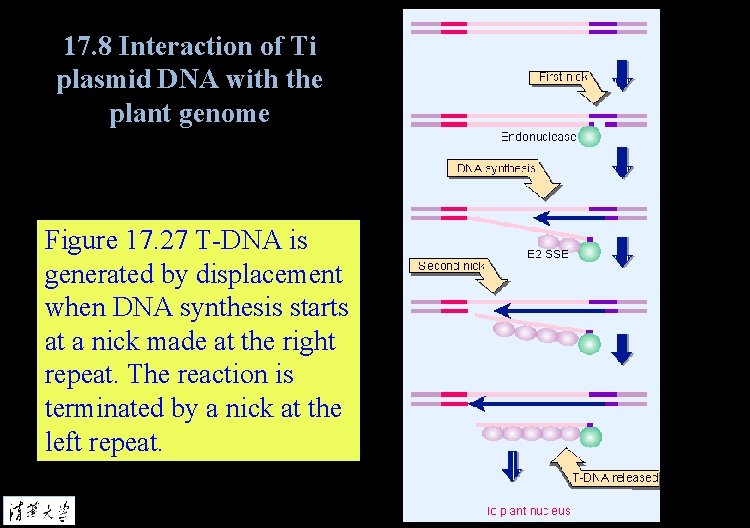
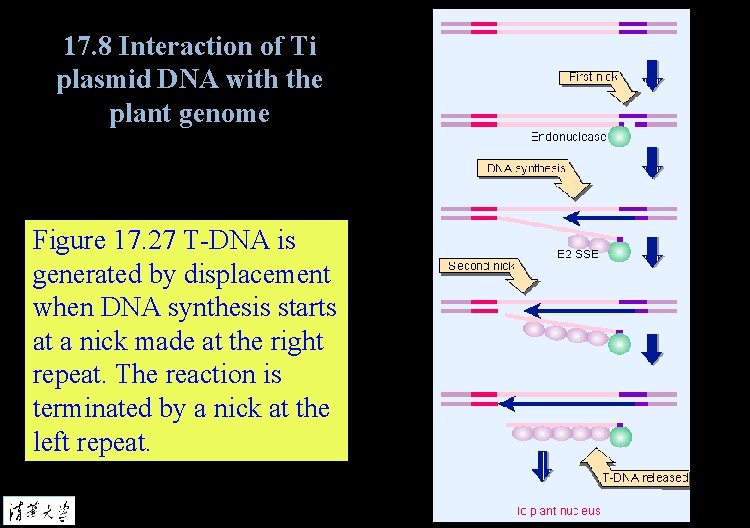
17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 27 T-DNA is generated by displacement when DNA synthesis starts at a nick made at the right repeat. The reaction is terminated by a nick at the left repeat.

17. 8 Interaction of Ti plasmid DNA with the plant genome Figure 17. 27 T-DNA is generated by displacement when DNA synthesis starts at a nick made at the right repeat. The reaction is terminated by a nick at the left repeat.

17. 9 Selection of amplified genomic sequences Amplification refers to the production of additional copies of a chromosomal sequence, found as intrachromosomal or extrachromosomal DNA.

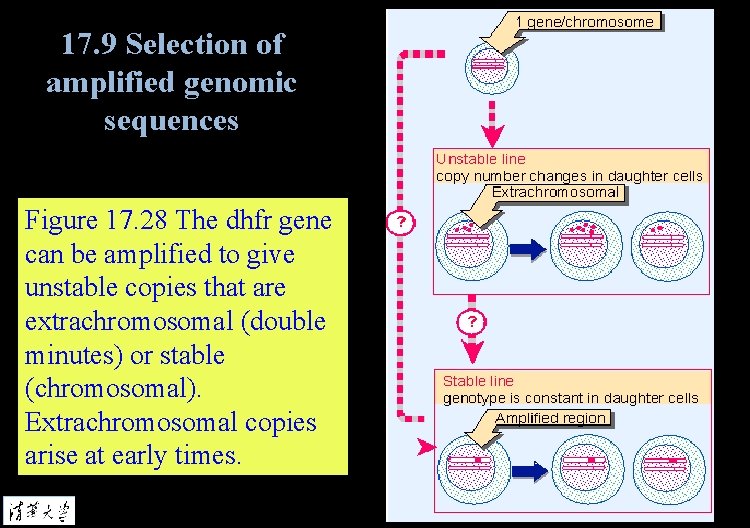
17. 9 Selection of amplified genomic sequences Figure 17. 28 The dhfr gene can be amplified to give unstable copies that are extrachromosomal (double minutes) or stable (chromosomal). Extrachromosomal copies arise at early times.

17. 9 Selection of amplified genomic sequences Figure 17. 29 Amplified copies of the dhfr gene produce a homogeneously staining region (HSR) in the chromosome. Photograph kindly provided by Robert Schimke.

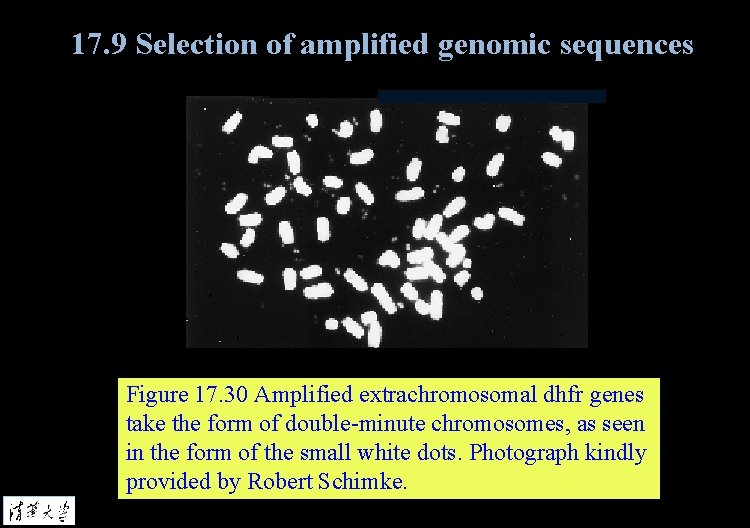
17. 9 Selection of amplified genomic sequences Figure 17. 30 Amplified extrachromosomal dhfr genes take the form of double-minute chromosomes, as seen in the form of the small white dots. Photograph kindly provided by Robert Schimke.

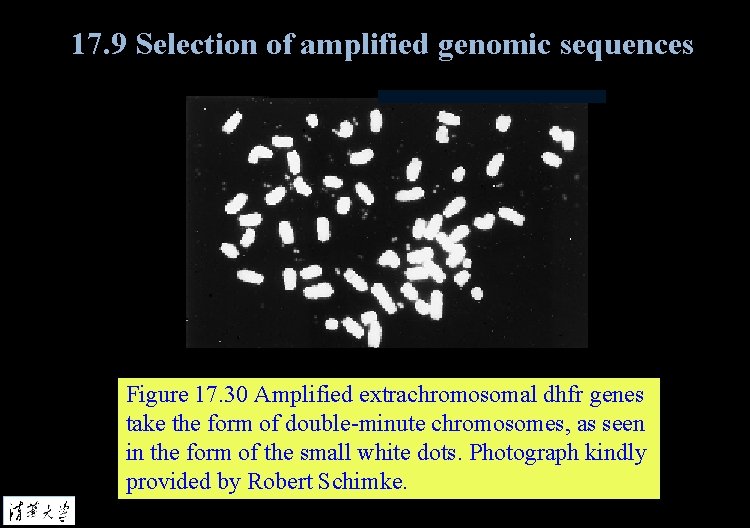
17. 9 Selection of amplified genomic sequences Figure 17. 30 Amplified extrachromosomal dhfr genes take the form of double-minute chromosomes, as seen in the form of the small white dots. Photograph kindly provided by Robert Schimke.

17. 10 Exogenous sequences can be introduced into cells and animals by transfection Transfection of eukaryotic cells is the acquisition of new genetic markers by incorporation of added DNA. Transgenic animals are created by introducing new DNA sequences into the germline via addition to the egg.

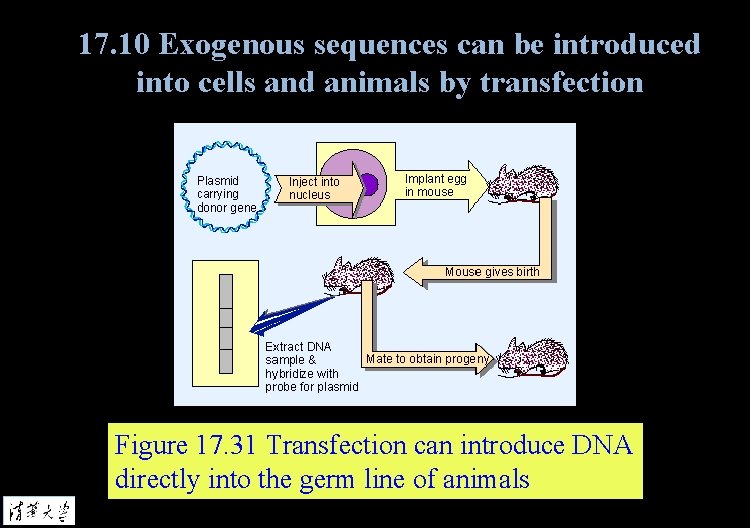
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 31 Transfection can introduce DNA directly into the germ line of animals

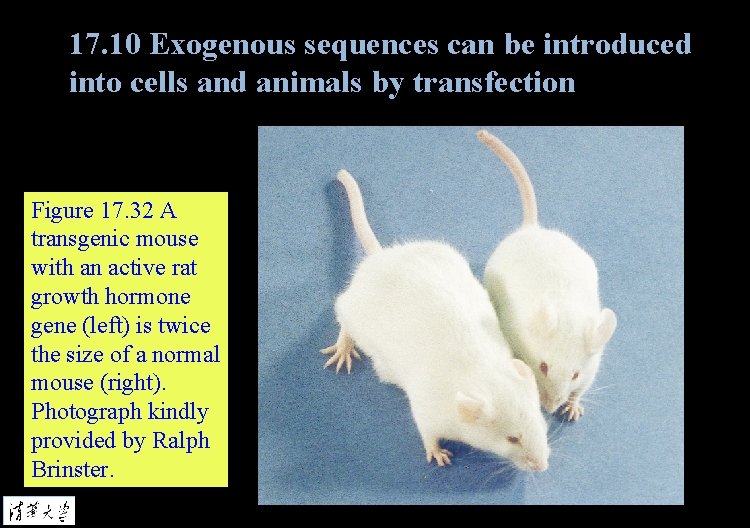
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 32 A transgenic mouse with an active rat growth hormone gene (left) is twice the size of a normal mouse (right). Photograph kindly provided by Ralph Brinster.

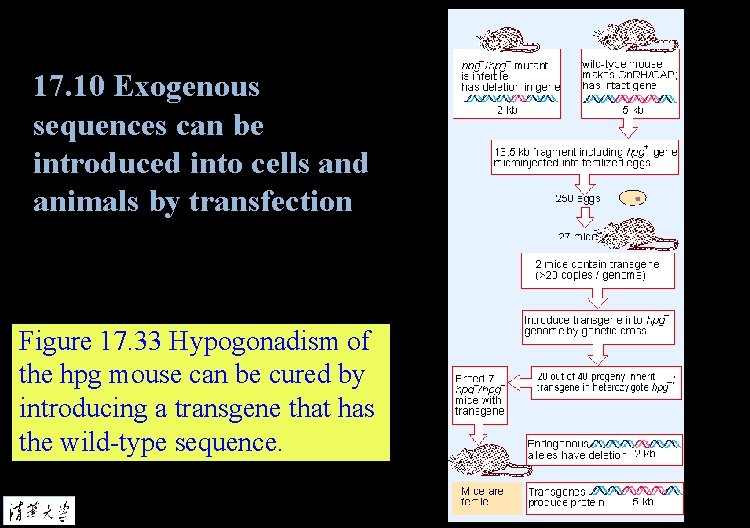
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 33 Hypogonadism of the hpg mouse can be cured by introducing a transgene that has the wild-type sequence.

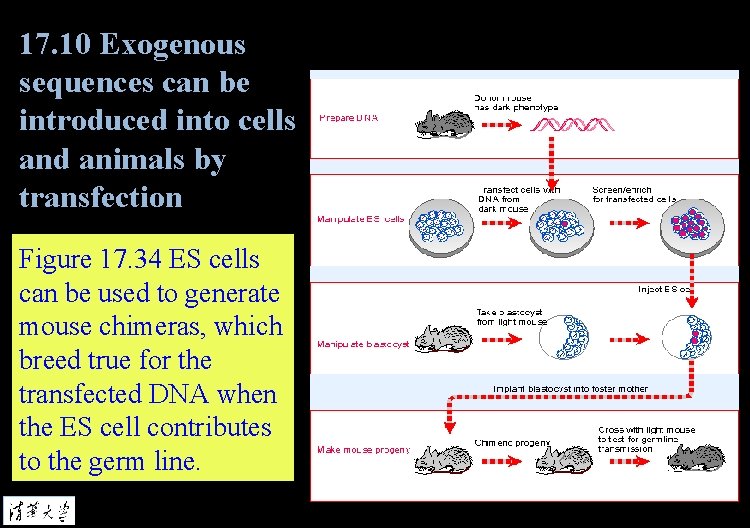
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 34 ES cells can be used to generate mouse chimeras, which breed true for the transfected DNA when the ES cell contributes to the germ line.

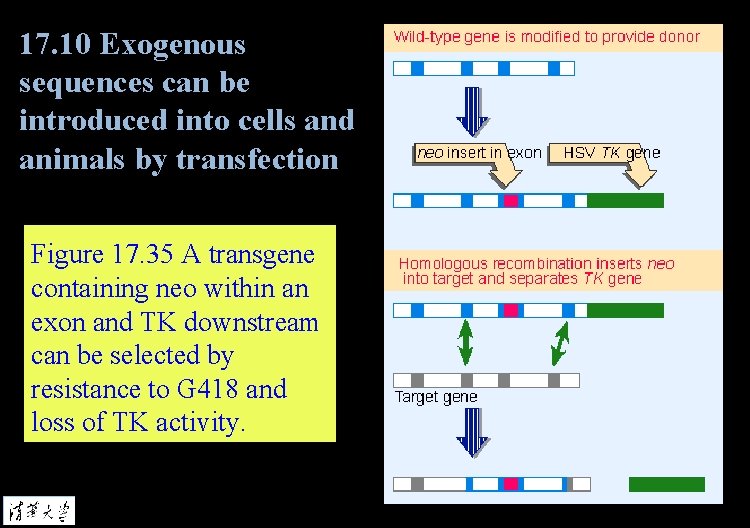
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 35 A transgene containing neo within an exon and TK downstream can be selected by resistance to G 418 and loss of TK activity.

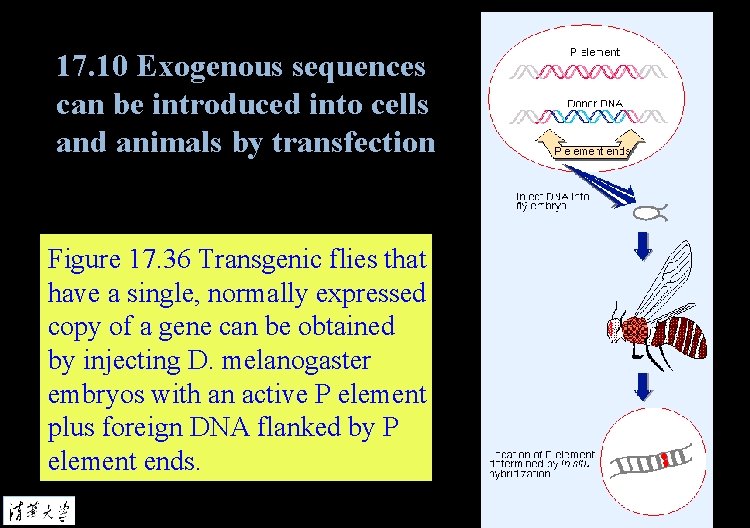
17. 10 Exogenous sequences can be introduced into cells and animals by transfection Figure 17. 36 Transgenic flies that have a single, normally expressed copy of a gene can be obtained by injecting D. melanogaster embryos with an active P element plus foreign DNA flanked by P element ends.

17. 11 Summary • Yeast mating type is determined by whether the MAT locus carries the a or sequence. • Additional, silent copies of the matingtype sequences are carried at the loci HML and HMRa. • Trypanosomes carry >1000 sequences coding for varieties of the surface antigen.

17. 11 Summary • Agrobacteria induce tumor formation in wounded plant cells. The wounded cells secrete phenolic compounds that activate vir genes carried by the Ti plasmid of the bacterium. • Endogenous sequences may become amplified in cultured cells. Exposure to methotrexate leads to the accumulation of cells that have additional copies of the dhfr gene. • New sequences of DNA may be introduced into a cultured cell by transfection or into an animal egg by microinjection.
 Cosine rule variations
Cosine rule variations Cope elimination
Cope elimination Carbonium ion rearrangement
Carbonium ion rearrangement Rearranging atoms worksheet answer key
Rearranging atoms worksheet answer key Openreach line plant rearrangement
Openreach line plant rearrangement Main difference between hofmann and curtius rearrangement
Main difference between hofmann and curtius rearrangement Arranging sentences in order
Arranging sentences in order Semipinacol rearrangement
Semipinacol rearrangement Diels alder
Diels alder Mclafferty rearrangement
Mclafferty rearrangement Imine hydrolysis
Imine hydrolysis Enediol rearrangement
Enediol rearrangement Replication fork
Replication fork Bioflix activity dna replication dna replication diagram
Bioflix activity dna replication dna replication diagram Coding dna and non coding dna
Coding dna and non coding dna Enzyme involved in dna replication
Enzyme involved in dna replication Dna rna protein synthesis homework #2 dna replication
Dna rna protein synthesis homework #2 dna replication Molecular genetics section 1 dna the genetic material
Molecular genetics section 1 dna the genetic material Chapter 11 dna and genes
Chapter 11 dna and genes Chapter 12 section 1: dna: the genetic material
Chapter 12 section 1: dna: the genetic material Dna and genes chapter 11
Dna and genes chapter 11 Chapter 12 molecular genetics
Chapter 12 molecular genetics Chapter 12 dna and rna
Chapter 12 dna and rna Chapter 12 section 3 dna rna and protein
Chapter 12 section 3 dna rna and protein Chapter 12 dna and rna
Chapter 12 dna and rna Chapter 12 dna the genetic material
Chapter 12 dna the genetic material Gcat dna
Gcat dna Section 12-1 dna
Section 12-1 dna Body paragraph
Body paragraph Dna model using yarn of different colors
Dna model using yarn of different colors Separating dna
Separating dna Application dna
Application dna Genghis khan dna map
Genghis khan dna map Varning
Varning Desnaturalizacion del dna
Desnaturalizacion del dna Forms of dna
Forms of dna Transcription end result
Transcription end result Chargaff's rule of base pairing
Chargaff's rule of base pairing Dna stand for
Dna stand for Dna replication is semi-conservative
Dna replication is semi-conservative Strawberry dna extraction materials
Strawberry dna extraction materials Dna itsepalvelu
Dna itsepalvelu 5 examples of palindromic dna sequences
5 examples of palindromic dna sequences Dna transkripsi
Dna transkripsi Relationships and biodiversity lab answers
Relationships and biodiversity lab answers Unlike dnarna contains
Unlike dnarna contains Dna vaccines pros and cons
Dna vaccines pros and cons Characteristic of dna
Characteristic of dna Dna stands for deoxyribonucleic acid
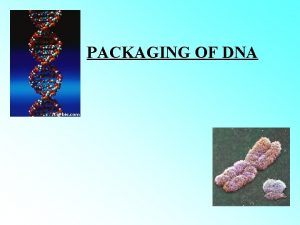
Dna stands for deoxyribonucleic acid Packaging of dna helix
Packaging of dna helix Structer of dna
Structer of dna