CANCER OF UNKNOWN PRIMARY SITE NICHOLAS PAVLIDIS MD



















![MOLECULAR [Microarray ANALYSIS Platforms] > 80 – 90 % accuracy MOLECULAR [Microarray ANALYSIS Platforms] > 80 – 90 % accuracy](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-36.jpg)









![Data : 1988 – 2010 No pts : 515 [Low grade = 231 (45%)] Data : 1988 – 2010 No pts : 515 [Low grade = 231 (45%)]](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-68.jpg)



![HISTOLOGIC SPECTRUM OF LIVER METASTASES Histology Mousseau et al [Bull Cancer 1991] Ayoub et HISTOLOGIC SPECTRUM OF LIVER METASTASES Histology Mousseau et al [Bull Cancer 1991] Ayoub et](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-75.jpg)









- Slides: 91

CANCER OF UNKNOWN PRIMARY SITE NICHOLAS PAVLIDIS, MD, Ph. D, FRCP (Edin) PROFESSOR OF MEDICAL ONCOLOGY MEDICAL SCHOOL, UNIVERSITY OF IOANNINA GREECE Barcelona , June 2012

CANCER OF UNKNOWN PRIMARY ( CUP ) 1) DEFINITION 2) EPIDEMIOLOGY 3) BIOLOGY 4) PATHOLOGY 5) NATURAL HISTORY 6) DIAGNOSTIC APPROACH 7) TREATMENT

IS THERE A DEFINITION FOR CANCER OF UNKNOWN PRIMARY ORIGIN ?

THE DEFINITION In 1970’s All patients presented with histologically confirmed metastatic carcinoma in whom a complete medical history, careful physical examination, chest x-ray, full blood count, stool occult blood testing and urinalysis did not identify the primary site.

CLINICAL AND LABORATORY DATA REQUIRED TO DEFINE A PATIENT AS HAVING A CUP ü Histologically confirmed metastatic cancer ü Detailed medical history ü Complete physical examination (plus pelvic and rectal exam) ü Chest radiography ü Full blood count ü Biochemistry ü Urinalysis ü Stool occult blood testing ü Histopathology review and use of immunohistochemistry ü Computed tomography of chest, abdomen and pelvis ü Mammography or MRI (in certain cases). ü PET – scan (in certain cases).






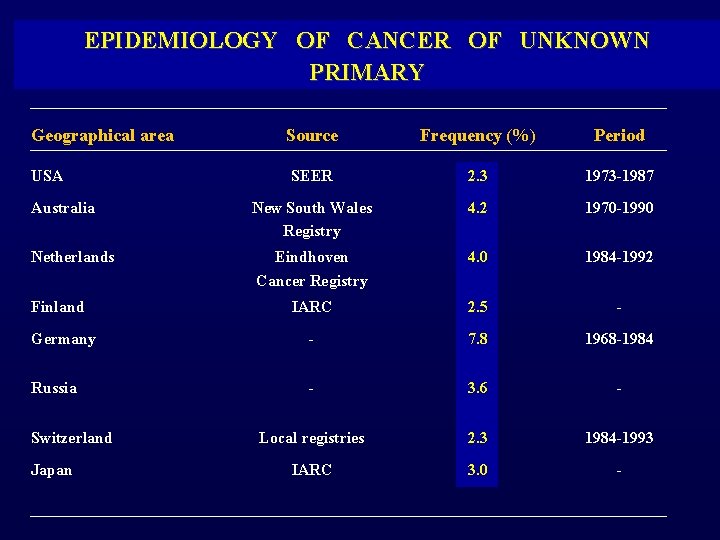
WHAT IS THE INCIDENCE OF CANCER OF UNKNOWN PRIMARY SITE ?

EPIDEMIOLOGY OF CANCER OF UNKNOWN PRIMARY Geographical area Source Frequency (%) Period SEER 2. 3 1973 -1987 Australia New South Wales Registry 4. 2 1970 -1990 Netherlands Eindhoven Cancer Registry 4. 0 1984 -1992 IARC 2. 5 - Germany - 7. 8 1968 -1984 Russia - 3. 6 - Local registries 2. 3 1984 -1993 IARC 3. 0 - USA Finland Switzerland Japan

THE BIOLOGY OF CANCER OF UNKNOWN PRIMARY

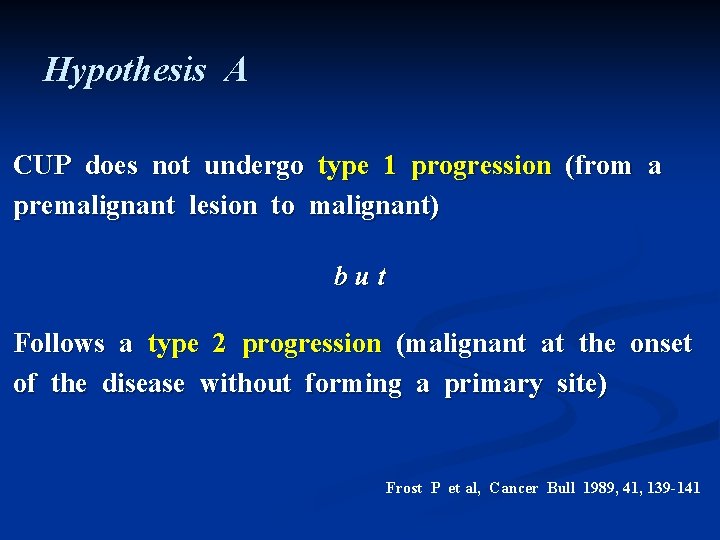
Hypothesis A CUP does not undergo type 1 progression (from a premalignant lesion to malignant) but Follows a type 2 progression (malignant at the onset of the disease without forming a primary site) Frost P et al, Cancer Bull 1989, 41, 139 -141

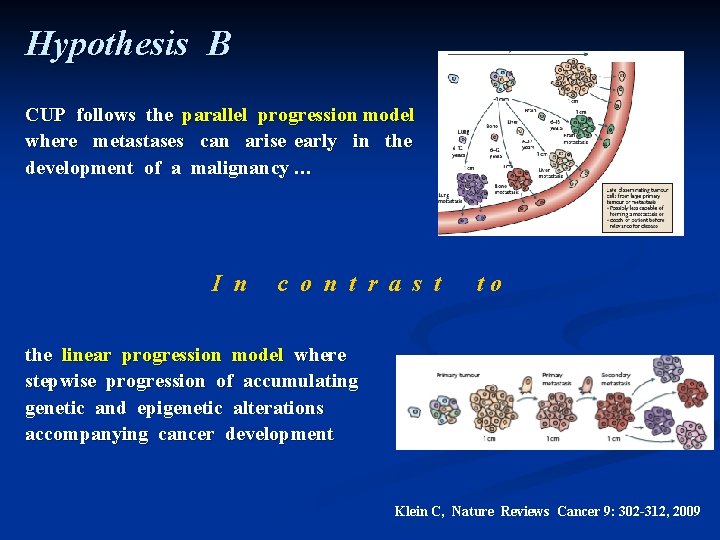
Hypothesis B CUP follows the parallel progression model where metastases can arise early in the development of a malignancy … I n c o n t r a s t to the linear progression model where stepwise progression of accumulating genetic and epigenetic alterations accompanying cancer development Klein C, Nature Reviews Cancer 9: 302 -312, 2009

TRANSLATIONAL RESEARCH ON CUP BIOLOGY 1. Chromosomal Instability 2. Oncogenes – Oncoproteins 3. Tumour and Metastasis Suppressor Genes 4. Angiogenesis 5. Metalloproteinases 6. Hypoxia 7. Epithelial Mesenchymal Transition and Stemness 8. Signaling Pathways

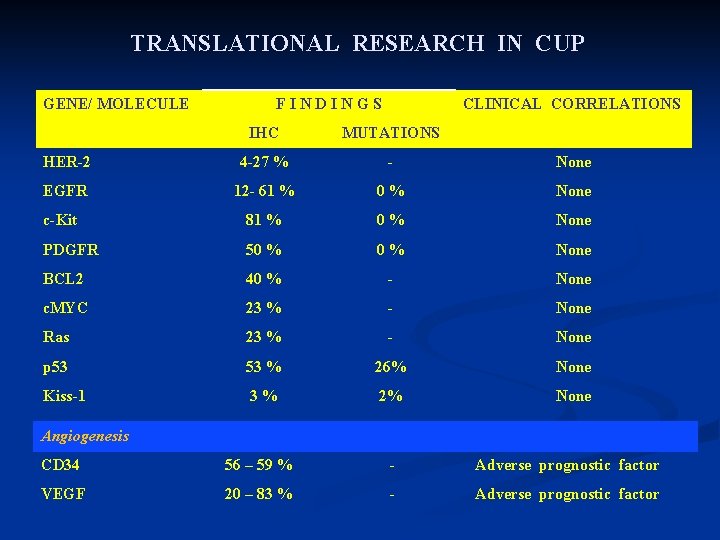
TRANSLATIONAL RESEARCH IN CUP GENE/ MOLECULE F I N D I N G S CLINICAL CORRELATIONS IHC MUTATIONS HER-2 4 -27 % - None EGFR 12 - 61 % 0 % None c-Kit 81 % 0 % None PDGFR 50 % None BCL 2 40 % - None c. MYC 23 % - None Ras 23 % - None p 53 53 % 26% None Kiss-1 3 % 2% None Angiogenesis CD 34 56 – 59 % - Adverse prognostic factor VEGF 20 – 83 % - Adverse prognostic factor

Matrix Metalloproteinase MMP-2 4 % - None MMP -9 36 % - None TIMP-1 44 % - Adverse prognostic factor Hypoxia GLUT-1 HIF 1 a 25 % - COX-2 Adverse prognostic factor in squamous cervical CUP EMT E-Cadherin 78. 8 % - SNAIL 61. 9 % - Vimentin 23. 2 % - N-Cadherin 13. 8 % Oct-4 0% • EMT phenotype was seen in 816% of CUP • Adverse prognostic factor

Signaling Pathways c. MET 42% 30 % p. MARK 54% - Notch 1 -3 2 -73 % - Jagged 1 22 % - PTEN 50 % - p. AKT 73 % - p. RPS 6 60 % - p 21 61% - Cyclin D 1 44% - • c. MET favourable prognosis • p. MAPK adverse prognosis • Notch-3 adverse prognosis • p 21 favourable prognosis • p. AKT or p. RPS 6 adverse prognosis • p. MAPK + p. AKT adverse prognosis

THE NATURAL HISTORY OF CANCER OF UNKNOWN PRIMARY SITE

FUNDAMENTAL CHARACTERISTICS v Early dissemination v Clinical absence of primary at presentation v Aggressiveness v Unpredictable metastatic pattern

UNPREDICTABLE METASTATIC PATTERN v Refers to the differences in the incidence of metastatic sites at diagnosis between known and unknown primary carcinomas Example ü Pancreatic cancer presenting as CUP has 4 -fold higher incidence to affect bones, and 30% incidence to appear with lung metastases.

Cancer of Unknown Primary Site : One or more Diseases ?

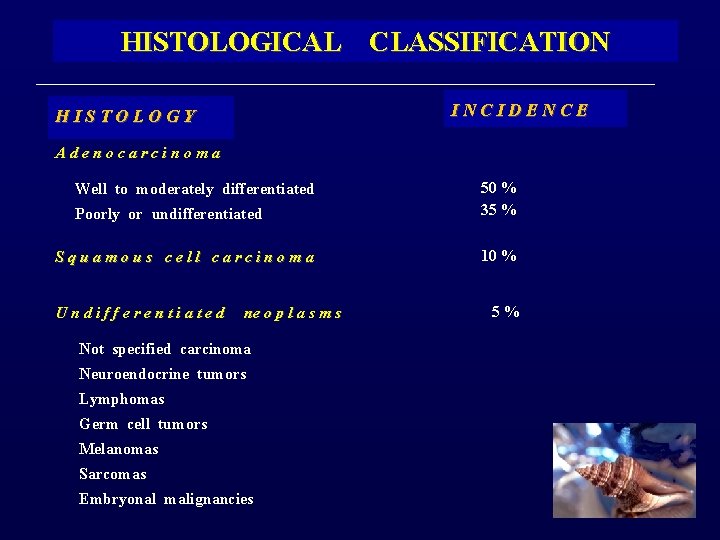
HISTOLOGICAL CLASSIFICATION HISTOLOGY Adenocarcinoma Well to moderately differentiated INCIDENCE Poorly or undifferentiated 50 % 35 % Squamous cell carcinoma 10 % U n d i f f e r e n t i a t e d ne o p l a s m s Not specified carcinoma Neuroendocrine tumors Lymphomas Germ cell tumors Melanomas Sarcomas Embryonal malignancies 5 %

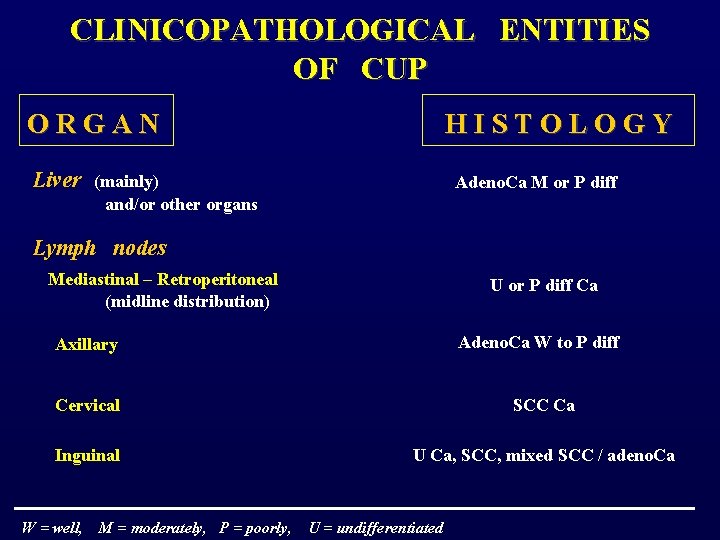
CLINICOPATHOLOGICAL ENTITIES OF CUP O R G A N H I S T O L O G Y Liver (mainly) Adeno. Ca M or P diff and/or other organs Lymph nodes Mediastinal – Retroperitoneal U or P diff Ca (midline distribution) Adeno. Ca W to P diff Axillary Cervical SCC Ca Inguinal U Ca, SCC, mixed SCC / adeno. Ca W = well, M = moderately, P = poorly, U = undifferentiated

Peritoneal cavity Peritoneal adenocarcinomatosis Papillary or serous adeno. Ca in females ( ± psammoma bodies ) Malignant ascites of other Mucin adeno. Ca M or P diff unknown origin ( ± signet ring cells ) Lungs Pulmonary metastases Pleural effusion W = well, M = moderately, P = poorly, Adeno. Ca various diff Adeno. Ca M or P diff U = undifferentiated

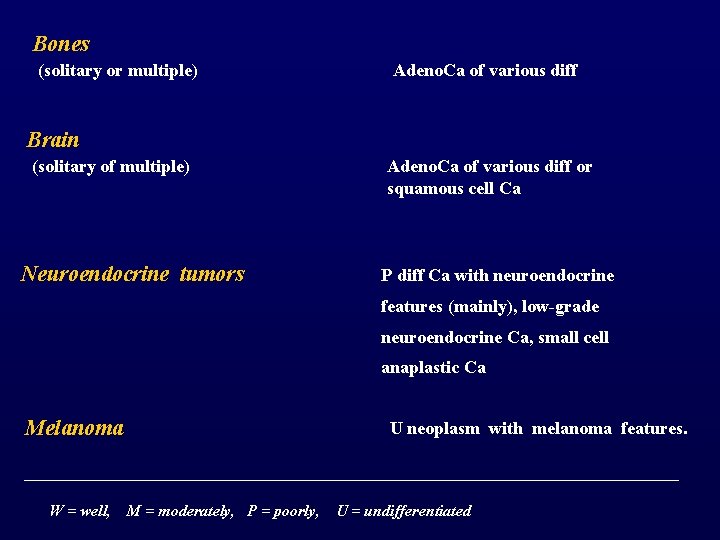
Bones (solitary or multiple) Adeno. Ca of various diff Brain (solitary of multiple) Neuroendocrine tumors Adeno. Ca of various diff or squamous cell Ca P diff Ca with neuroendocrine features (mainly), low-grade neuroendocrine Ca, small cell anaplastic Ca Melanoma W = well, U neoplasm with melanoma features. M = moderately, P = poorly, U = undifferentiated

WHAT IS THE OPTIMAL INVESTIGATIONAL DIAGNOSTIC APPROACH FOR THE IDENTIFICATION OF THE PRIMARY TUMOR ?

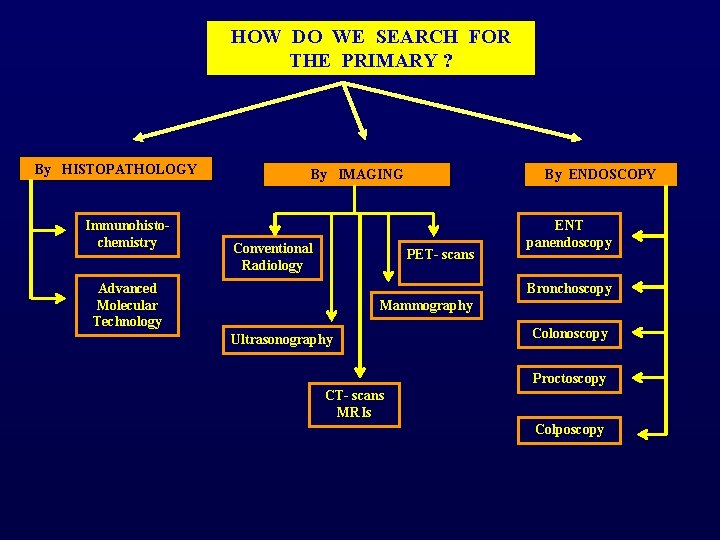
HOW DO WE SEARCH FOR THE PRIMARY ? By HISTOPATHOLOGY Immunohistochemistry By IMAGING Conventional Radiology By ENDOSCOPY PET- scans Advanced Molecular Technology ENT panendoscopy Bronchoscopy Mammography Ultrasonography Colonoscopy Proctoscopy CT- scans MRIs Colposcopy

By HISTOPATHOLOGY

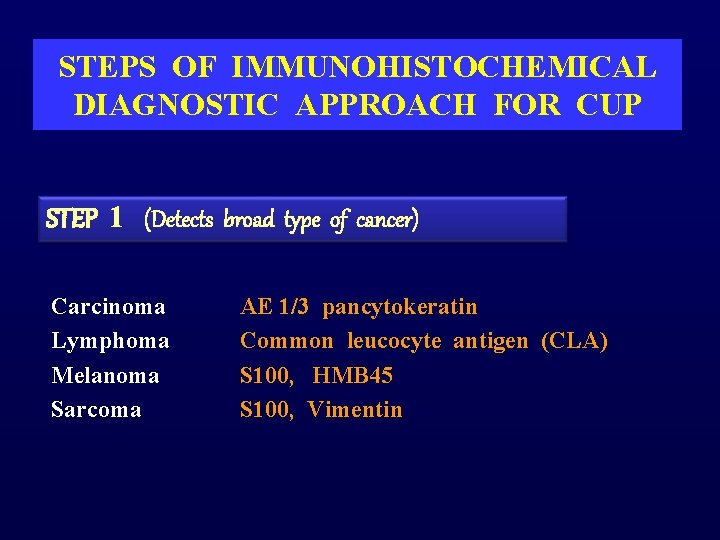
STEPS OF IMMUNOHISTOCHEMICAL DIAGNOSTIC APPROACH FOR CUP STEP 1 (Detects broad type of cancer) Carcinoma Lymphoma Melanoma Sarcoma AE 1/3 pancytokeratin Common leucocyte antigen (CLA) S 100, HMB 45 S 100, Vimentin

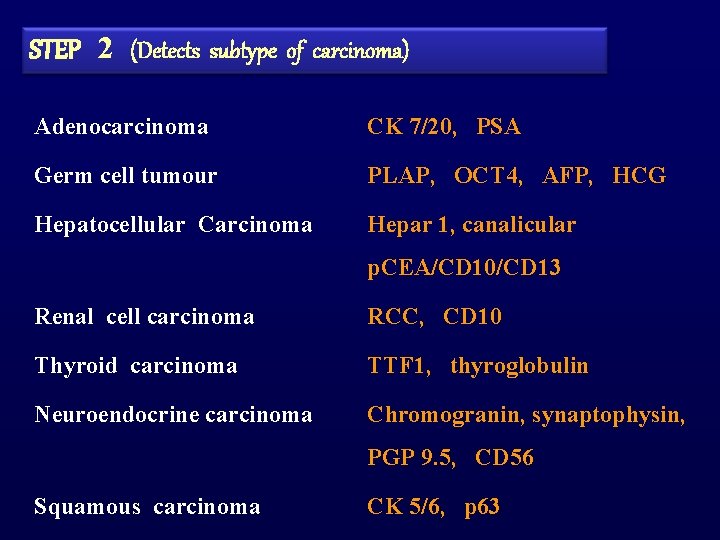
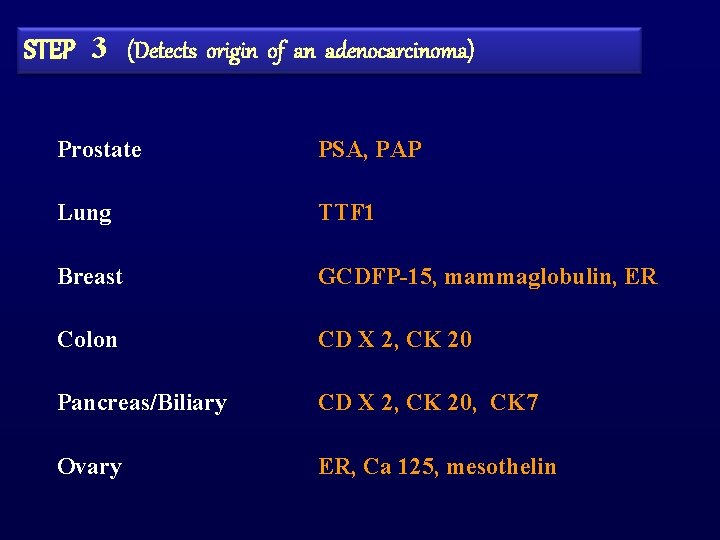
STEP 2 (Detects subtype of carcinoma) Adenocarcinoma CK 7/20, PSA Germ cell tumour PLAP, OCT 4, AFP, HCG Hepatocellular Carcinoma Hepar 1, canalicular p. CEA/CD 10/CD 13 Renal cell carcinoma RCC, CD 10 Thyroid carcinoma TTF 1, thyroglobulin Neuroendocrine carcinoma Chromogranin, synaptophysin, PGP 9. 5, CD 56 Squamous carcinoma CK 5/6, p 63

STEP 3 (Detects origin of an adenocarcinoma) Prostate PSA, PAP Lung TTF 1 Breast GCDFP-15, mammaglobulin, ER Colon CD X 2, CK 20 Pancreas/Biliary CD X 2, CK 20, CK 7 Ovary ER, Ca 125, mesothelin

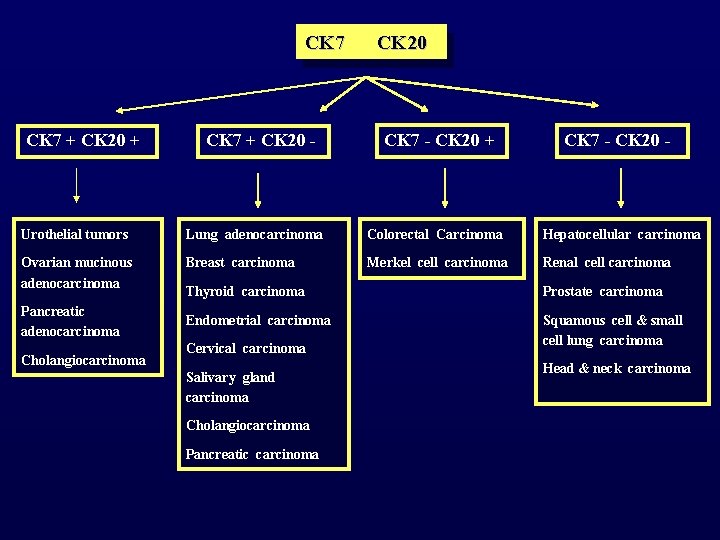
CYTOKERATINS (CKS) Monoclonal antibodies against cytokeratin polypeptides CK 7 and CK 20

CK 7 + CK 20 + CK 7 + CK 20 - CK 20 CK 7 - CK 20 + CK 7 - CK 20 - Urothelial tumors Lung adenocarcinoma Colorectal Carcinoma Hepatocellular carcinoma Ovarian mucinous adenocarcinoma Breast carcinoma Merkel cell carcinoma Renal cell carcinoma Pancreatic adenocarcinoma Cholangiocarcinoma Thyroid carcinoma Prostate carcinoma Endometrial carcinoma Squamous cell & small cell lung carcinoma Cervical carcinoma Salivary gland carcinoma Cholangiocarcinoma Pancreatic carcinoma Head & neck carcinoma
![MOLECULAR Microarray ANALYSIS Platforms 80 90 accuracy MOLECULAR [Microarray ANALYSIS Platforms] > 80 – 90 % accuracy](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-36.jpg)
MOLECULAR [Microarray ANALYSIS Platforms] > 80 – 90 % accuracy

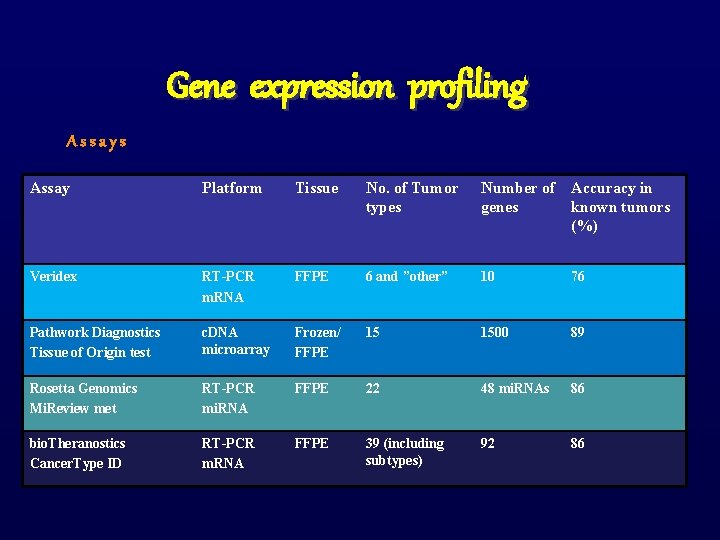
Gene expression profiling Assays Assay Platform Tissue No. of Tumor types Number of Accuracy in genes known tumors (%) Veridex RT-PCR m. RNA FFPE 6 and ”other” 10 76 Pathwork Diagnostics Tissue of Origin test c. DNA microarray Frozen/ FFPE 15 1500 89 Rosetta Genomics Mi. Review met RT-PCR mi. RNA FFPE 22 48 mi. RNAs 86 bio. Theranostics Cancer. Type ID RT-PCR m. RNA FFPE 39 (including subtypes) 92 86

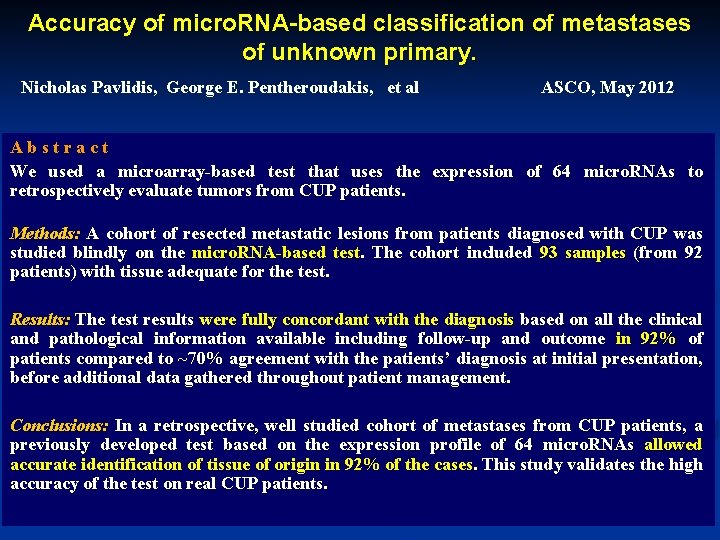
Accuracy of micro. RNA-based classification of metastases of unknown primary. Nicholas Pavlidis, George E. Pentheroudakis, et al ASCO, May 2012 A b s t r a c t We used a microarray-based test that uses the expression of 64 micro. RNAs to retrospectively evaluate tumors from CUP patients. Methods: A cohort of resected metastatic lesions from patients diagnosed with CUP was studied blindly on the micro. RNA-based test. The cohort included 93 samples (from 92 patients) with tissue adequate for the test. Results: The test results were fully concordant with the diagnosis based on all the clinical and pathological information available including follow-up and outcome in 92% of patients compared to ~70% agreement with the patients’ diagnosis at initial presentation, before additional data gathered throughout patient management. Conclusions: In a retrospective, well studied cohort of metastases from CUP patients, a previously developed test based on the expression profile of 64 micro. RNAs allowed accurate identification of tissue of origin in 92% of the cases. This study validates the high accuracy of the test on real CUP patients.

By IMAGING

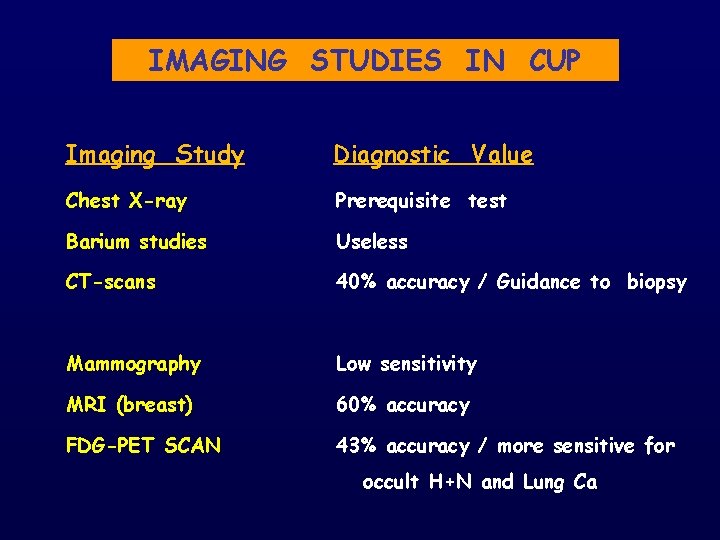
IMAGING STUDIES IN CUP Imaging Study Diagnostic Value Chest X-ray Prerequisite test Barium studies Useless CT-scans 40% accuracy / Guidance to biopsy Mammography Low sensitivity MRI (breast) 60% accuracy FDG-PET SCAN 43% accuracy / more sensitive for occult H+N and Lung Ca

By ENDOSCOPY

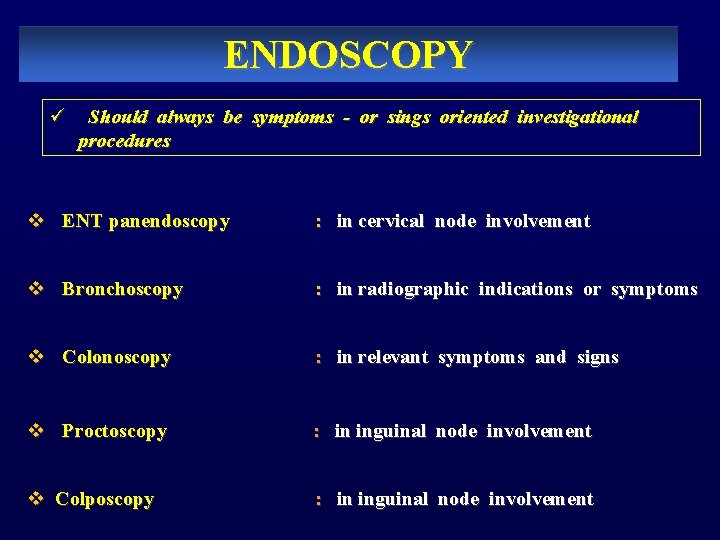
ENDOSCOPY ü Should always be symptoms - or sings oriented investigational procedures v ENT panendoscopy : in cervical node involvement v Bronchoscopy : in radiographic indications or symptoms v Colonoscopy : in relevant symptoms and signs v Proctoscopy v Colposcopy : in inguinal node involvement

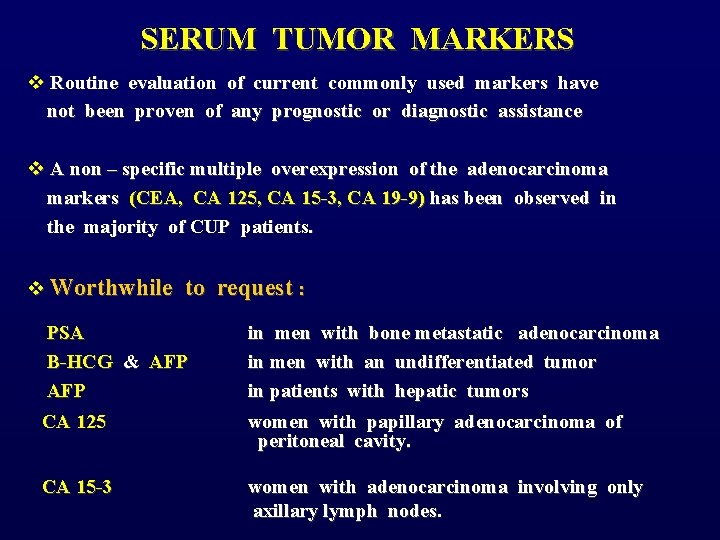
SERUM TUMOR MARKERS v Routine evaluation of current commonly used markers have not been proven of any prognostic or diagnostic assistance v A non – specific multiple overexpression of the adenocarcinoma markers (CEA, CA 125, CA 15 -3, CA 19 -9) has been observed in the majority of CUP patients. v Worthwhile to request : PSA Β-HCG & AFP CA 125 CA 15 -3 in men with bone metastatic adenocarcinoma in men with an undifferentiated tumor in patients with hepatic tumors women with papillary adenocarcinoma of peritoneal cavity. women with adenocarcinoma involving only axillary lymph nodes

HOW OFTEN CAN THE PRIMARY TUMOR BE INDENTIFIED ?

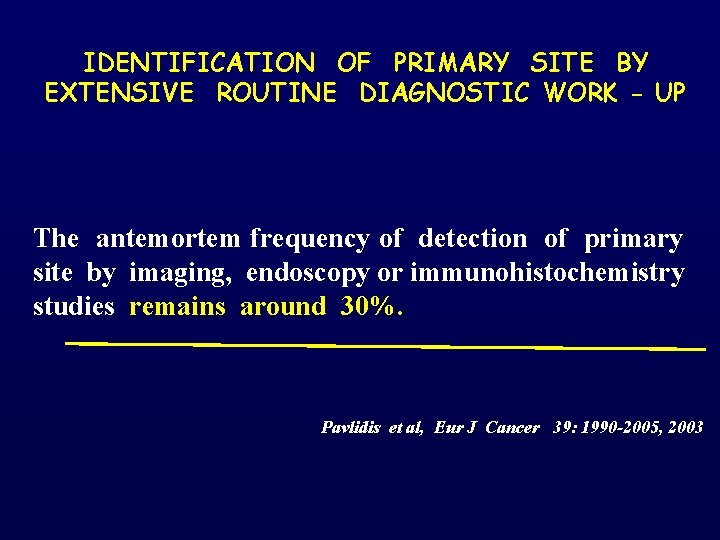
IDENTIFICATION OF PRIMARY SITE BY EXTENSIVE ROUTINE DIAGNOSTIC WORK - UP The antemortem frequency of detection of primary site by imaging, endoscopy or immunohistochemistry studies remains around 30%. Pavlidis et al, Eur J Cancer 39: 1990 -2005, 2003


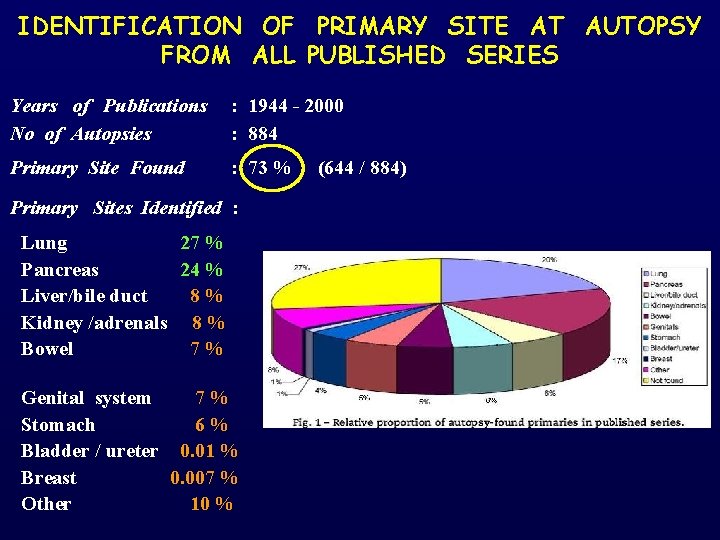
IDENTIFICATION OF PRIMARY SITE AT AUTOPSY FROM ALL PUBLISHED SERIES Years of Publications : 1944 - 2000 No of Autopsies : 884 Primary Site Found : 73 % (644 / 884) Primary Sites Identified : Lung 27 % Pancreas 24 % Liver/bile duct 8 % Kidney /adrenals 8 % Bowel 7 % Genital system Stomach Bladder / ureter Breast Other 7 % 6 % 0. 01 % 0. 007 % 10 %

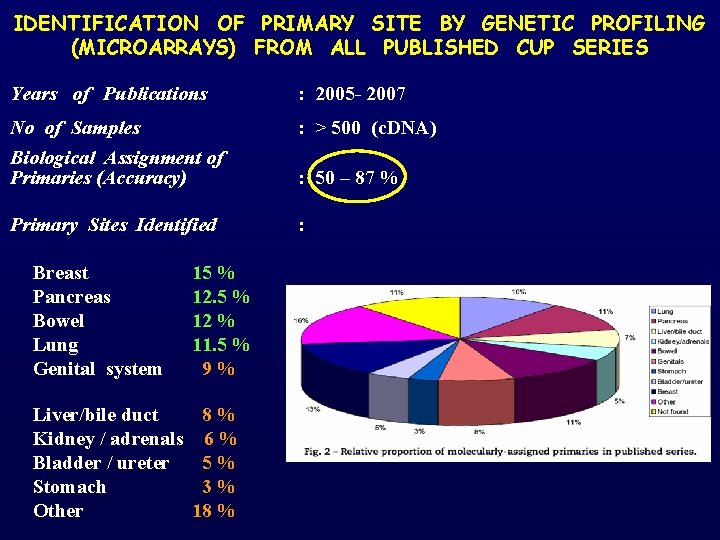
IDENTIFICATION OF PRIMARY SITE BY GENETIC PROFILING (MICROARRAYS) FROM ALL PUBLISHED CUP SERIES Years of Publications : 2005 - 2007 No of Samples : > 500 (c. DNA) Biological Assignment of Primaries (Accuracy) : 50 – 87 % Primary Sites Identified : Breast Pancreas Bowel Lung Genital system 15 % 12 % 11. 5 % 9 % Liver/bile duct 8 % Kidney / adrenals 6 % Bladder / ureter 5 % Stomach 3 % Other 18 %

WHAT IS THE OPTIMAL THERAPEUTIC APPROACH OF CANCER OF UNKNOWN PRIMARY ?

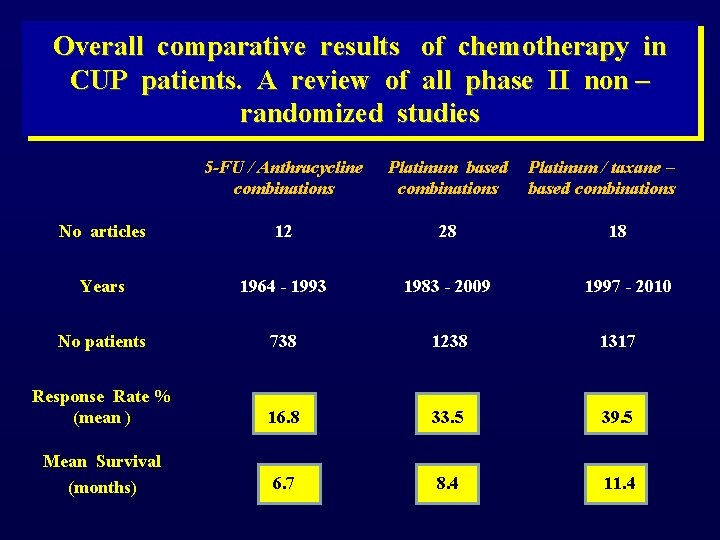
Overall comparative results of chemotherapy in CUP patients. A review of all phase II non – randomized studies 5 -FU / Anthracycline combinations Platinum based combinations Platinum / taxane – based combinations No articles 12 28 18 Years 1964 - 1993 1983 - 2009 1997 - 2010 No patients 738 1238 1317 Response Rate % (mean ) 16. 8 33. 5 39. 5 Mean Survival (months) 6. 7 8. 4 11. 4

DO WE HAVE EFFECTIVE DRUGS FOR CANCER OF UNKNOWN PRIMARY OR WE JUST HAVE RESPONSIVE SUBSETS OF PATIENTS ?

2003 , 199 : 9 3 DIAG NOS TIC 05 0 2 0– AND TH OF A ERAPEU T NU N. Pa NKN IC MAN vlidis AG OWN , E. B riaso PRIM EMENT ulis, OF C ARY J. Ha ANC insw ER orth, E. A. Grec o

WHAT IS CANCER OF UNKNOWN PRIMARY ? Lung-hidden CUP Kidney-hidden CUP Pancreas-hidden CUP Gastric-hidden CUP Liver-hidden CUP Colon-hidden CUP Prostate-hidden CUP Breast-hidden CUP

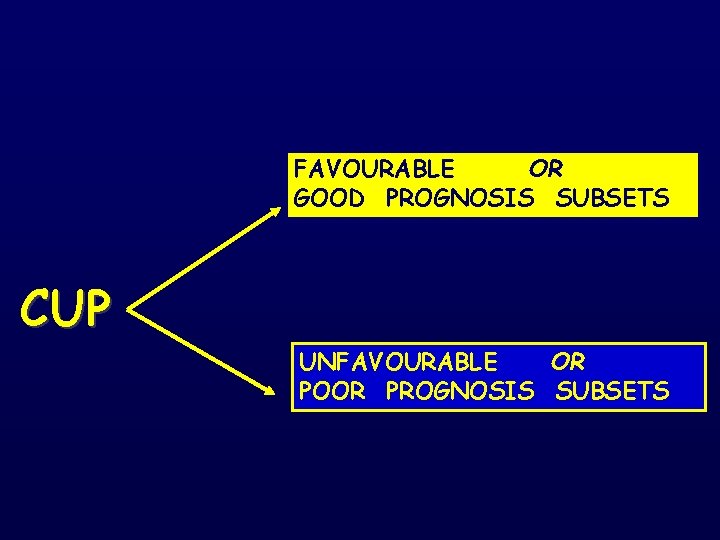
FAVOURABLE OR GOOD PROGNOSIS SUBSETS CUP UNFAVOURABLE OR POOR PROGNOSIS SUBSETS

THE FAVOURABLE SUBSETS OR GOOD PROGNOSIS SUBSETS

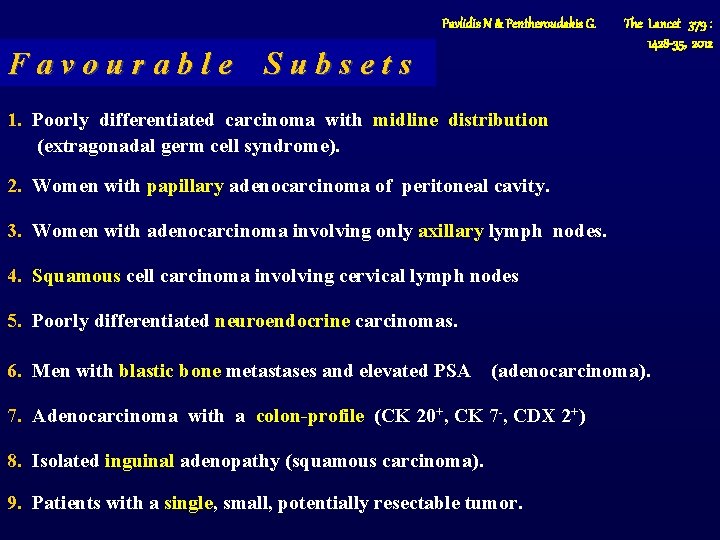
Pavlidis N & Pentheroudakis G. Favourable Subsets The Lancet 379 : 1428 -35, 2012 1. Poorly differentiated carcinoma with midline distribution (extragonadal germ cell syndrome). 2. Women with papillary adenocarcinoma of peritoneal cavity. 3. Women with adenocarcinoma involving only axillary lymph nodes. 4. Squamous cell carcinoma involving cervical lymph nodes 5. Poorly differentiated neuroendocrine carcinomas. 6. Men with blastic bone metastases and elevated PSA (adenocarcinoma). 7. Adenocarcinoma with a colon-profile (CK 20+, CK 7 -, CDX 2+) 8. Isolated inguinal adenopathy (squamous carcinoma). 9. Patients with a single, small, potentially resectable tumor.

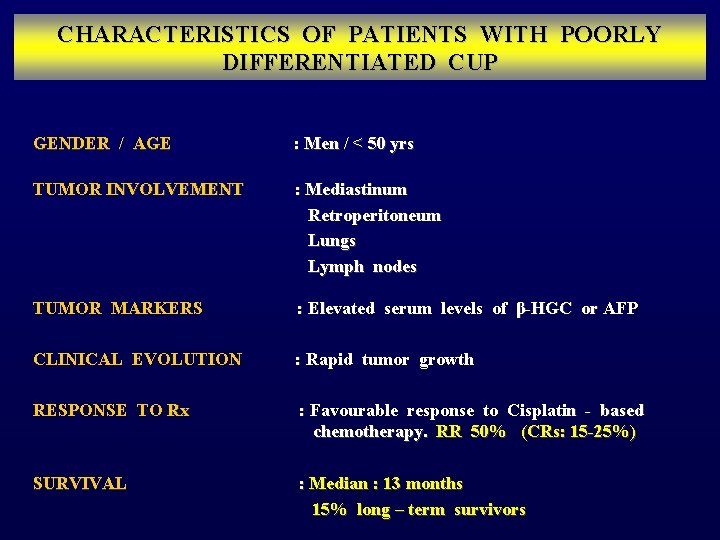
CHARACTERISTICS OF PATIENTS WITH POORLY DIFFERENTIATED CUP GENDER / AGE TUMOR INVOLVEMENT TUMOR MARKERS : Men / < 50 yrs : Mediastinum Retroperitoneum Lungs Lymph nodes : Elevated serum levels of β-HGC or AFP CLINICAL EVOLUTION : Rapid tumor growth RESPONSE TO Rx : Favourable response to Cisplatin - based chemotherapy. RR 50% (CRs: 15 -25%) SURVIVAL : Median : 13 months 15% long – term survivors

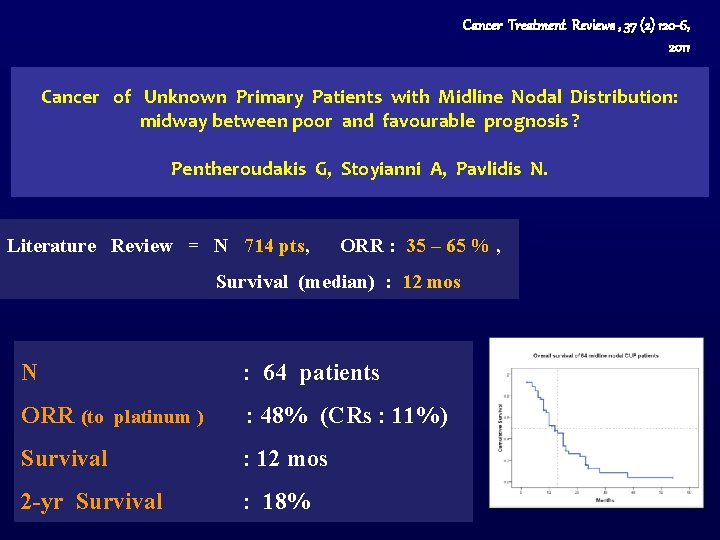
Cancer Treatment Reviews , 37 (2) 120 -6, 2011 Cancer of Unknown Primary Patients with Midline Nodal Distribution: midway between poor and favourable prognosis ? Pentheroudakis G, Stoyianni A, Pavlidis N. Literature Review = N 714 pts, ORR : 35 – 65 % , Survival (median) : 12 mos N : 64 patients ORR (to platinum ) : 48% (CRs : 11%) Survival : 12 mos 2 -yr Survival : 18%

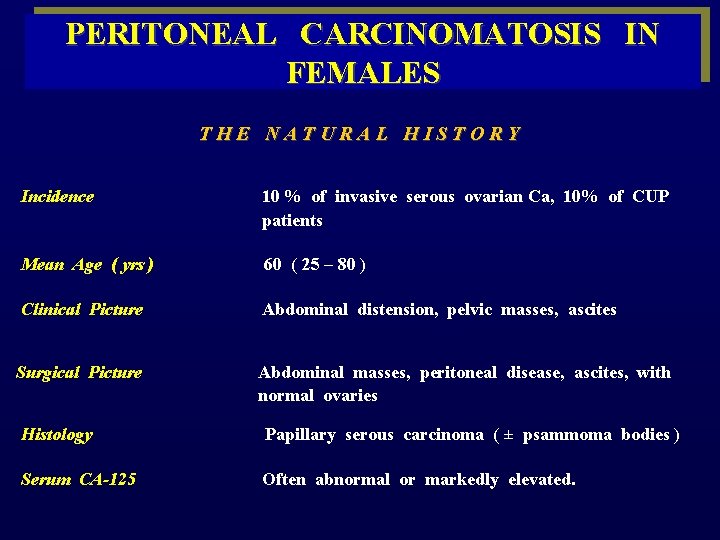
PERITONEAL CARCINOMATOSIS IN FEMALES T H E N A T U R A L H I S T O R Y Incidence 10 % of invasive serous ovarian Ca, 10% of CUP patients Mean Age ( yrs ) 60 ( 25 – 80 ) Clinical Picture Abdominal distension, pelvic masses, ascites Surgical Picture Histology Serum CA-125 Abdominal masses, peritoneal disease, ascites, with normal ovaries Papillary serous carcinoma ( ± psammoma bodies ) Often abnormal or markedly elevated.

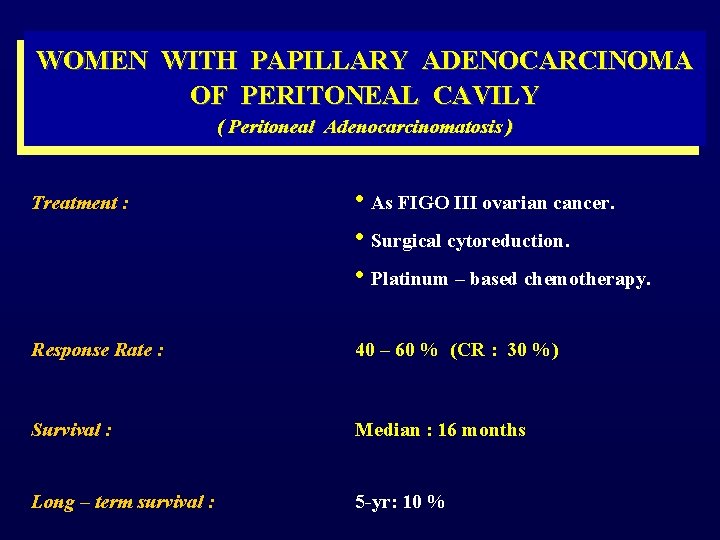
WOMEN WITH PAPILLARY ADENOCARCINOMA OF PERITONEAL CAVILY ( Peritoneal Adenocarcinomatosis ) Treatment : • As FIGO III ovarian cancer. • Surgical cytoreduction. • Platinum – based chemotherapy. Response Rate : 40 – 60 % (CR : 30 %) Survival : Median : 16 months Long – term survival : 5 -yr: 10 %

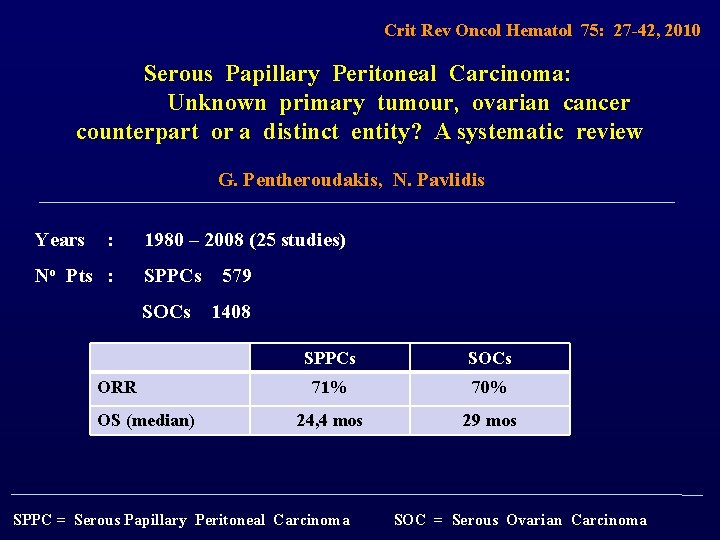
Crit Rev Oncol Hematol 75: 27 -42, 2010 Serous Papillary Peritoneal Carcinoma: Unknown primary tumour, ovarian cancer counterpart or a distinct entity? A systematic review G. Pentheroudakis, N. Pavlidis Years : 1980 – 2008 (25 studies) No Pts : SPPCs 579 SOCs 1408 ORR OS (median) SPPCs SOCs 71% 70% 24, 4 mos 29 mos SPPC = Serous Papillary Peritoneal Carcinoma SOC = Serous Ovarian Carcinoma

ISOLATED AXILLARY NODAL METASTASES FROM AN OCCULT PRIMARY BREAST CANCER

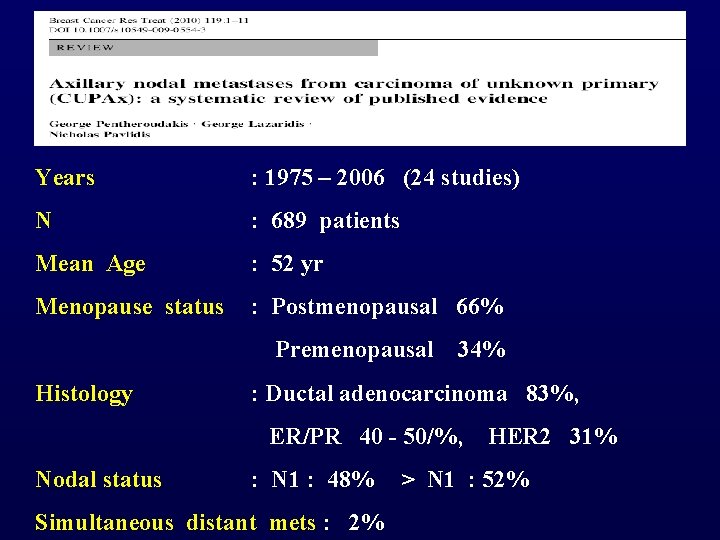
Years : 1975 – 2006 (24 studies) N : 689 patients Mean Age : 52 yr Menopause status : Postmenopausal 66% Premenopausal 34% Histology : Ductal adenocarcinoma 83%, ER/PR 40 - 50/%, HER 2 31% Nodal status : N 1 : 48% > N 1 : 52% Simultaneous distant mets : 2%

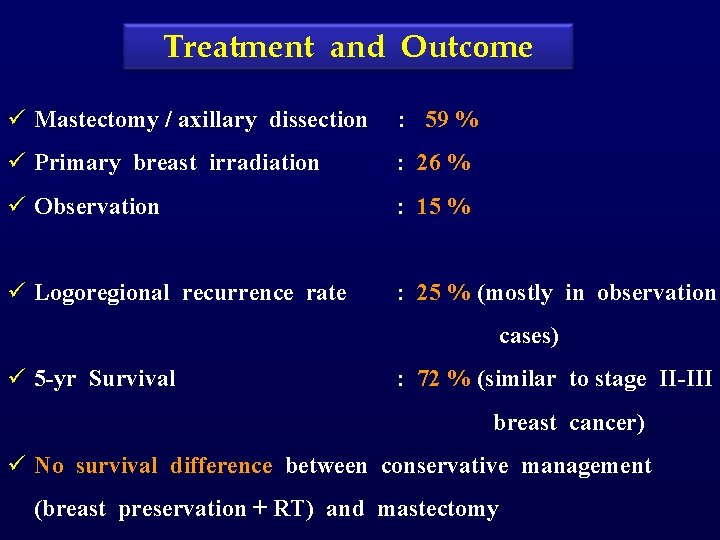
Treatment and Outcome ü Mastectomy / axillary dissection : 59 % ü Primary breast irradiation : 26 % ü Observation : 15 % ü Logoregional recurrence rate : 25 % (mostly in observation cases) ü 5 -yr Survival : 72 % (similar to stage II-III breast cancer) ü No survival difference between conservative management (breast preservation + RT) and mastectomy

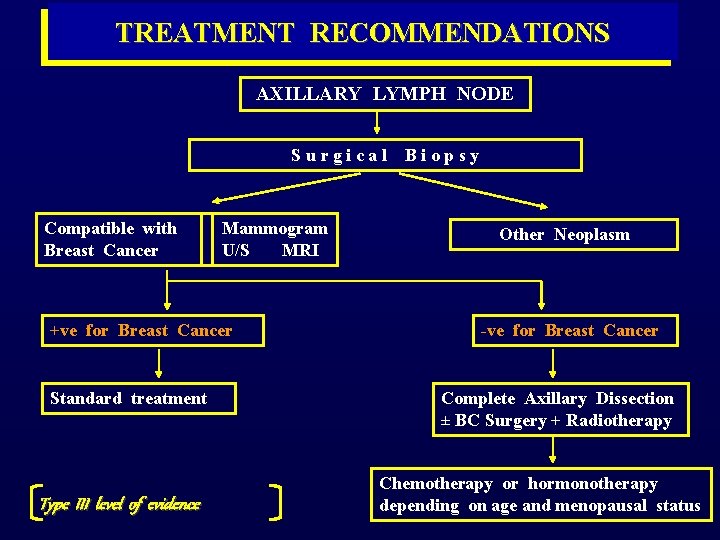
TREATMENT RECOMMENDATIONS AXILLARY LYMPH NODE S u r g i c a l B i o p s y Compatible with Breast Cancer Mammogram U/S MRI +ve for Breast Cancer Standard treatment Type III level of evidence Other Neoplasm -ve for Breast Cancer Complete Axillary Dissection ± BC Surgery + Radiotherapy Chemotherapy or hormonotherapy depending on age and menopausal status

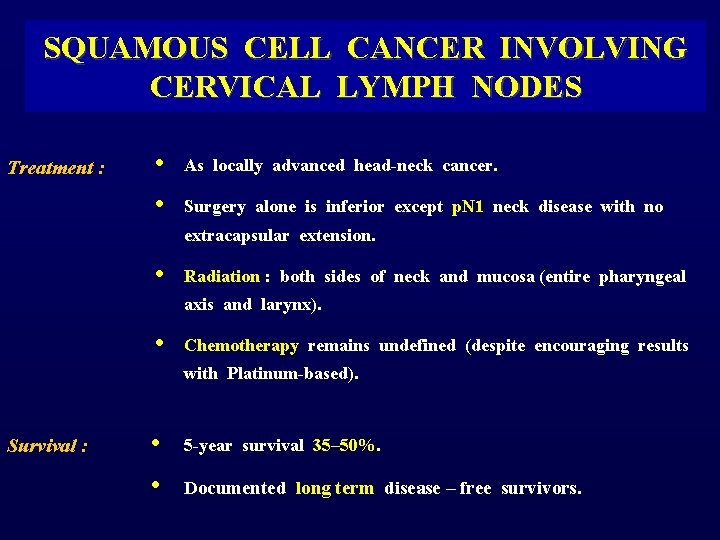
SQUAMOUS CELL CANCER INVOLVING CERVICAL LYMPH NODES Treatment : • • As locally advanced head-neck cancer. Surgery alone is inferior except p. N 1 neck disease with no extracapsular extension. • Radiation : both sides of neck and mucosa (entire pharyngeal axis and larynx). • Chemotherapy remains undefined (despite encouraging results with Platinum-based). Survival : • 5 -year survival 35– 50%. • Documented long term disease – free survivors.

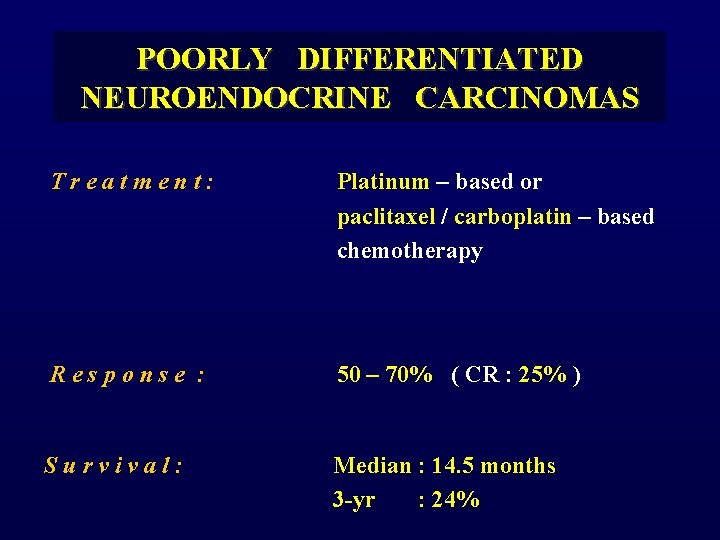
POORLY DIFFERENTIATED NEUROENDOCRINE CARCINOMAS Treatment: Platinum – based or paclitaxel / carboplatin – based chemotherapy Response : 50 – 70% ( CR : 25% ) Survival: Median : 14. 5 months 3 -yr : 24%
![Data 1988 2010 No pts 515 Low grade 231 45 Data : 1988 – 2010 No pts : 515 [Low grade = 231 (45%)]](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-68.jpg)
Data : 1988 – 2010 No pts : 515 [Low grade = 231 (45%)] Chemotherapy (Platinum based) : 65% Response rate : 50 -60% (CR: 20 - 30%) Median survival : 15. 5 months (11. 6 – 40)

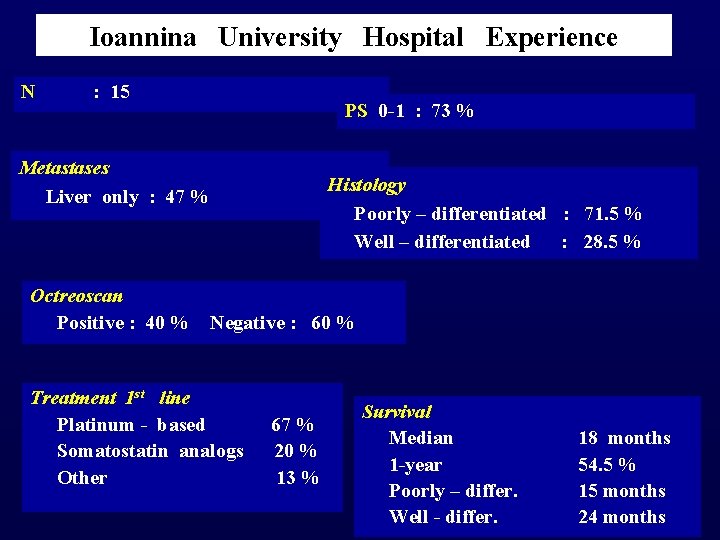
Ioannina University Hospital Experience N : 15 Metastases Liver only : 47 % PS 0 -1 : 73 % Histology Poorly – differentiated : 71. 5 % Well – differentiated : 28. 5 % Octreoscan Positive : 40 % Negative : 60 % Treatment 1 st line Platinum - based 67 % Somatostatin analogs 20 % Other 13 % Survival Median 1 -year Poorly – differ. Well - differ. 18 months 54. 5 % 15 months 24 months

OTHER FAVOURABLE CUP SUBSETS ü Men with adenocarcinoma blastic bone metastases (and elevated PSA) Rx = Treat as metastatic prostate cancer ü Isolated inguinal lymphadenopathy from squamous cell carcinoma Rx = Dissection ± radiotherapy ü Single metastatic site Rx = Dissection ± radiotherapy

THE UNFAVOURABLE SUBSETS OR POOR PROGNOSIS SUBSETS

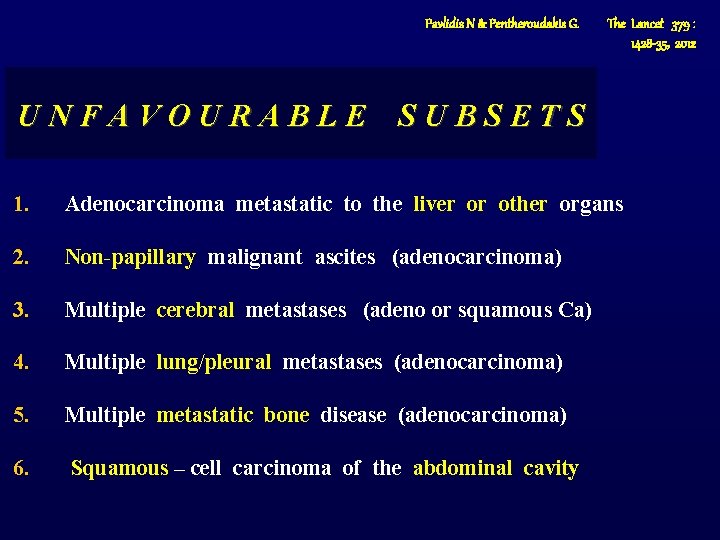
Pavlidis N & Pentheroudakis G. The Lancet 379 : 1428 -35, 2012 UNFAVOURABLE SUBSETS 1. Adenocarcinoma metastatic to the liver or other organs 2. Non-papillary malignant ascites (adenocarcinoma) 3. Multiple cerebral metastases (adeno or squamous Ca) 4. Multiple lung/pleural metastases (adenocarcinoma) 5. Multiple metastatic bone disease (adenocarcinoma) 6. Squamous – cell carcinoma of the abdominal cavity

Greco F, Pavlidis N. Semin Oncol, 2009

THE SUBSET OF ADENOCARCINOMA METASTATIC TO THE LIVER
![HISTOLOGIC SPECTRUM OF LIVER METASTASES Histology Mousseau et al Bull Cancer 1991 Ayoub et HISTOLOGIC SPECTRUM OF LIVER METASTASES Histology Mousseau et al [Bull Cancer 1991] Ayoub et](https://slidetodoc.com/presentation_image/7bbe551b832502731f80d2caf43eb42d/image-75.jpg)
HISTOLOGIC SPECTRUM OF LIVER METASTASES Histology Mousseau et al [Bull Cancer 1991] Ayoub et al [JCO 1998] Hogan et al [Clin Radiol 2002] Poussel et al Lazaridis et al [Gastr Clin Biol [Cancer Treat Rev 2005] 2008] Total (N= 91) (N=365) (N=88) (N=118) (N=49) (N=711) Adenocarcinoma 78% 61% 79. 5% 58% 69% Undifferentiated 12% 27% 3. 5% 20% 24% 20% Neuroendocrine - 9% 9% 14% 6% 9% Squamous 6% 2% 4. 5% 4% 0% 4% Others 4% 1% 3. 5% 4% - 3%

OVERALL RESULTS OF CHEMOTHERAPY IN CUP PATIENTS WITH LIVER METASTASES No of trials : 5 (1991, 1998, 2002, 2005, 2008) No of patients : 711 Response rate : < 20% Median survival : 5. 5 months Bull Cancer 1991, J Clin Oncol 1998, Clin Radiol 2002, Gastroent Clin Biol 2005, Cancer Treat Rev 2008

DO WE HAVE ANY EDIVENCE THAT TARGETED TREATMENT IS DRASTIC IN CUP PATIENTS ?

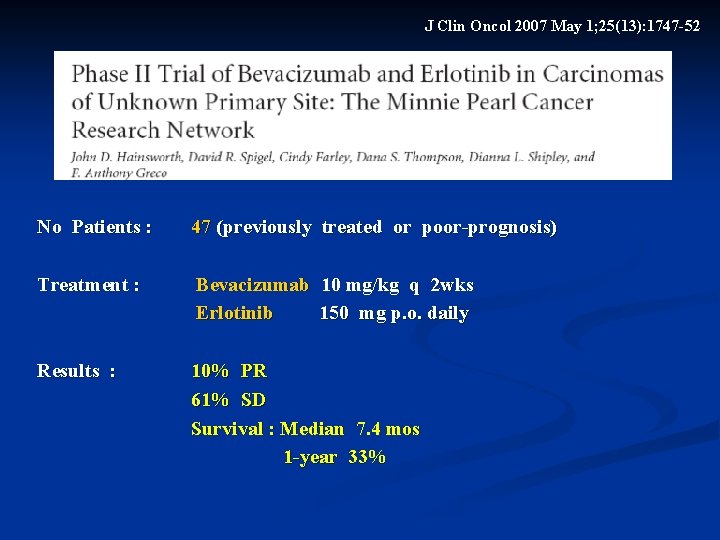
J Clin Oncol 2007 May 1; 25(13): 1747 -52 No Patients : 47 (previously treated or poor-prognosis) Treatment : Bevacizumab 10 mg/kg q 2 wks Erlotinib 150 mg p. o. daily Results : 10% PR 61% SD Survival : Median 7. 4 mos 1 -year 33%

Oncologist 2009, 14(12): 1189 -97 No Patients : 60 Regimen : Carboplatin / paclitaxel / Bevacizumab / Erlotinib As first-line and maintenance (Bev/Erlot) Treatment : 49 pts completed 4 cycles 44 pts continued maintenance bevacizumab/erlotinib Results : 53% major responses 41% stable disease PFS - median : 8 mos 1 -year : 38% Survival – median: 12. 6 mos 2 -year : 27%

DOES THE IDENTIFICATION OF PRIMARY SITE BY MOLECULAR PROFILING IMPROVE PATIENTS’ OUTCOME ? ? WHAT IS THE EVIDENCE TODAY ?

Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site (CUP): Results of a prospective Sarah Cannon Research Institute Trial F. Anthony Greco, MD 1, 2; Mark S. Rubin, MD 1, 3; David R. Spigel, MD 1, 2; Samuel Raby 1; Thabiso Chirwa 1; Raven Quinn, MS 1; Catherine A. Schnabel, Ph. D. 4; Mark G. Erlander, Ph. D. 4; John D. Hainsworth, MD 1, 2 1 Sarah Cannon Research Institute (SCRI), Nashville, TN ; 2 Tennessee Oncology, PLLC, Nashville, TN; 3 Florida Cancer Specialists/SCRI, Ft Myers, FL; 4 bio. Theranostics, Inc. , San Diego, CA

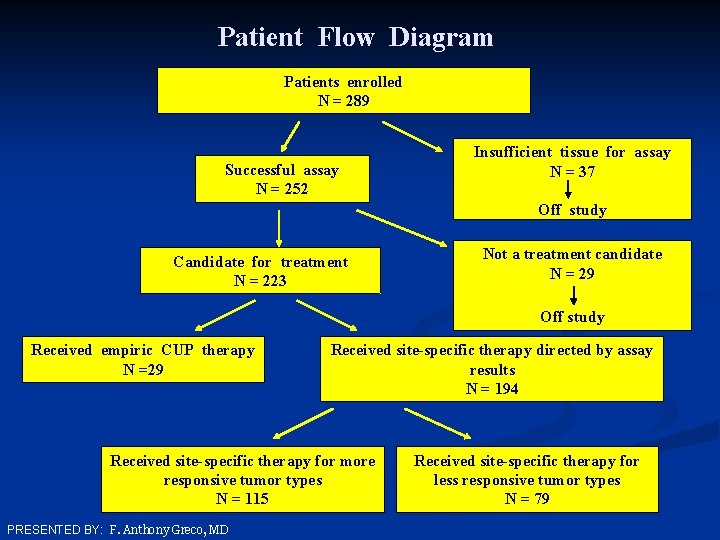
Patient Flow Diagram Patients enrolled N = 289 Successful assay N = 252 Insufficient tissue for assay N = 37 Off study Candidate for treatment N = 223 Not a treatment candidate N = 29 Off study Received empiric CUP therapy N =29 Received site-specific therapy directed by assay results N = 194 Received site-specific therapy for more responsive tumor types N = 115 PRESENTED BY: F. Anthony Greco, MD Received site-specific therapy for less responsive tumor types N = 79

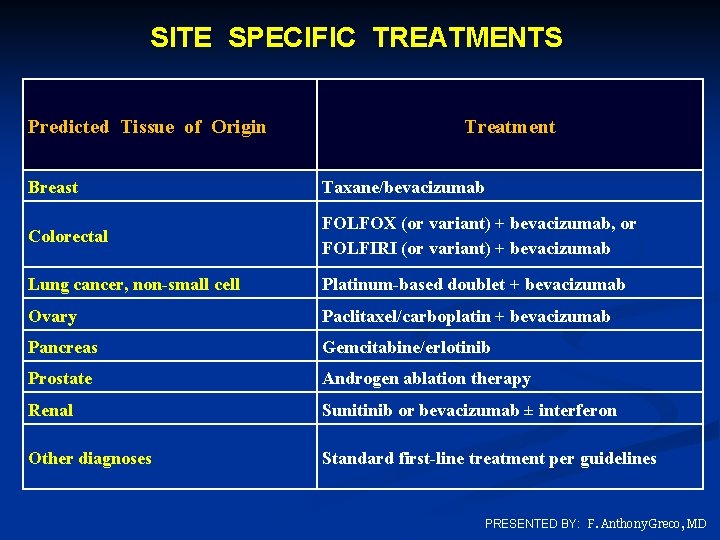
SITE SPECIFIC TREATMENTS Predicted Tissue of Origin Treatment Breast Taxane/bevacizumab Colorectal FOLFOX (or variant) + bevacizumab, or FOLFIRI (or variant) + bevacizumab Lung cancer, non-small cell Platinum-based doublet + bevacizumab Ovary Paclitaxel/carboplatin + bevacizumab Pancreas Gemcitabine/erlotinib Prostate Androgen ablation therapy Renal Sunitinib or bevacizumab ± interferon Other diagnoses Standard first-line treatment per guidelines PRESENTED BY: F. Anthony Greco, MD

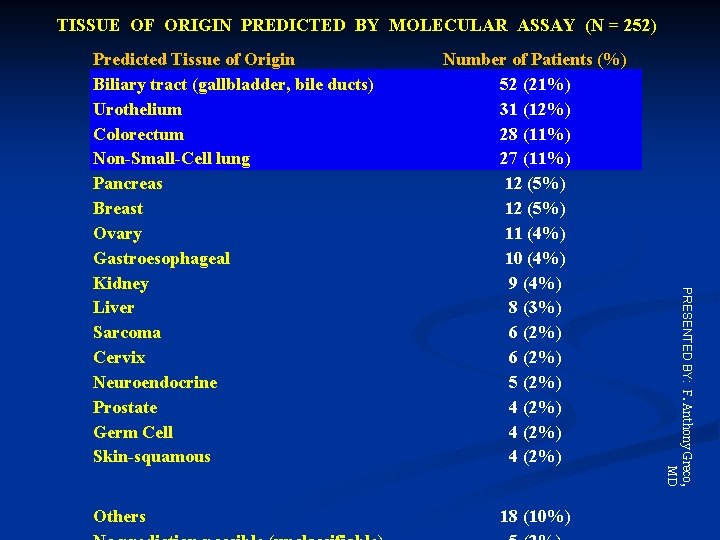
TISSUE OF ORIGIN PREDICTED BY MOLECULAR ASSAY (N = 252) Others Number of Patients (%) 52 (21%) 31 (12%) 28 (11%) 27 (11%) 12 (5%) 11 (4%) 10 (4%) 9 (4%) 8 (3%) 6 (2%) 5 (2%) 4 (2%) 18 (10%) PRESENTED BY: F. Anthony Greco, MD Predicted Tissue of Origin Biliary tract (gallbladder, bile ducts) Urothelium Colorectum Non-Small-Cell lung Pancreas Breast Ovary Gastroesophageal Kidney Liver Sarcoma Cervix Neuroendocrine Prostate Germ Cell Skin-squamous

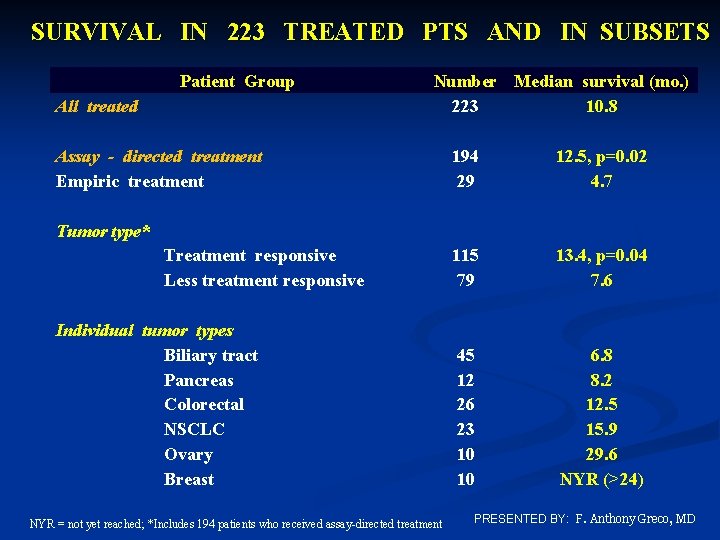
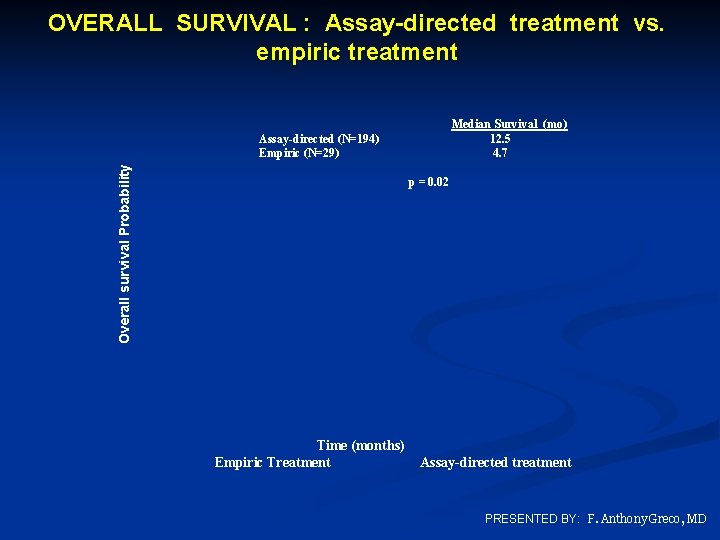
SURVIVAL IN 223 TREATED PTS AND IN SUBSETS Patient Group All treated Number Median survival (mo. ) 223 10. 8 Assay - directed treatment Empiric treatment 194 29 12. 5, p=0. 02 4. 7 115 79 13. 4, p=0. 04 7. 6 45 12 26 23 10 10 6. 8 8. 2 12. 5 15. 9 29. 6 NYR (>24) Tumor type* Treatment responsive Less treatment responsive Individual tumor types Biliary tract Pancreas Colorectal NSCLC Ovary Breast NYR = not yet reached; *Includes 194 patients who received assay-directed treatment PRESENTED BY: F. Anthony Greco, MD

OVERALL SURVIVAL : Assay-directed treatment vs. empiric treatment Overall survival Probability Assay-directed (N=194) Empiric (N=29) Median Survival (mo) 12. 5 4. 7 p = 0. 02 Time (months) Empiric Treatment Assay-directed treatment PRESENTED BY: F. Anthony Greco, MD

Ongoing Clinical Trials on CUP Trial Phase Regimens Country CUP-ONE II Epi / Cis / Capec ± Vandetanib UK GEFCAPI 04 III Cis / Gemc vs standard chemo based France on molecular diagnosis of the primary

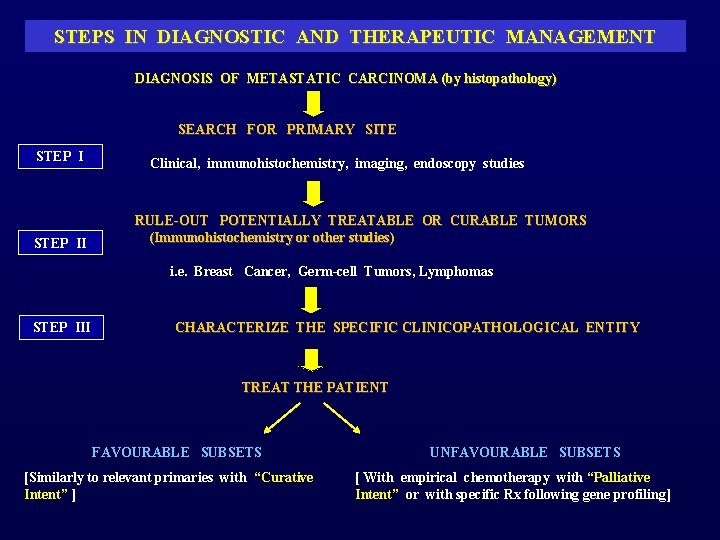
STEPS IN DIAGNOSTIC AND THERAPEUTIC MANAGEMENT DIAGNOSIS OF METASTATIC CARCINOMA (by histopathology) SEARCH FOR PRIMARY SITE STEP I Clinical, immunohistochemistry, imaging, endoscopy studies RULE-OUT POTENTIALLY TREATABLE OR CURABLE TUMORS (Immunohistochemistry or other studies) STEP II i. e. Breast Cancer, Germ-cell Tumors, Lymphomas STEP III CHARACTERIZE THE SPECIFIC CLINICOPATHOLOGICAL ENTITY TREAT THE PATIENT FAVOURABLE SUBSETS [Similarly to relevant primaries with “Curative Intent” ] UNFAVOURABLE SUBSETS [ With empirical chemotherapy with “Palliative Intent” or with specific Rx following gene profiling]

89

THE IOANNINA CUP TEAM

Thank you
 Nicholas pavlidis
Nicholas pavlidis Michalis pavlidis
Michalis pavlidis Hot site cold site warm site disaster recovery
Hot site cold site warm site disaster recovery Magenta cyan and yellow are the ____ color. *
Magenta cyan and yellow are the ____ color. * Shell shick
Shell shick Nicholas possessive
Nicholas possessive How did nicholas novikov describe the united states
How did nicholas novikov describe the united states Nicholas nickleby cda
Nicholas nickleby cda Nicholas lemonias
Nicholas lemonias St angela school regina
St angela school regina Nicholas spykman rimland theory
Nicholas spykman rimland theory Evolution of the contemporary political pattern
Evolution of the contemporary political pattern Nicholas carlini
Nicholas carlini St james azurewebsites
St james azurewebsites Nicholas michalak
Nicholas michalak Nicholas bevins
Nicholas bevins Charles nicholas umbc
Charles nicholas umbc Domino theory
Domino theory Dr nicholas robinson
Dr nicholas robinson Nicholas barbaro
Nicholas barbaro Nicholas spykman rimland theory
Nicholas spykman rimland theory Dr nicholas gibbins
Dr nicholas gibbins Nicholas lemonias
Nicholas lemonias Nicholas seeliger, md flpen 32413
Nicholas seeliger, md flpen 32413 Carré magique de kaldor
Carré magique de kaldor Nicholas oddy
Nicholas oddy Nicholas belotti
Nicholas belotti Three golden balls
Three golden balls P-11212
P-11212 Dr nicholas tsourmas
Dr nicholas tsourmas Nicholas okon do
Nicholas okon do Ion george nicholas alexander lambrino
Ion george nicholas alexander lambrino Nicholas gordon poet
Nicholas gordon poet Nicholas gardner
Nicholas gardner Define czar nicholas ii
Define czar nicholas ii Giunc
Giunc Istituto nicholas green
Istituto nicholas green Nicholas bloom stanford
Nicholas bloom stanford Nicholas financial las vegas
Nicholas financial las vegas Nicholas schroder
Nicholas schroder Istituto comprensivo nicholas green ascoli satriano
Istituto comprensivo nicholas green ascoli satriano Nicholas ruozzi
Nicholas ruozzi Nicholas lemonias
Nicholas lemonias Russian czar family tree
Russian czar family tree Nicholas solomos md
Nicholas solomos md Anarchy is what states make of it
Anarchy is what states make of it Nicholas tunney
Nicholas tunney Nicholas tunney
Nicholas tunney Nicholas nethercote
Nicholas nethercote Nicholas carr
Nicholas carr George rowlands warwick
George rowlands warwick Ted nicholas
Ted nicholas Nicholas alden riggle
Nicholas alden riggle Nicholas bishop industry
Nicholas bishop industry Apa style of referencing
Apa style of referencing What is cohesion
What is cohesion Samuel nicholas
Samuel nicholas Nicholas carr
Nicholas carr Nicholas eastham
Nicholas eastham Nicholas jabbour
Nicholas jabbour Nicholas bartzen
Nicholas bartzen Cannabis
Cannabis Nicholas moeller
Nicholas moeller Joanne bretherton
Joanne bretherton Nicholas edward stroustrup
Nicholas edward stroustrup Dr nick gall
Dr nick gall Americanhoney93
Americanhoney93 Ivan bogeski
Ivan bogeski The unknown world worksheet answers
The unknown world worksheet answers Guessing meaning of unfamiliar words
Guessing meaning of unfamiliar words Separate result unknown
Separate result unknown This photo by unknown author is licensed under
This photo by unknown author is licensed under The unknown citizen literary devices pdf
The unknown citizen literary devices pdf Unknown self johari window
Unknown self johari window Separate result unknown
Separate result unknown What motivates people to explore
What motivates people to explore Lesson 10 unknown angle proofs
Lesson 10 unknown angle proofs Truncated sentences examples
Truncated sentences examples Separate result unknown
Separate result unknown Unknown words
Unknown words Stable prediction across unknown environments
Stable prediction across unknown environments Fear of the unknown theme
Fear of the unknown theme Unknown home employee ngt 06-2 (1)
Unknown home employee ngt 06-2 (1) Kode icd 10 basal cell carcinoma
Kode icd 10 basal cell carcinoma Agent a chapter 2
Agent a chapter 2 Unknown white male
Unknown white male Answers
Answers Unknown vice lord
Unknown vice lord Omniscient agent
Omniscient agent An unknown gas composed of homonuclear diatomic molecules
An unknown gas composed of homonuclear diatomic molecules This photo by unknown author is licensed under cc by-nc-nd.
This photo by unknown author is licensed under cc by-nc-nd. This photo by unknown author is licensed under cc by-nc-nd
This photo by unknown author is licensed under cc by-nc-nd