An Overview of Mouse Genetics Reference Material t














- Slides: 76

An Overview of Mouse Genetics

Reference Material t Mouse Genetics, Concepts and Applications. 1995. Silver, L. M. Oxford University Press, New York, NY, 1995, pp 1 -362. t Genetic Variants and Strains of the Laboratory Mouse, 3 rd Edition. 1996. Edited by M. F. Lyon, S. Rastan and S. D. M. Brown. Oxford University Press, New York, NY, 2 Volumes, pp. 1 -1807.

Reference Material (Out of Print; Often in Libraries) t The Mouse in Biomedical Research. 1981. Edited by H. L. Foster, J. D. Small and J. G. Fox. Academic Press, New York, NY. 4 Volumes. t Inbred Strains in Biomedical Research. 1979. Festing, M. F. W. Oxford University Press, New York, NY. t Origins of Inbred Mice. 1978. Morse III, H. C. Academic Press, New York, NY.

Genetics I: From Fancy Mice to Mendel t Brief History of the Laboratory Mouse t Details and Definitions t Genetic Resources t Gene Mapping t Annotating the Mouse Genome

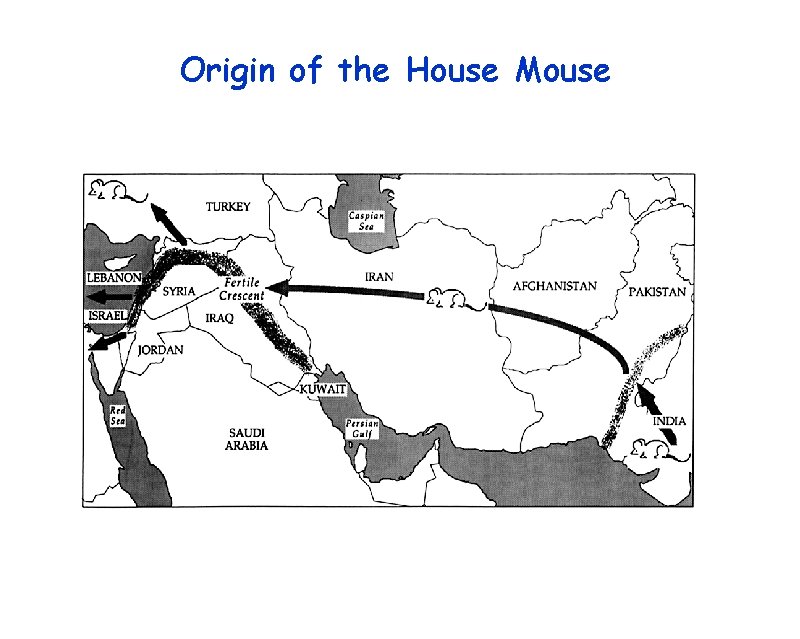
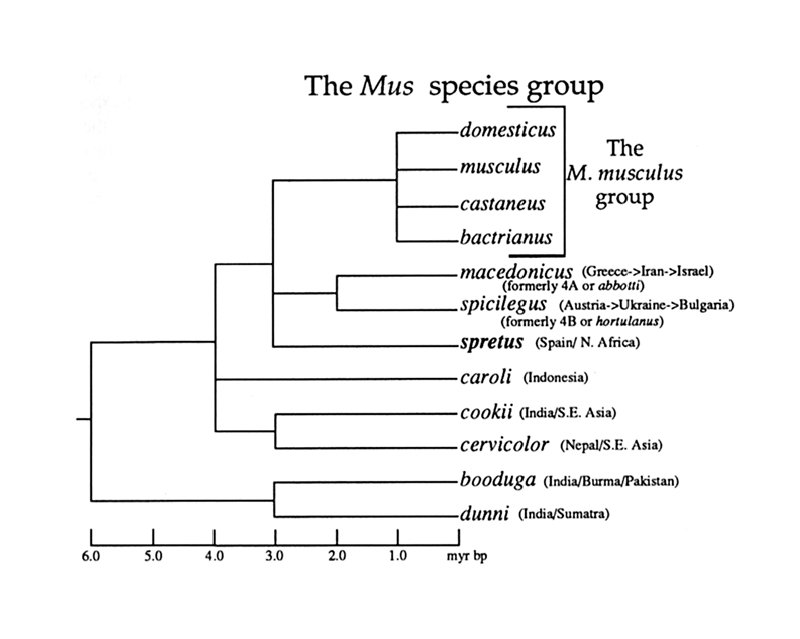
Origin of the House Mouse

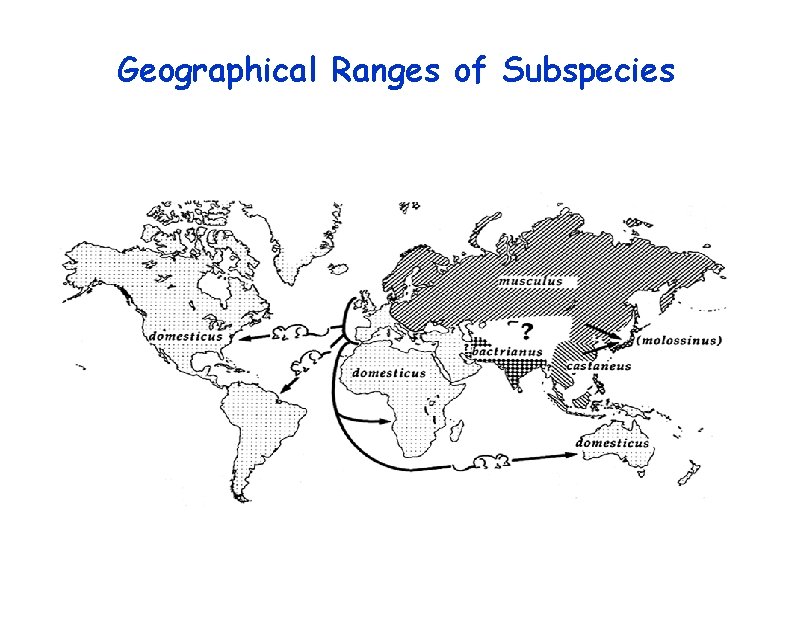
Geographical Ranges of Subspecies


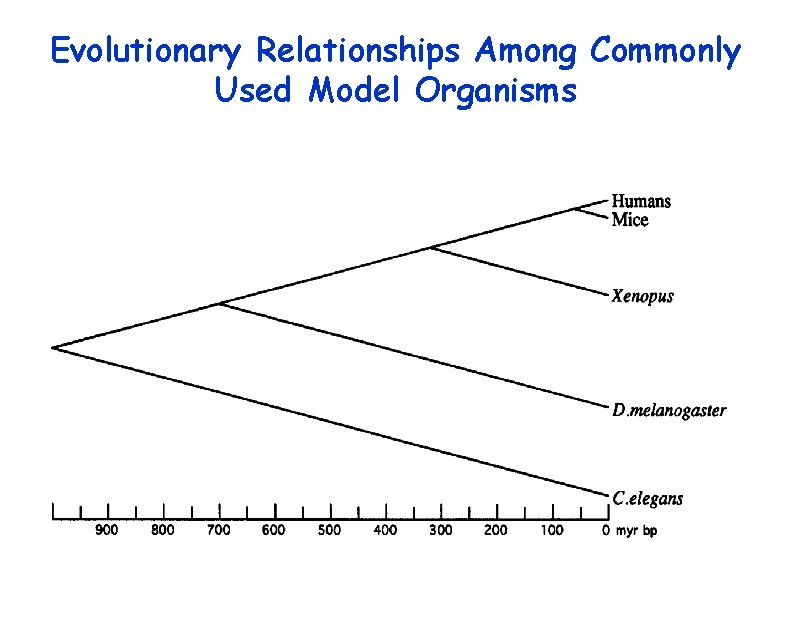
Evolutionary Relationships Among Commonly Used Model Organisms

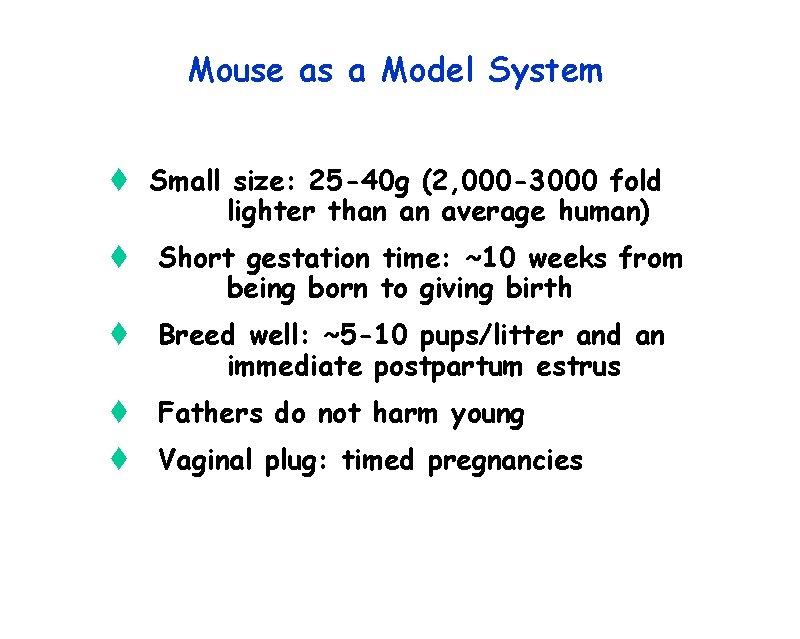
Mouse as a Model System t Small size: 25 -40 g (2, 000 -3000 fold lighter than an average human) t Short gestation time: ~10 weeks from being born to giving birth t Breed well: ~5 -10 pups/litter and an immediate postpartum estrus t Fathers do not harm young t Vaginal plug: timed pregnancies

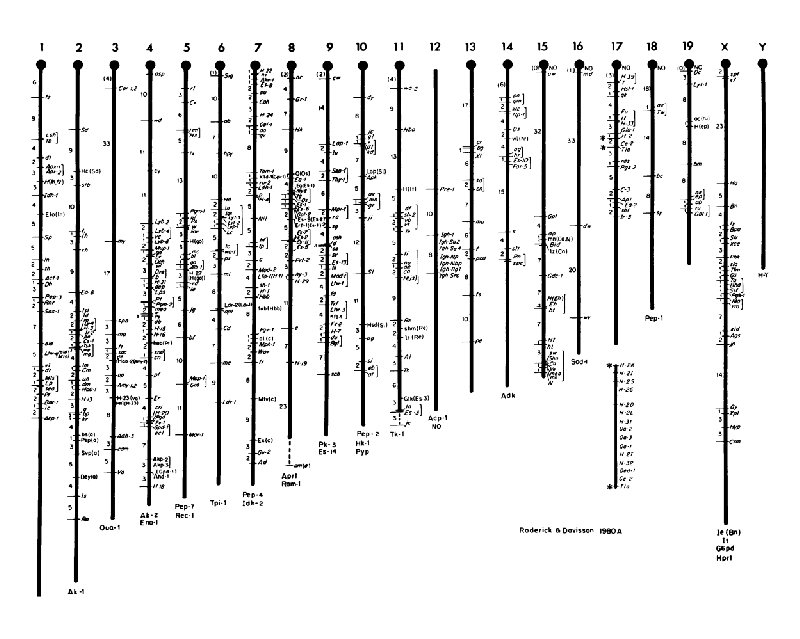
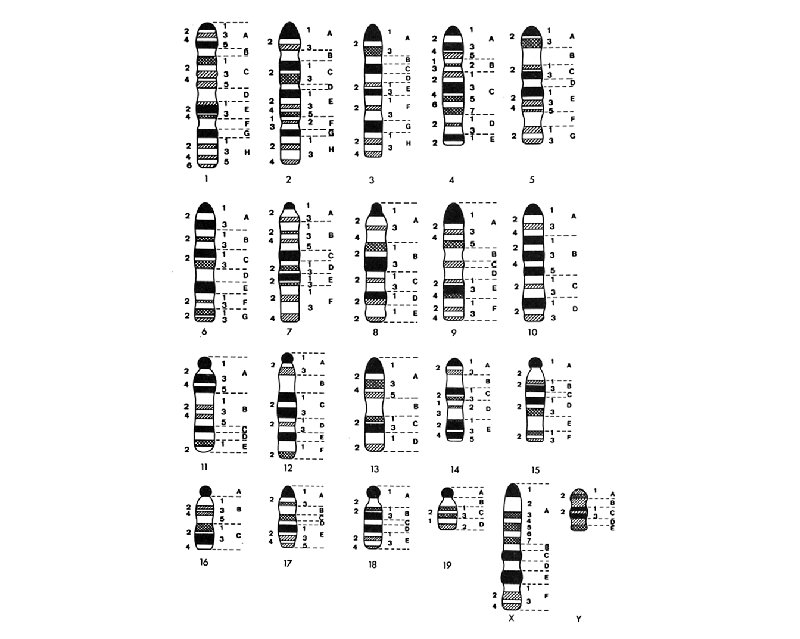
Mouse Genome t 3 x 109 base pairs t ~1450 c. M t ~2 Mb/c. M t ~25, 000 genes t Organized into 19 autosomes and the X and Y Chromosome t Chromosomes are acrocentric and show a continual decrease in size


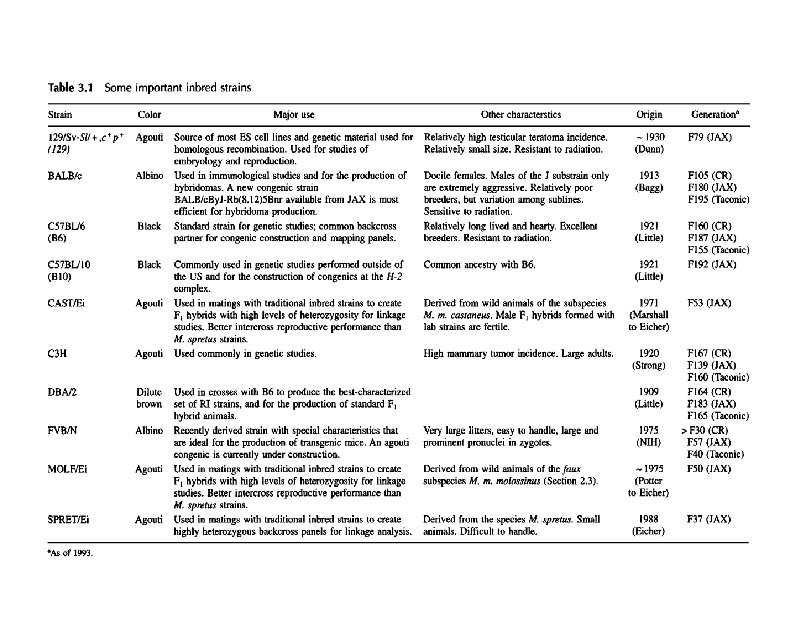
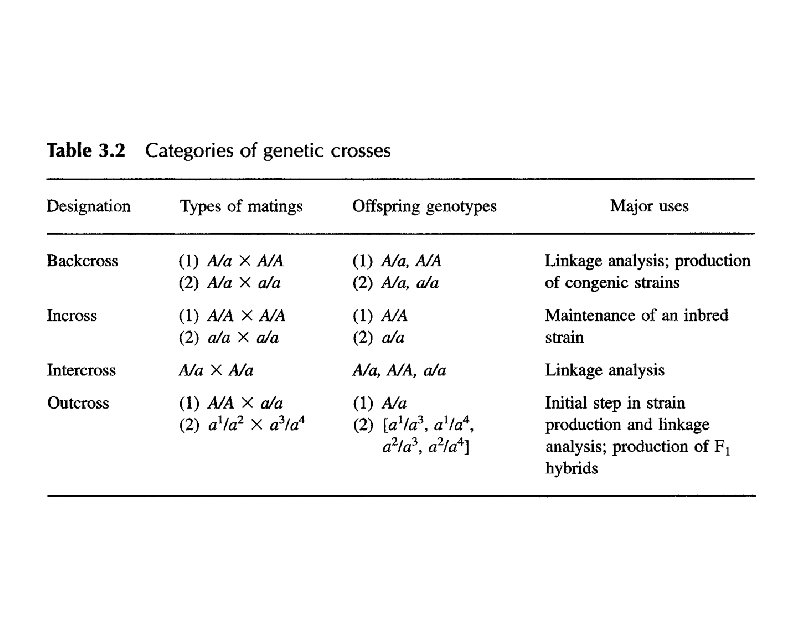
Genetic Resources t Outbred Stocks t Inbred Strains and Substrains t F 1 Hybrids t Mutant Strains

Outbred Stocks t Genetically undefined t Bred to maintain a maximal level of heterozygosity in all animals t Long life spans, high disease resistance, large and frequent litters, low neonatal mortality, rapid growth, large size, CHEAP t Examples: CD 1, Swiss Webster, ICR, NIH t Useful in experiments where genotype does NOT matter (biochemical purification, stud males) t Should NEVER be used in any breeding experiment because of the genetic uncertainty

Inbred Strains t All members of a strain are genetically identical t Bred by strict brother X sister matings for at least 20 generations (Note: ~3 -4 generations/year; at least 5 years)


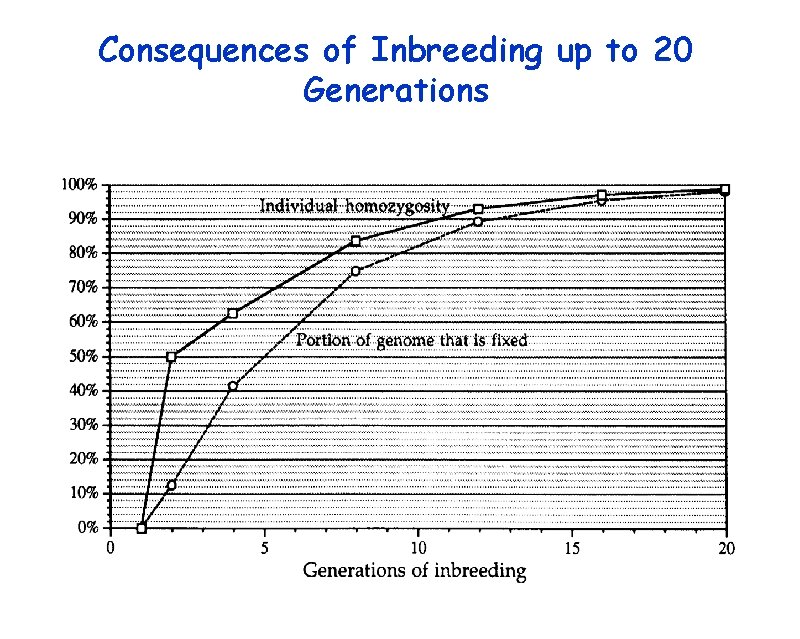
Consequences of Inbreeding up to 20 Generations

Beck J. A. Nat. Genet. 2000; 21: 23 -25




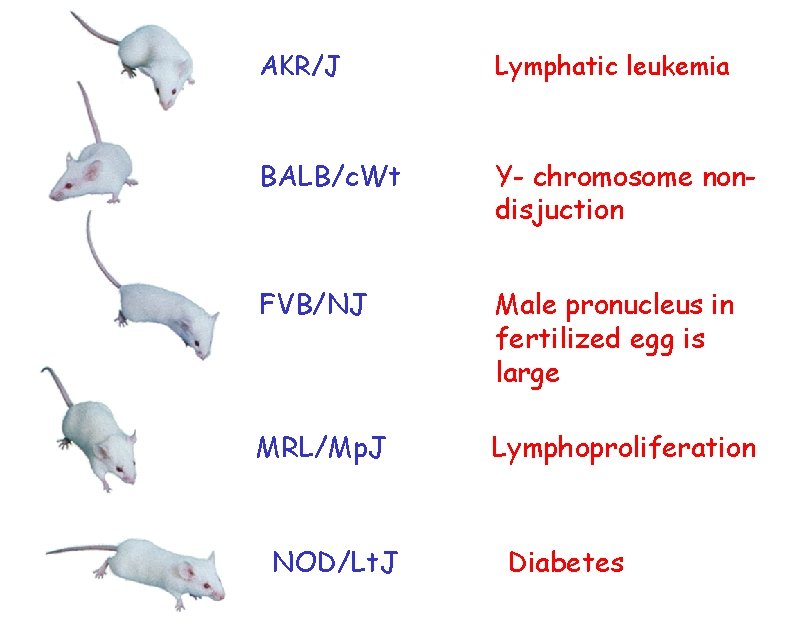
FVB/NJ AKR/J NOD/Lt. J BALB/c. Wt MRL/Mp. J

AKR/J Lymphatic leukemia BALB/c. Wt Y- chromosome nondisjuction FVB/NJ Male pronucleus in fertilized egg is large MRL/Mp. J Lymphoproliferation NOD/Lt. J Diabetes

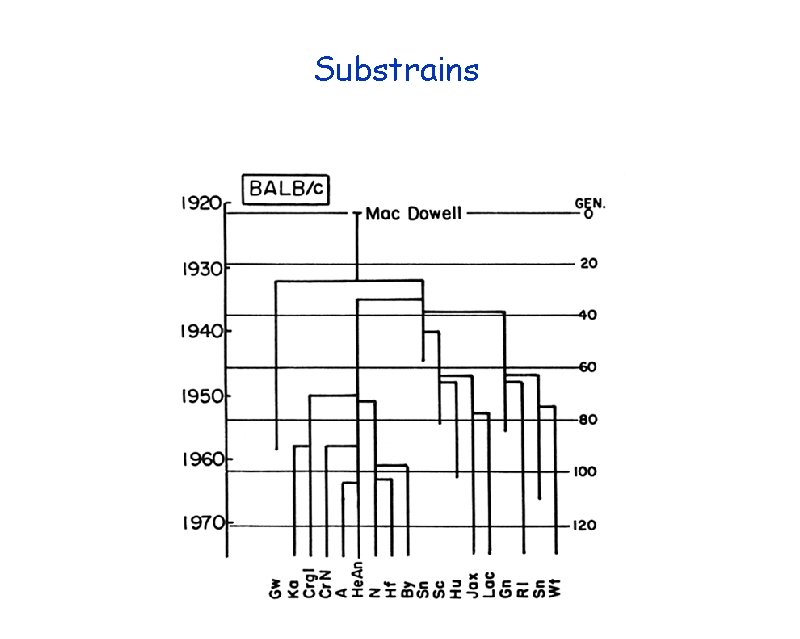
Substrains

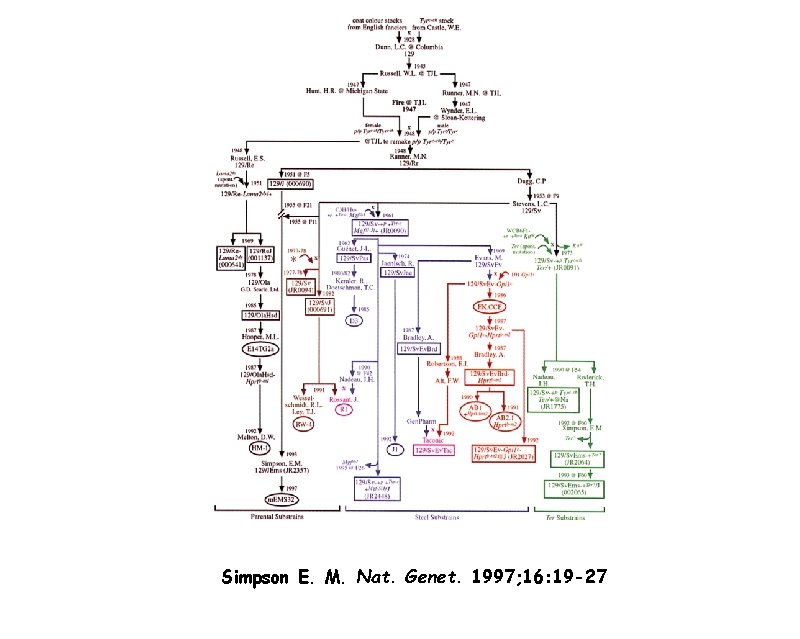
Simpson E. M. Nat. Genet. 1997; 16: 19 -27

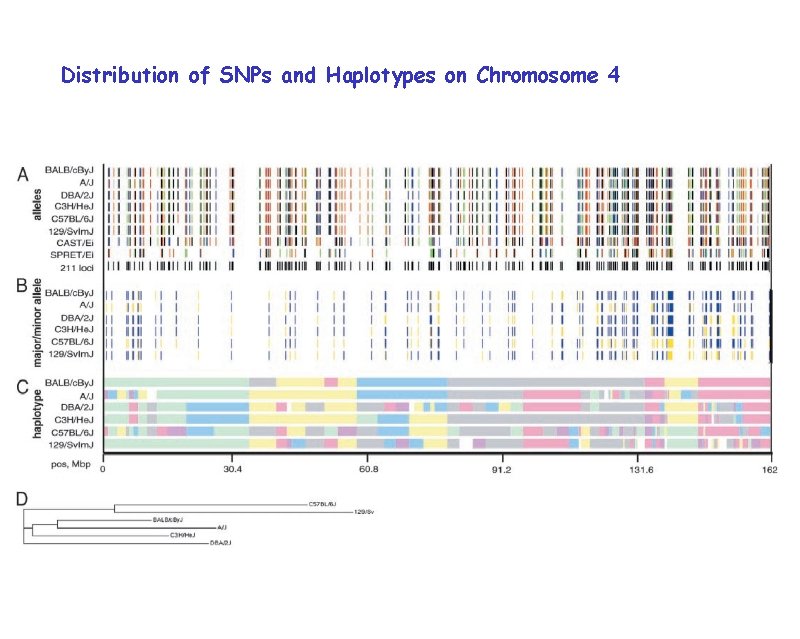
Distribution of SNPs and Haplotypes on Chromosome 4

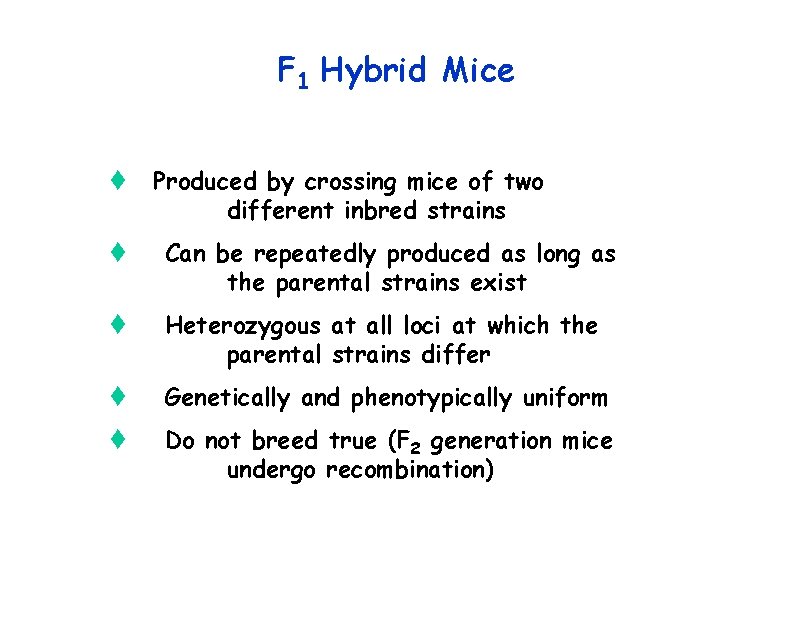
F 1 Hybrid Mice t Produced by crossing mice of two different inbred strains t Can be repeatedly produced as long as the parental strains exist t Heterozygous at all loci at which the parental strains differ t Genetically and phenotypically uniform t Do not breed true (F 2 generation mice undergo recombination)

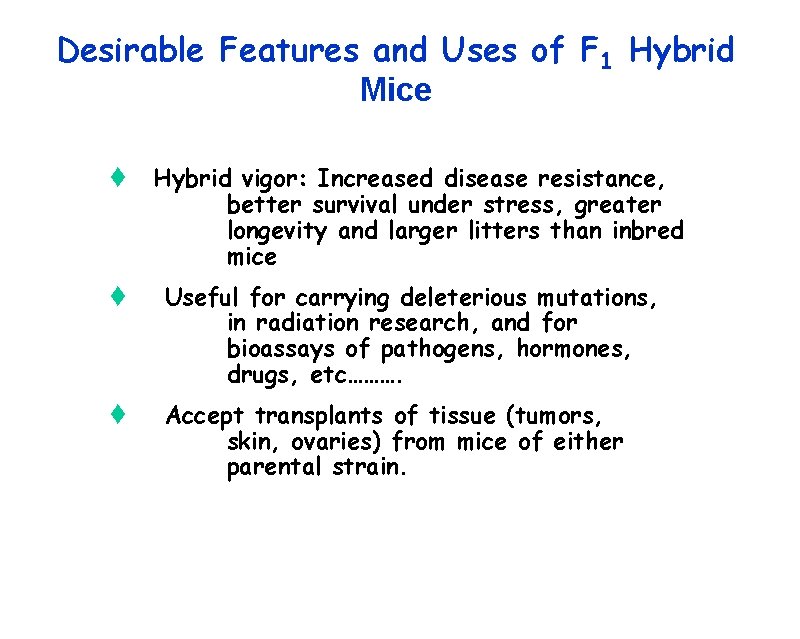
Desirable Features and Uses of F 1 Hybrid Mice t Hybrid vigor: Increased disease resistance, better survival under stress, greater longevity and larger litters than inbred mice t Useful for carrying deleterious mutations, in radiation research, and for bioassays of pathogens, hormones, drugs, etc………. t Accept transplants of tissue (tumors, skin, ovaries) from mice of either parental strain.

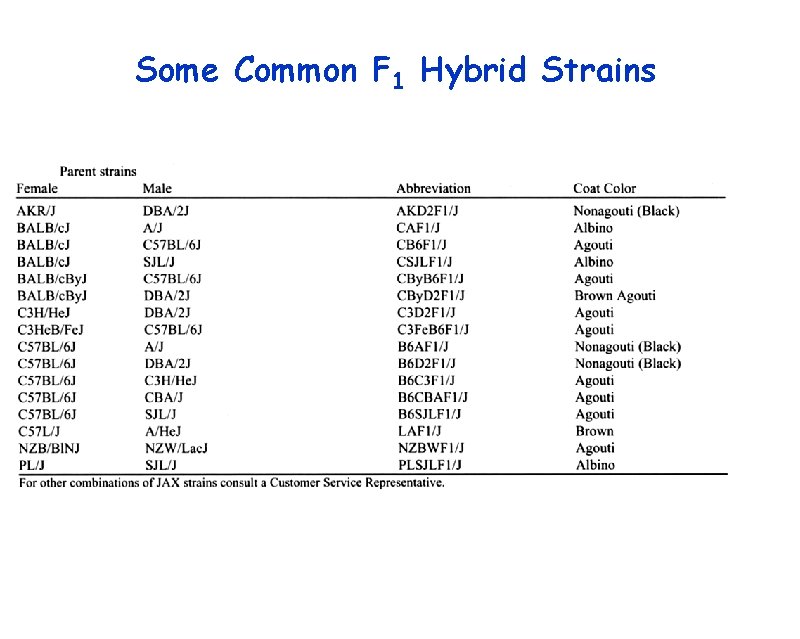
Some Common F 1 Hybrid Strains


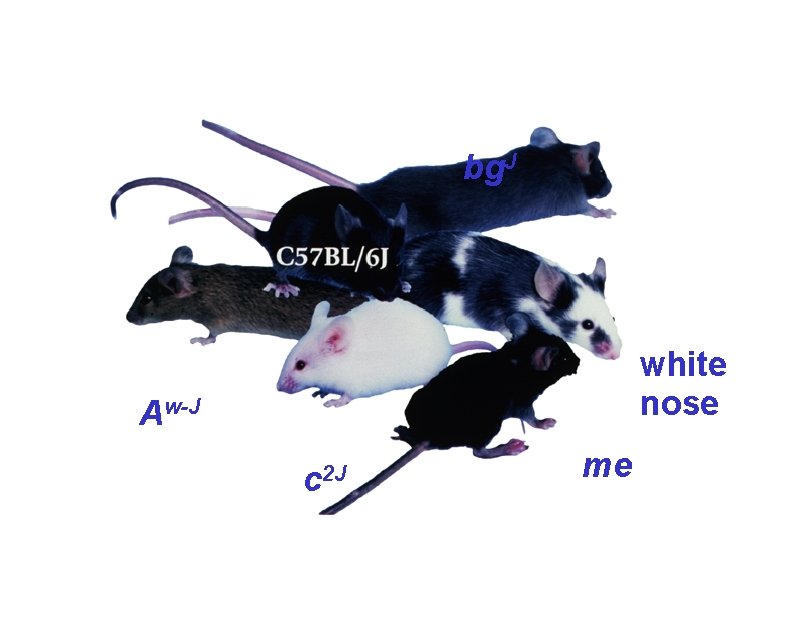
bg. J white nose Aw-J c 2 J me

“A Mutation” t What is it? : A variation from wild-type at a single locus t Mode of origin: Spontaneous or induced (Chemicals, Radiation, Gene Modification) t Mode of inheritance: dominant, semidominant, recessive (or not heritable) t Penetrance: complete or incomplete t Interaction with other mutations

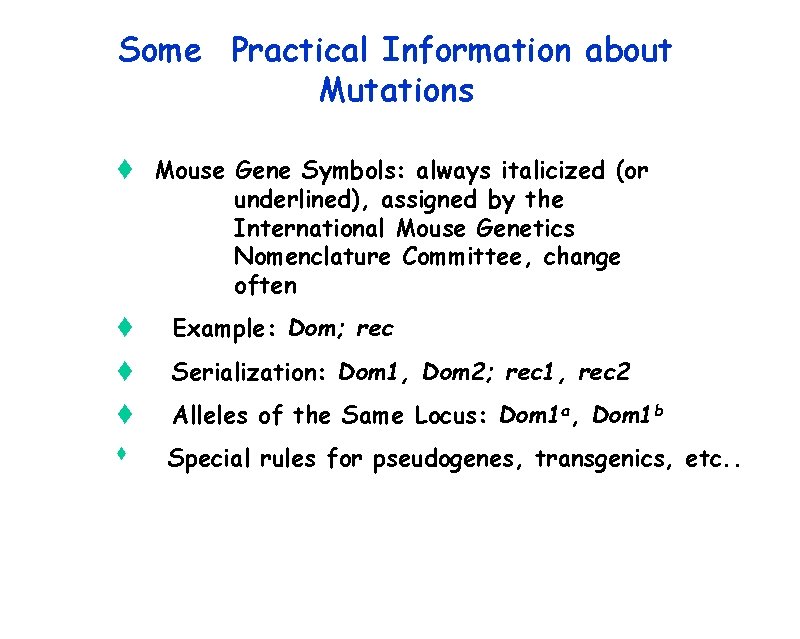
Some Practical Information about Mutations t Mouse Gene Symbols: always italicized (or underlined), assigned by the International Mouse Genetics Nomenclature Committee, change often t Example: Dom; rec t Serialization: Dom 1, Dom 2; rec 1, rec 2 t Alleles of the Same Locus: Dom 1 a, Dom 1 b t Special rules for pseudogenes, transgenics, etc. .

A Wealth of Information at…. t The Jackson Laboratory Home Page: http: //www. jax. org

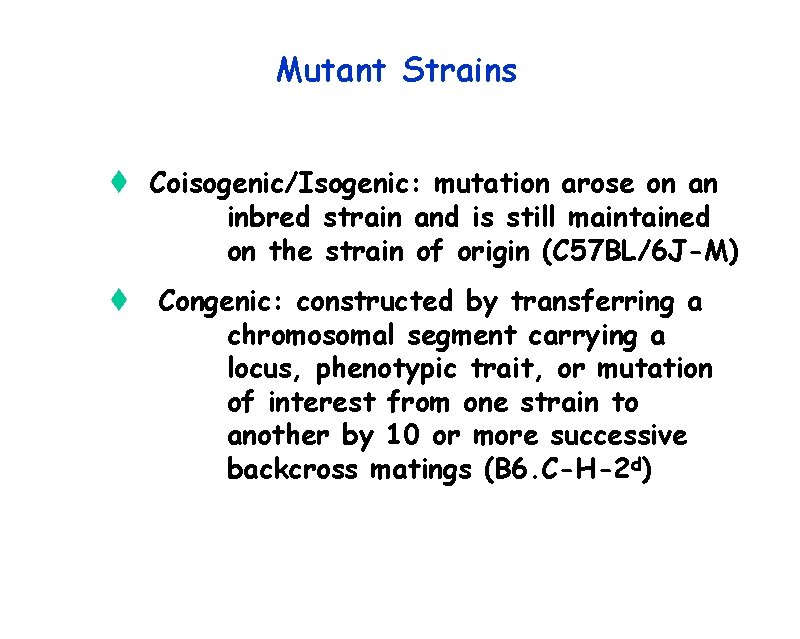
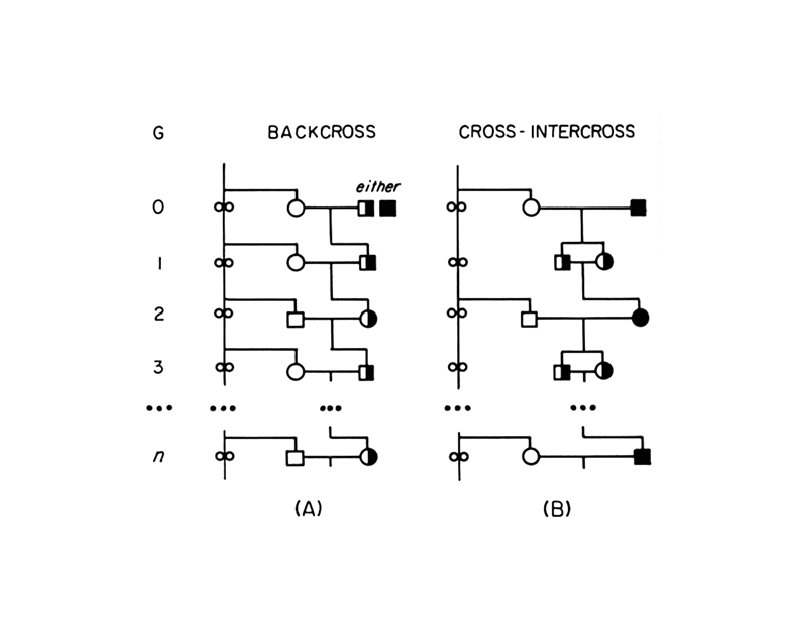
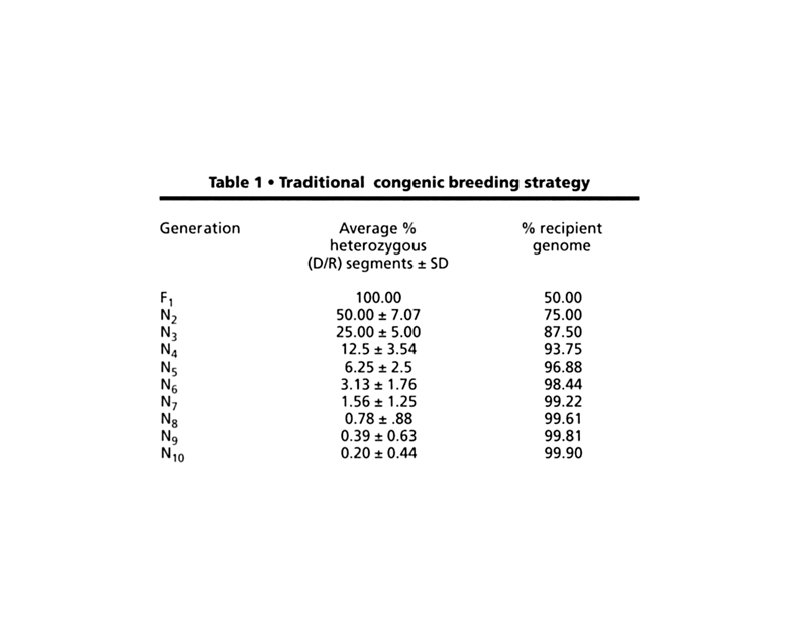
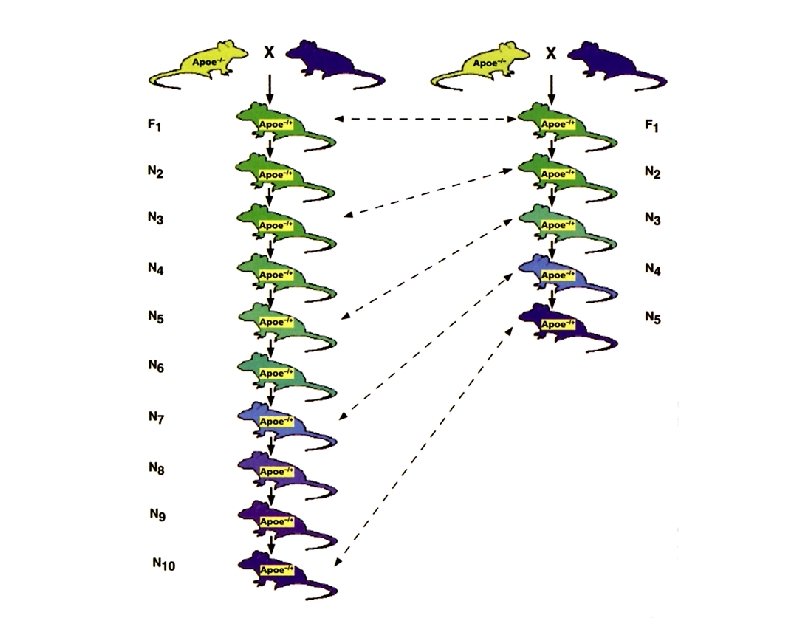
Mutant Strains t Coisogenic/Isogenic: mutation arose on an inbred strain and is still maintained on the strain of origin (C 57 BL/6 J-M) t Congenic: constructed by transferring a chromosomal segment carrying a locus, phenotypic trait, or mutation of interest from one strain to another by 10 or more successive backcross matings (B 6. C-H-2 d)



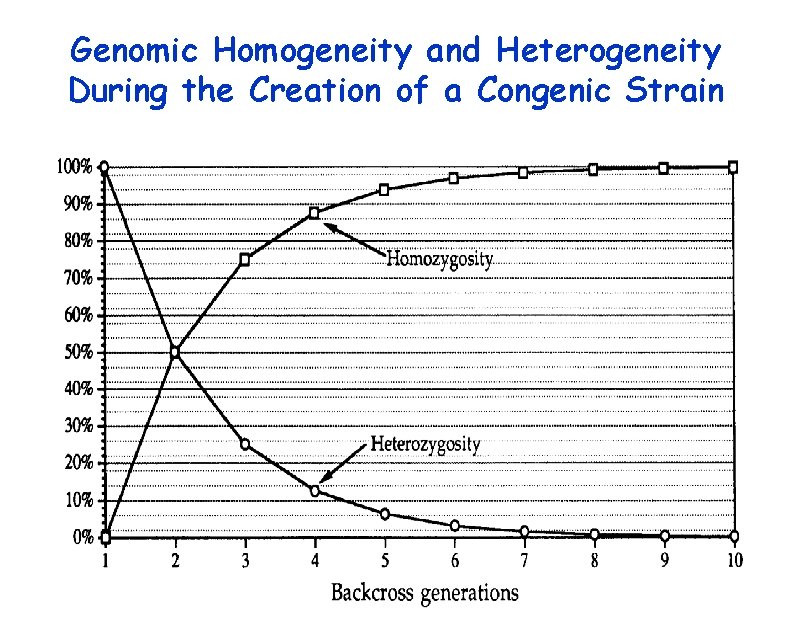
Genomic Homogeneity and Heterogeneity During the Creation of a Congenic Strain

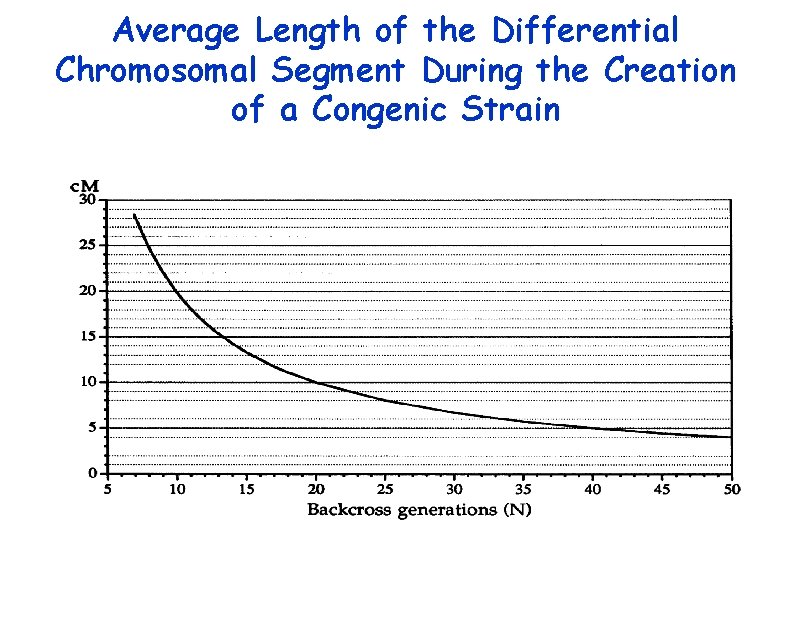
Average Length of the Differential Chromosomal Segment During the Creation of a Congenic Strain

Why Make Congenic Strains? t Evaluate phenotype of a mutation (single gene trait) on one or more well defined genetic backgrounds t Can be very useful in the analysis of complex traits

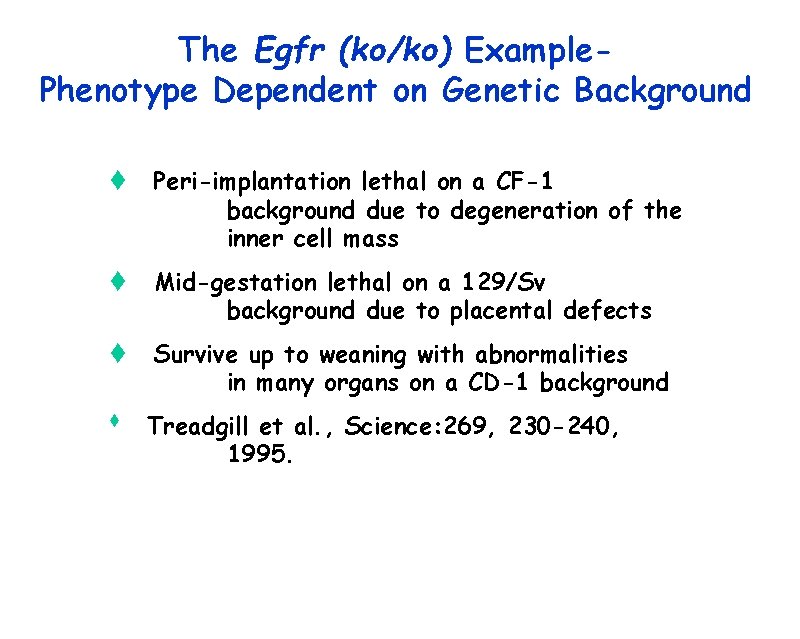
The Egfr (ko/ko) Example. Phenotype Dependent on Genetic Background t Peri-implantation lethal on a CF-1 background due to degeneration of the inner cell mass t Mid-gestation lethal on a 129/Sv background due to placental defects t Survive up to weaning with abnormalities in many organs on a CD-1 background t Treadgill et al. , Science: 269, 230 -240, 1995.



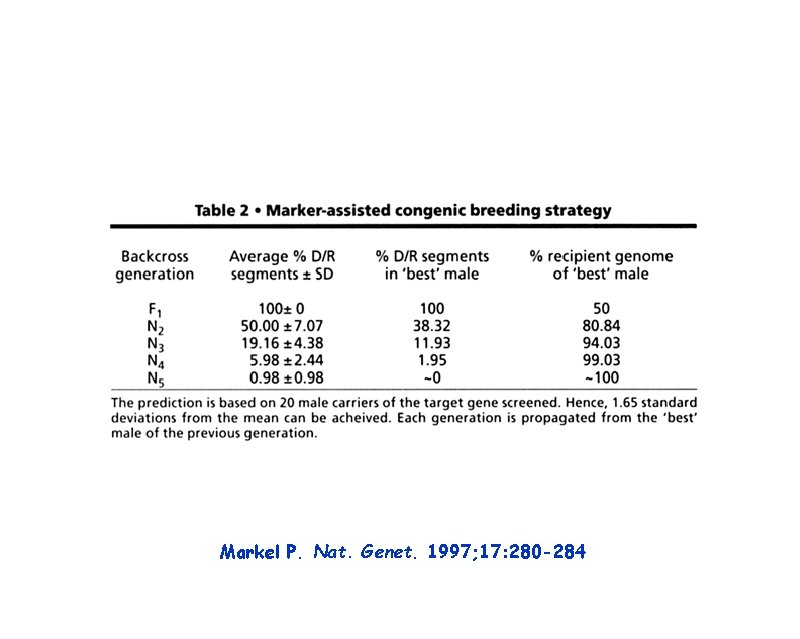
Markel P. Nat. Genet. 1997; 17: 280 -284

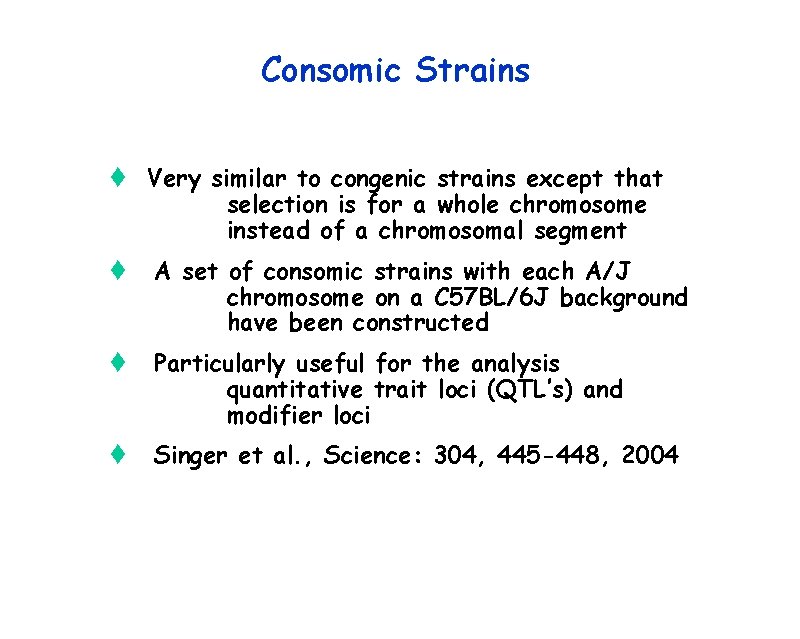
Consomic Strains t Very similar to congenic strains except that selection is for a whole chromosome instead of a chromosomal segment t A set of consomic strains with each A/J chromosome on a C 57 BL/6 J background have been constructed t Particularly useful for the analysis quantitative trait loci (QTL’s) and modifier loci t Singer et al. , Science: 304, 445 -448, 2004

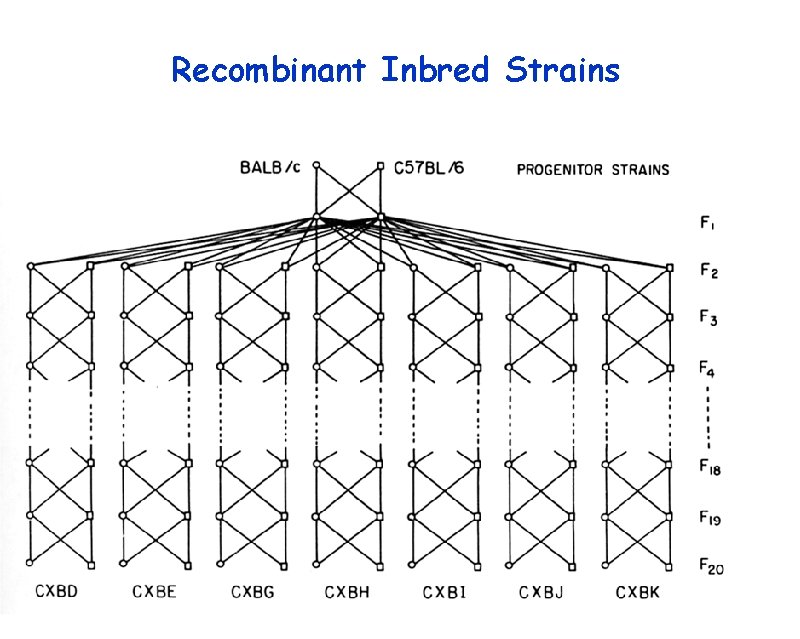
Recombinant Inbred Strains

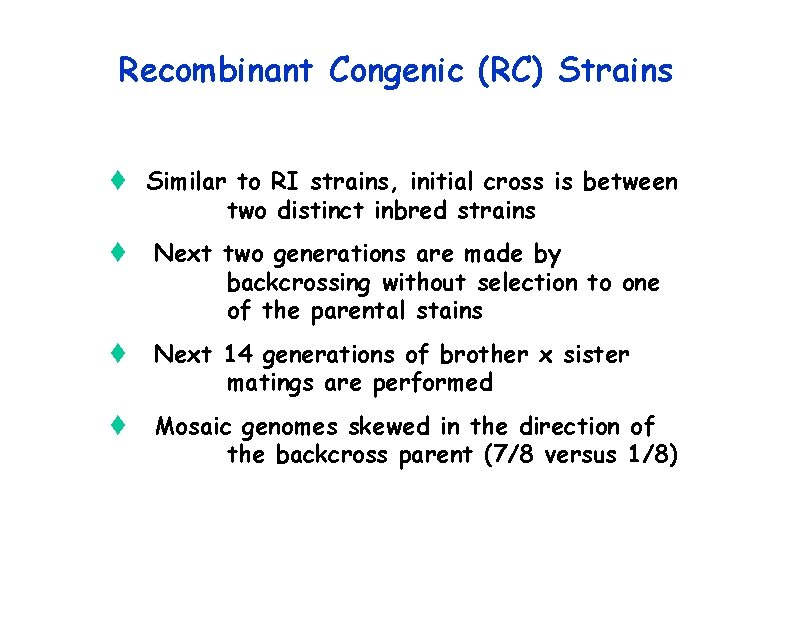
Recombinant Congenic (RC) Strains t Similar to RI strains, initial cross is between two distinct inbred strains t Next two generations are made by backcrossing without selection to one of the parental stains t Next 14 generations of brother x sister matings are performed t Mosaic genomes skewed in the direction of the backcross parent (7/8 versus 1/8)

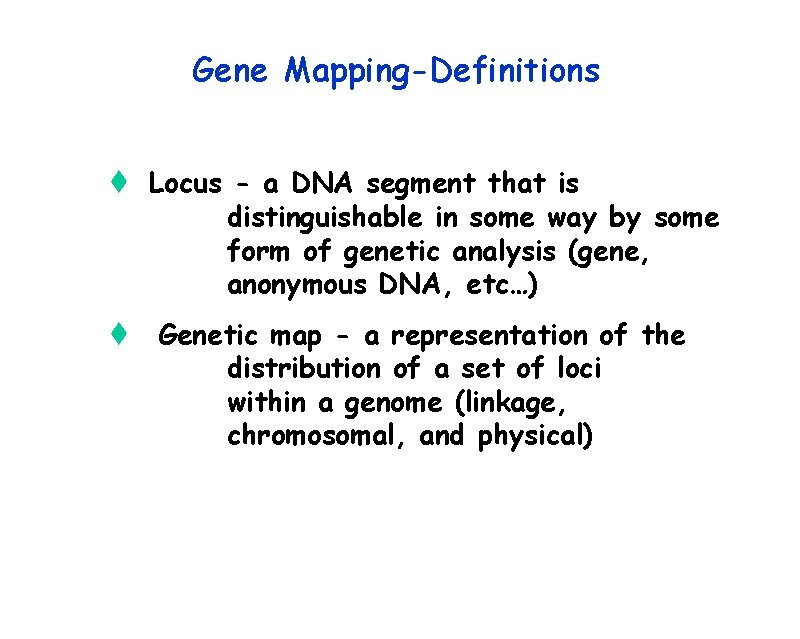
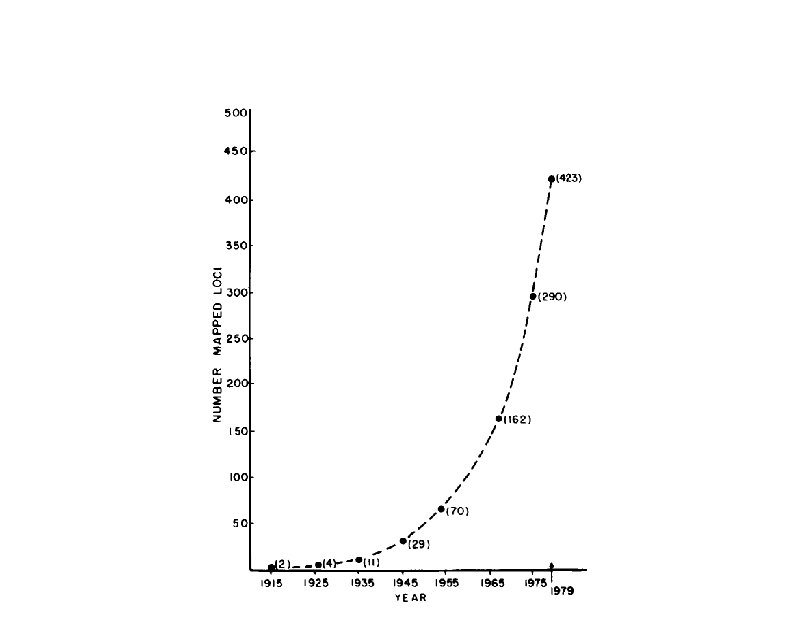
Gene Mapping-Definitions t Locus - a DNA segment that is distinguishable in some way by some form of genetic analysis (gene, anonymous DNA, etc…) t Genetic map - a representation of the distribution of a set of loci within a genome (linkage, chromosomal, and physical)

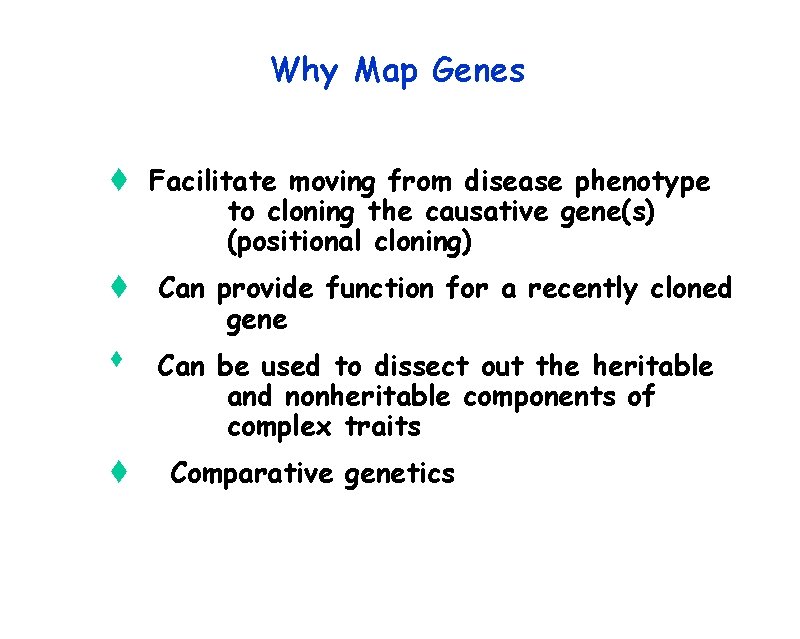
Why Map Genes t Facilitate moving from disease phenotype to cloning the causative gene(s) (positional cloning) t Can provide function for a recently cloned gene t t Can be used to dissect out the heritable and nonheritable components of complex traits Comparative genetics

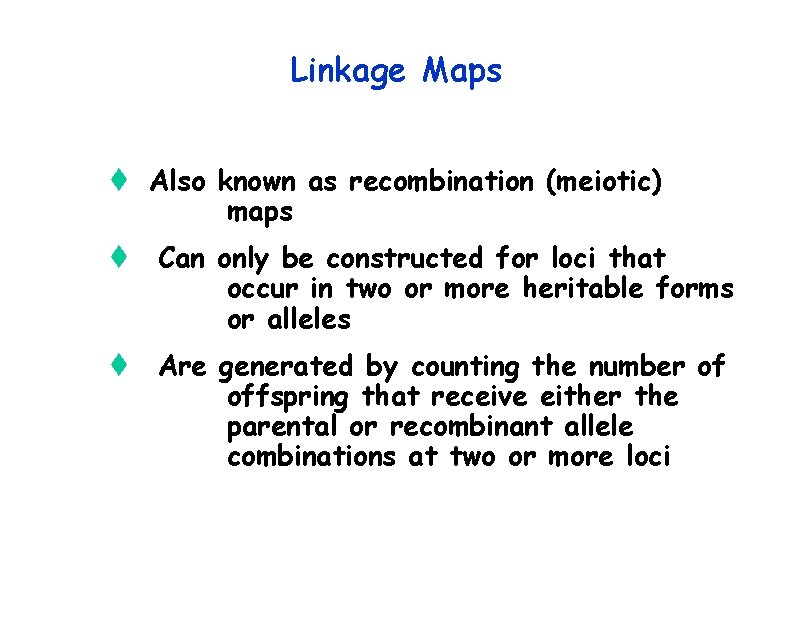
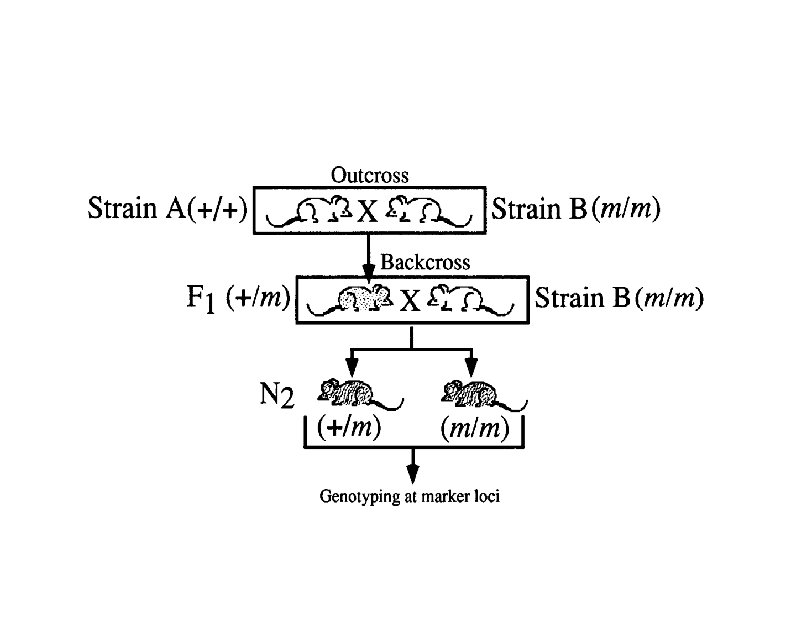
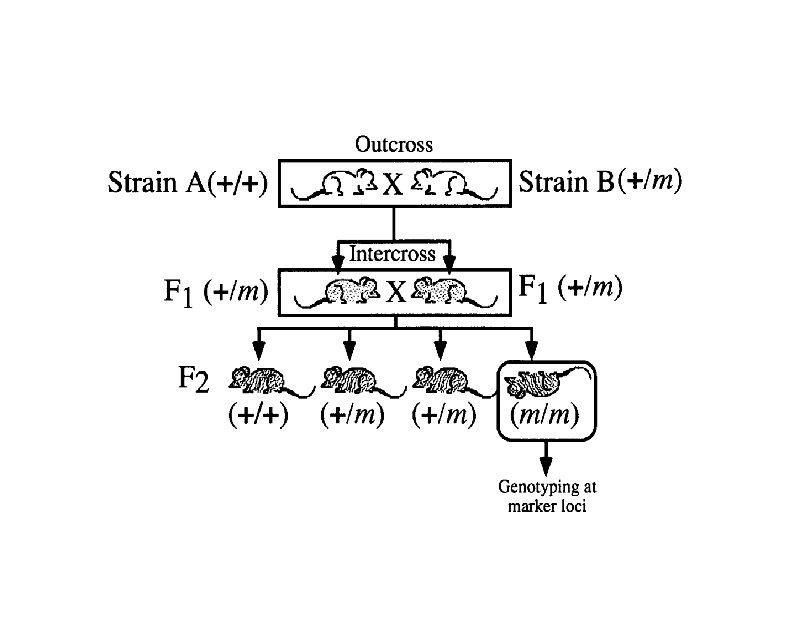
Linkage Maps t Also known as recombination (meiotic) maps t Can only be constructed for loci that occur in two or more heritable forms or alleles t Are generated by counting the number of offspring that receive either the parental or recombinant allele combinations at two or more loci

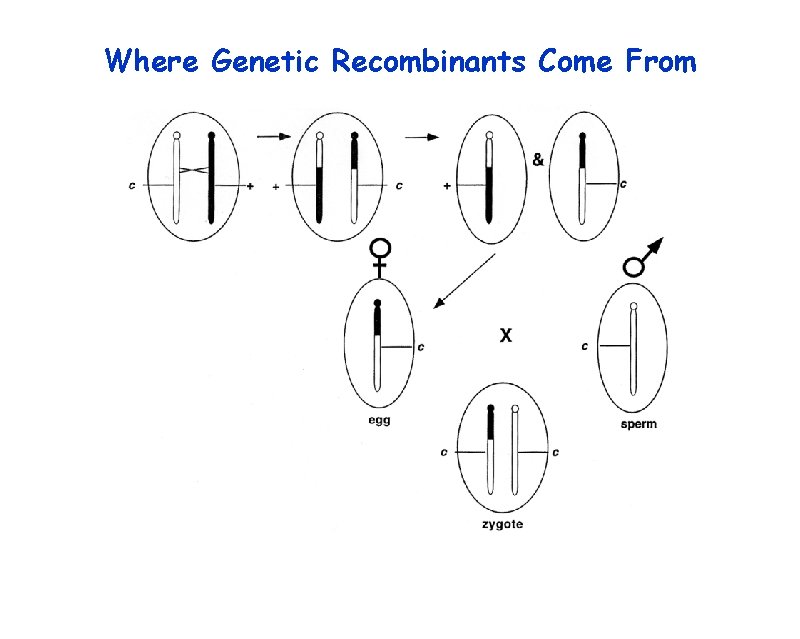
Where Genetic Recombinants Come From

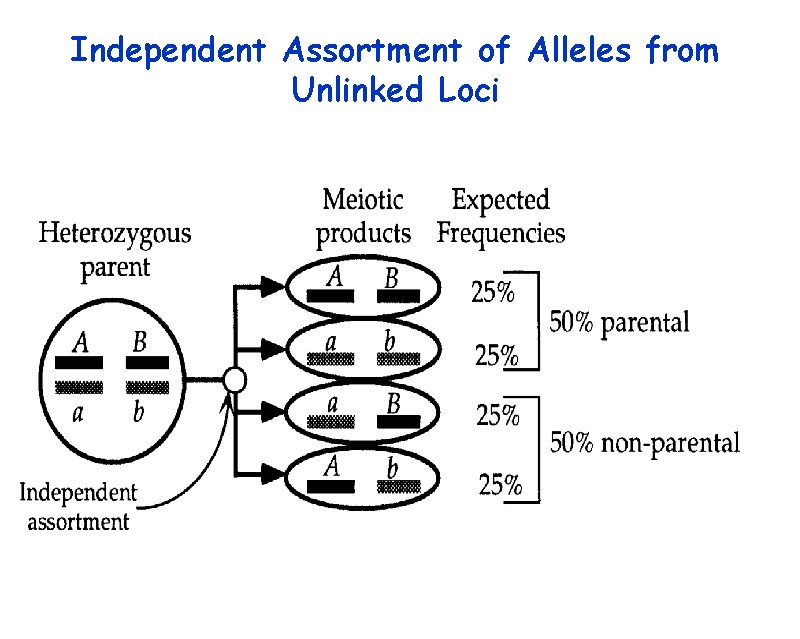
Independent Assortment of Alleles from Unlinked Loci

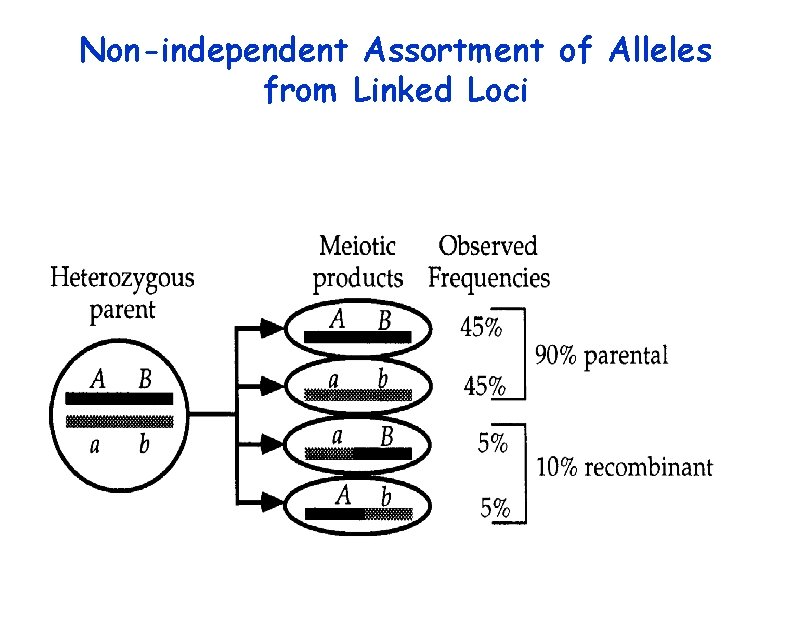
Non-independent Assortment of Alleles from Linked Loci

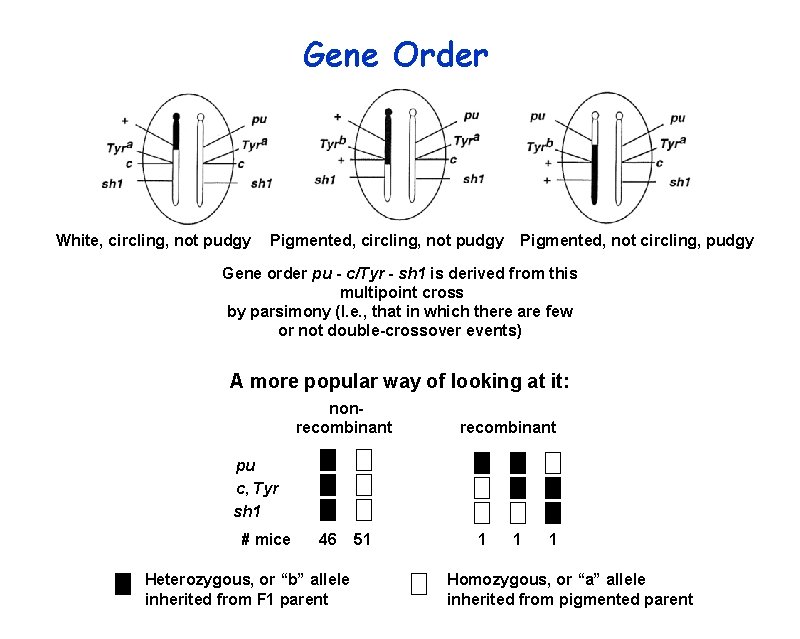
Gene Order White, circling, not pudgy Pigmented, not circling, pudgy Gene order pu - c/Tyr - sh 1 is derived from this multipoint cross by parsimony (I. e. , that in which there are few or not double-crossover events) A more popular way of looking at it: nonrecombinant pu c, Tyr sh 1 # mice 46 Heterozygous, or “b” allele inherited from F 1 parent 51 1 Homozygous, or “a” allele inherited from pigmented parent




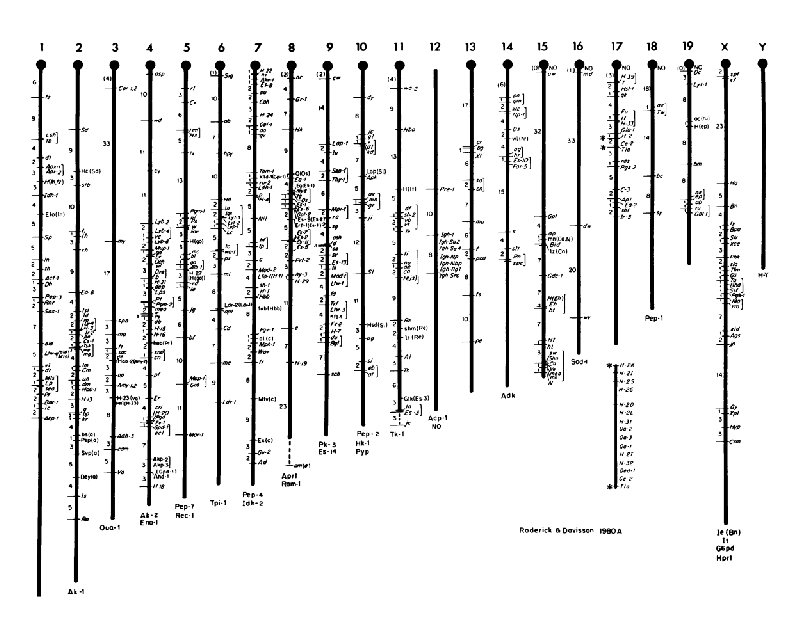
Live Linkage Map of the Mouse 10 th International Congress of Genetics Montreal, Canada. 1958.


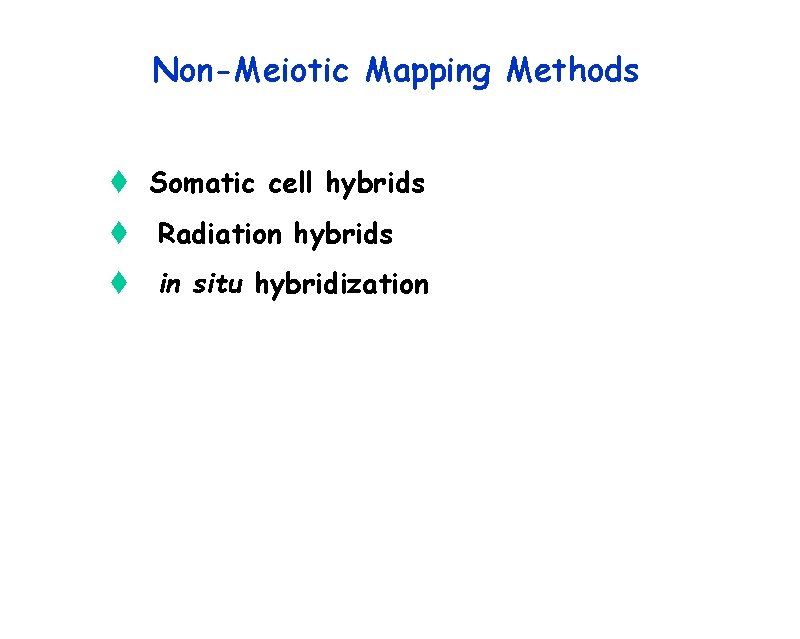
Non-Meiotic Mapping Methods t Somatic cell hybrids t Radiation hybrids t in situ hybridization



The Ultimate Physical Map t The sequence of the mouse genome t Public draft sequence (~96% of the euchromatic genome of C 57 BL/6 J excluding the Y chromosome) released in December 2002 t Sanger Center is completing the sequence of chromosomes 2, 4, 11, and X t Page lab has sequenced part of the mouse Y t The rest?

Functional Annotation of the Mouse Genome t Need: One or more independent mutations in every gene t Approach: Genotype to phenotype t Approach: Phenotype to Genotype

Genotype to Phenotype t Targeted mutations in each gene t Gene trap mutagenesis t Chemical mutagenesis of ES cells t Ultimately, must determine the phenotypic affect of the mutation, in vivo

Targeted mutations t Homologous recombination in ES cells t Advantages: can create exactly what you want; conditional or null allele, temporal regulation, point mutations, small deletions, etc. ; tend to know a lot about the gene which can help with interpreting phenotype t Disadvantages: time-consuming and very expensive if all ~25, 000 genes are to be mutated; must make assumptions about important functional domains

Targeted mutations the future? t Development of simpler/faster methods for construct building-”recombineering” and high throughput analysis t The Sanger Plan--generate 10, 000 - 15, 000 targeted mutations (probably conditional, not null) in a five year period; A FEW THOUSAND IN THE GERMLINE t Part of a larger European effort that will include a collection of null alleles, total exact numbers are unclear t US effort--discussion stage

Gene Trap Mutagenesis t A form of insertional mutagenesis in ES cells t Various vectors have been developed but all are designed to report the expression of the endogenous gene at the site of integration and provide a DNA tag for rapid identification of the disrupted gene t Lexicon: 60% coverage of the mouse genome from 200, 000 sequence tags t International Gene Trap Consortium (IGTC): 32% coverage in 27, 000 tags (variety of vectors may overcome insertion site preference); 20% do not overlap with Lexicon

Gene Trap Mutagenesis t Advantages: IGTC clones are available without restriction (see Nature Genet. 36, 543545, 2004); high throughput (1 new gene added every 35 tags); combination of vectors helps overcome integration site preference; like targeted mutations, insertions can be archived as ES cells, which is inexpensive t Disadvantages: Unclear what you have: null, hypomorphic, or neomorphic allele; most of the existing insertions have not been examined for phenotype, in vivo

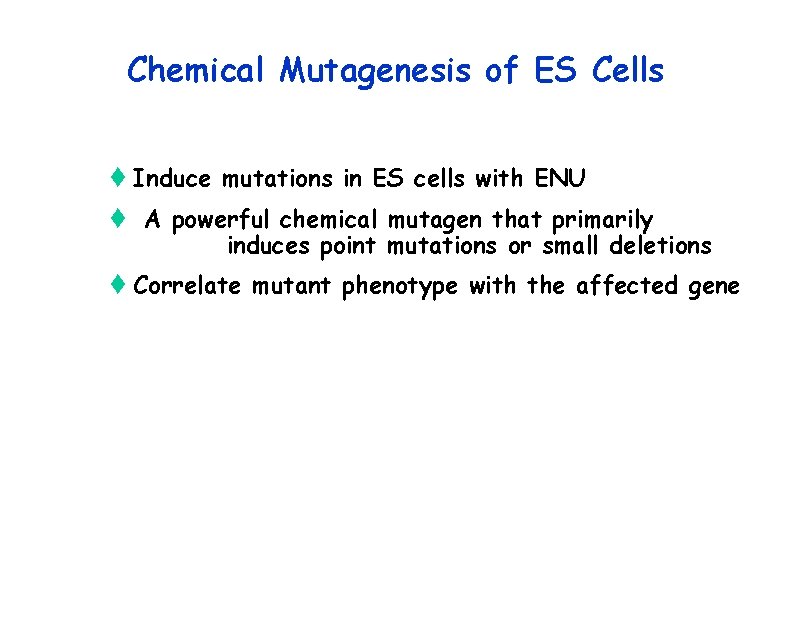
Gene Trap Mutagenesis the Future? t IGTC plans to additional tags ( until ~ saturation)) to expand the pool of tagged genes freely available t IGTC plans on additional refinement of the technology (new vectors, post-insertional modification of trapped loci to create additional/desired alleles of a tagged gene) t Likely will be up to individual investigators to put the mutations in the germ line and phenotype mice t Toronto Component of the IGTC has done phenotyping on some of the animals

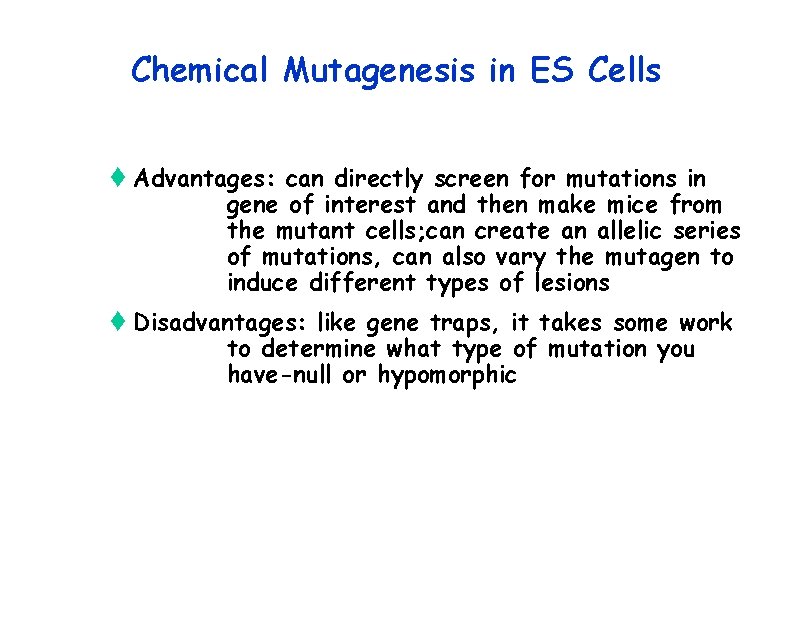
Chemical Mutagenesis of ES Cells t Induce mutations in ES cells with ENU t A powerful chemical mutagen that primarily induces point mutations or small deletions t Correlate mutant phenotype with the affected gene

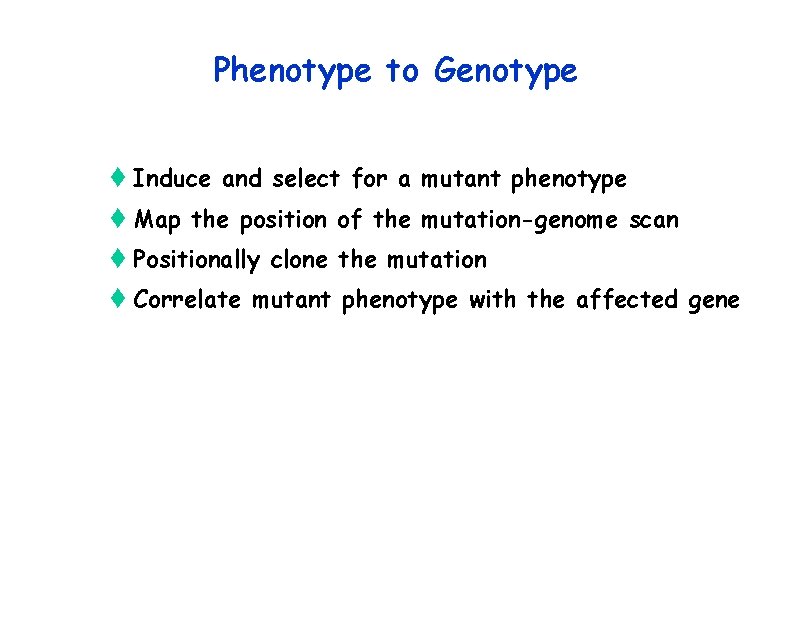
Chemical Mutagenesis in ES Cells t Advantages: can directly screen for mutations in gene of interest and then make mice from the mutant cells; can create an allelic series of mutations, can also vary the mutagen to induce different types of lesions t Disadvantages: like gene traps, it takes some work to determine what type of mutation you have-null or hypomorphic

Phenotype to Genotype t Induce and select for a mutant phenotype t Map the position of the mutation-genome scan t Positionally clone the mutation t Correlate mutant phenotype with the affected gene

ENU Mutagenesis, in vivo t A powerful chemical mutagen of spermatogonial stem cells t Primarily induces point mutations or small deletions t Can select for dominant, semidominant or recessive mutations; mutations can be gain or partial or complete loss of function t One mutation/locus in ~600 - 1000 gametes

Enu Mutagenesis Projects t International effort-large scale, genome wide t Centers in England, Germany, Japan, Toronto (http: //www. mut. har. mrc. ac. uk) t Jax, Northwestern, Oak Ridge, Baylor and others t A few smaller genome wide efforts, as well-Sloan Kettering

ENU Mutagenesis t Advantages-always have a phenotype, can select for particular organ system/stage of development/tissue type; can get hypomorphic alleles (new alleles of existing mutations) t Disadvantages-time consuming especially for recessive screens; works best with robust phenotypes; must go through a round of mapping but positional cloning is greatly simplified with the sequence of the genome; archiving and distributing the mutation is much more work than with ES cells

Issues for both Approaches t Phenotyping t Databases t Distribution