Alfred Hershey and Martha Chase Independent Functions of












































- Slides: 87

Alfred Hershey and Martha Chase: Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage Chelsea Bishop Emily Bonnell Leanne Dawe Stephanie Mayne Emily Porter

Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage ● Background ● Experiments Conducted ● 8 in total ● Discussion & Summary ● How Paper Impacted Future

ALFRED DAY HERSHEY

ALFRED DAY HERSHEY

ALFRED DAY HERSHEY

MARTHA CHASE

MARTHA CHASE

MARTHA CHASE

NOW FOR THE PAPER…

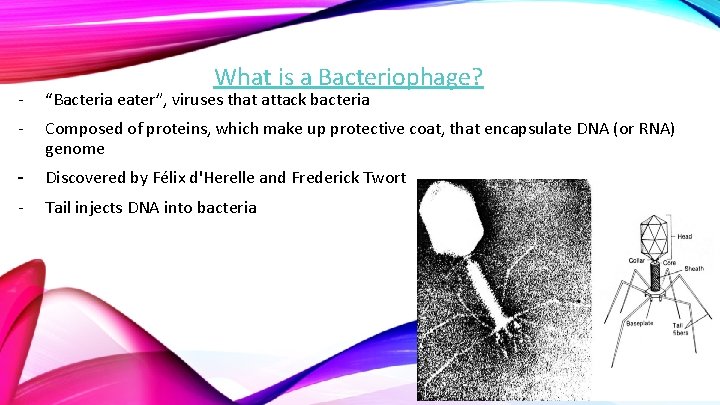
What is a Bacteriophage? - “Bacteria eater”, viruses that attack bacteria - Composed of proteins, which make up protective coat, that encapsulate DNA (or RNA) genome - Discovered by Félix d'Herelle and Frederick Twort - Tail injects DNA into bacteria

VS OR

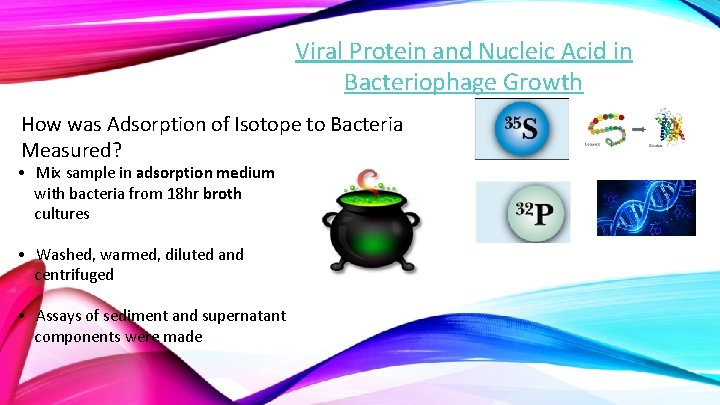
Viral Protein and Nucleic Acid in Bacteriophage Growth How was Adsorption of Isotope to Bacteria Measured? • Mix sample in adsorption medium with bacteria from 18 hr broth cultures • Washed, warmed, diluted and centrifuged • Assays of sediment and supernatant components were made

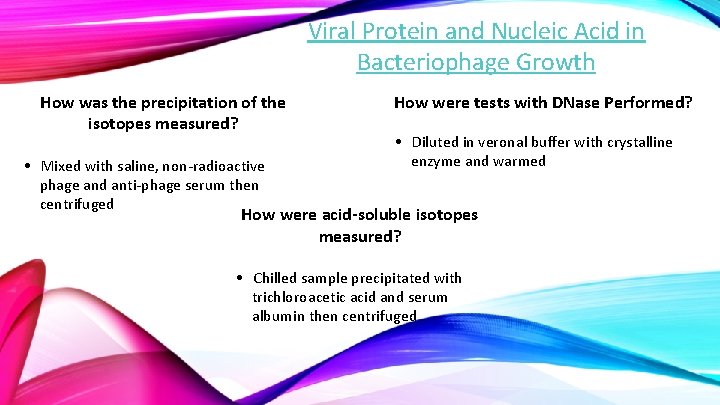
Viral Protein and Nucleic Acid in Bacteriophage Growth How was the precipitation of the isotopes measured? • Mixed with saline, non-radioactive phage and anti-phage serum then centrifuged How were tests with DNase Performed? • Diluted in veronal buffer with crystalline enzyme and warmed How were acid-soluble isotopes measured? • Chilled sample precipitated with trichloroacetic acid and serum albumin then centrifuged

The Chemical Morphology of Resting Phage Particles - Anderson 1949 Salt Water TIC O K M OS HOC S


OVERALL FOR EXPERIMENT ONE… The ghosts represent protein coats that surround the DNA of the intact particles The ghosts react with antiserum, protect the DNA from DNase and carry the organ of attachment to the bacteria This experiment characterizes the virus

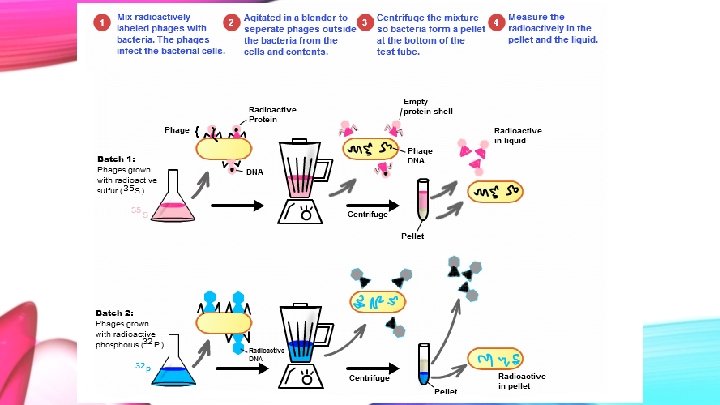
Removal of Phage Coats from Infected Bacteria ● The following experiments show that it is possible to separate the phage from the bacterial cell to determine what components of the phage are preserved in the bacterial cell.

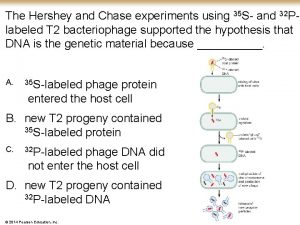
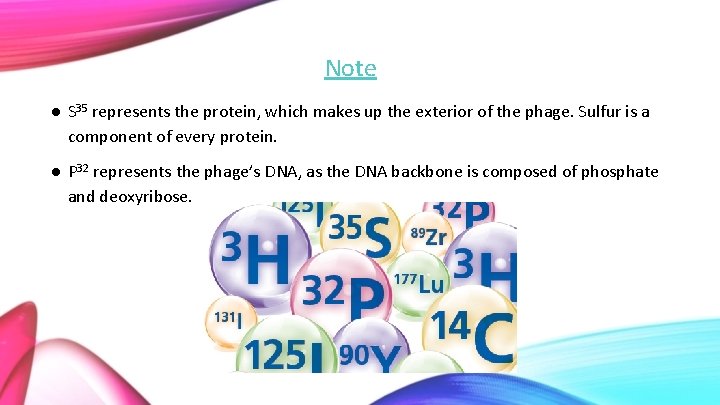
Methods ● Bacteria were infected with radioactive phage in a medium. ● The first experiment was run using S 35 labeled phages. ● The second experiment was conducted with P 32 labeled phages. ● Anything not absorbed by the bacterial medium was removed with the use of a centrifuge. ● The cells were re-suspended in Mg. SO 4, Ca. Cl 2 and gelatin containing water and spun in the Waring blendor.

Note ● S 35 represents the protein, which makes up the exterior of the phage. Sulfur is a component of every protein. ● P 32 represents the phage’s DNA, as the DNA backbone is composed of phosphate and deoxyribose.

● ● After 60 seconds of blending, the samples were cooled briefly and titrated to measure the bacteria capable of yielding phages. Samples were centrifuged and the amount of radiolabeled substance was measured.

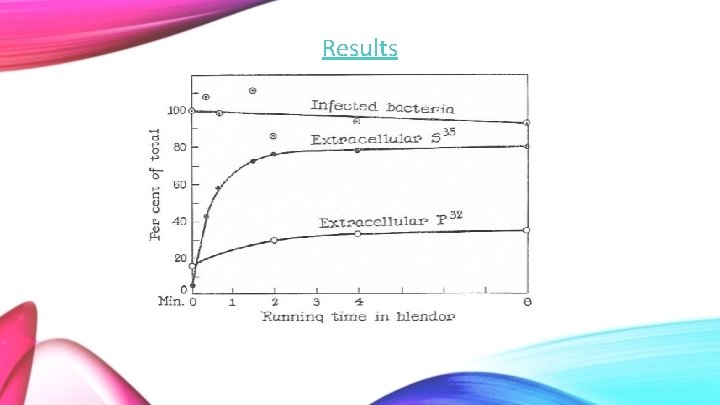
Results

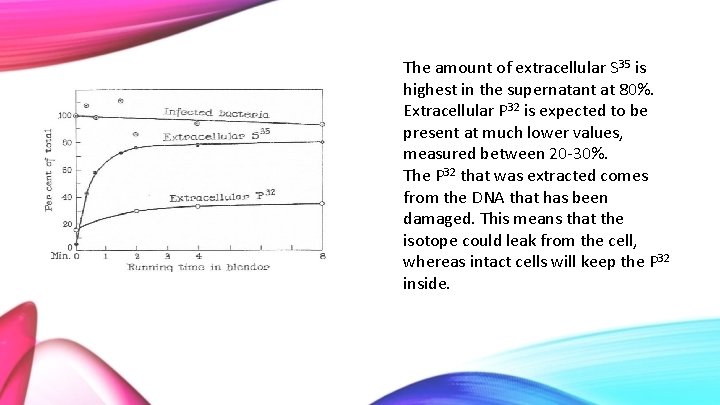
The amount of extracellular S 35 is highest in the supernatant at 80%. Extracellular P 32 is expected to be present at much lower values, measured between 20 -30%. The P 32 that was extracted comes from the DNA that has been damaged. This means that the isotope could leak from the cell, whereas intact cells will keep the P 32 inside.

Why was the sulfur isotope not 100% extracted ? !? !

- S 35 should theoretically be at 100% as none of the exterior bacteriophage shells would enter the bacterium cells. - It is most likely that some of the phage became stuck to the E. coli. - Anything that will come off in the minute time frame it was spun will be all that is released. The remainder will stay stuck to the bacteria cell, thus explaining why 100% of sulfur was not extracted in the supernatant.

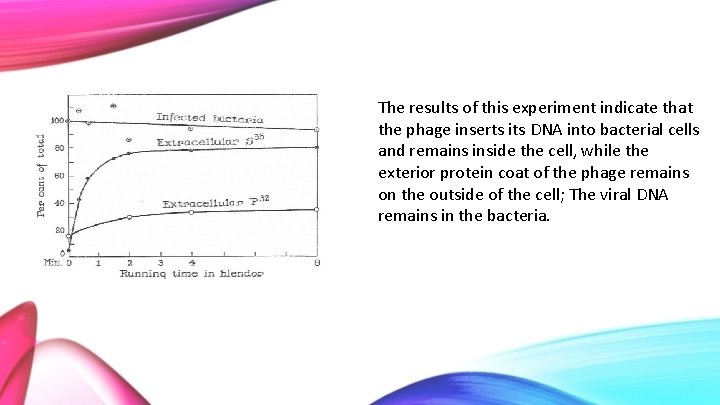
The results of this experiment indicate that the phage inserts its DNA into bacterial cells and remains inside the cell, while the exterior protein coat of the phage remains on the outside of the cell; The viral DNA remains in the bacteria.

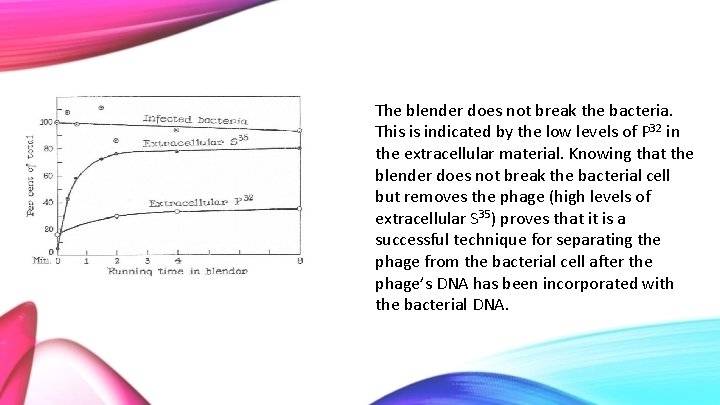
The blender does not break the bacteria. This is indicated by the low levels of P 32 in the extracellular material. Knowing that the blender does not break the bacterial cell but removes the phage (high levels of extracellular S 35) proves that it is a successful technique for separating the phage from the bacterial cell after the phage’s DNA has been incorporated with the bacterial DNA.


The results from the blender experiment allow for other experiments to be conducted on just the bacteria with the adsorbed phage DNA.


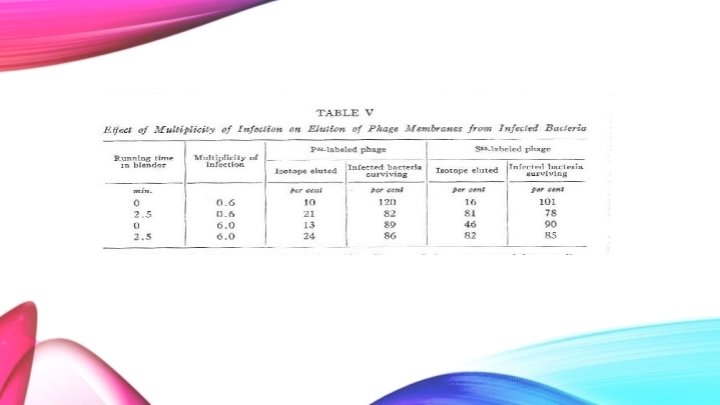
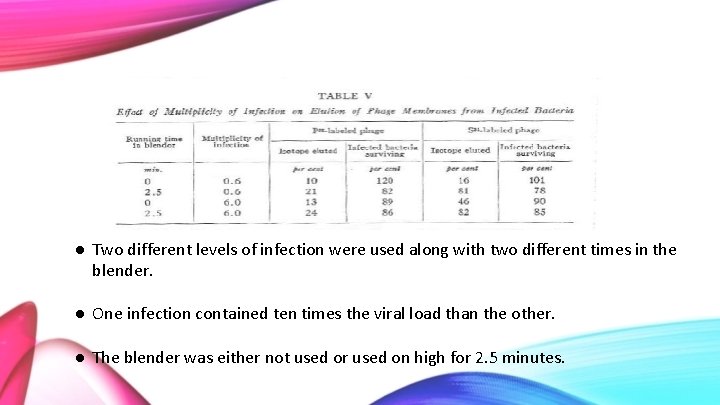
● Two different levels of infection were used along with two different times in the blender. ● One infection contained ten times the viral load than the other. ● The blender was either not used or used on high for 2. 5 minutes.

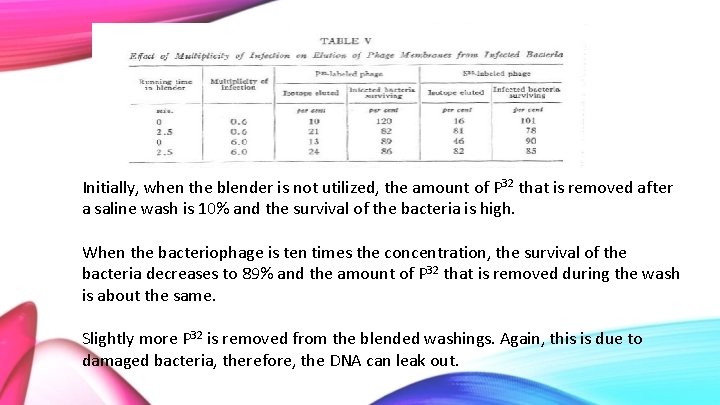
Initially, when the blender is not utilized, the amount of P 32 that is removed after a saline wash is 10% and the survival of the bacteria is high. When the bacteriophage is ten times the concentration, the survival of the bacteria decreases to 89% and the amount of P 32 that is removed during the wash is about the same. Slightly more P 32 is removed from the blended washings. Again, this is due to damaged bacteria, therefore, the DNA can leak out.

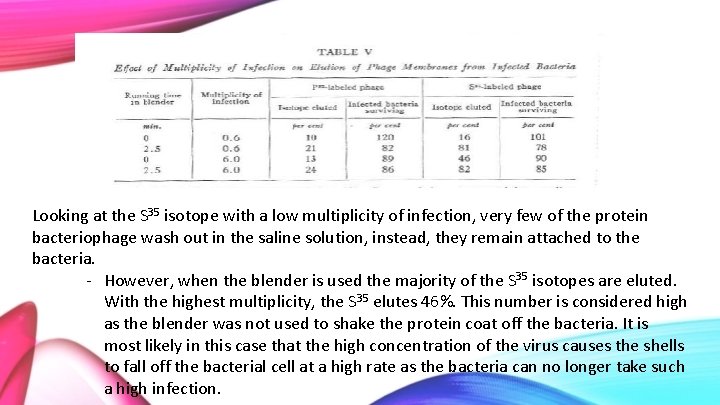
Looking at the S 35 isotope with a low multiplicity of infection, very few of the protein bacteriophage wash out in the saline solution, instead, they remain attached to the bacteria. - However, when the blender is used the majority of the S 35 isotopes are eluted. With the highest multiplicity, the S 35 elutes 46%. This number is considered high as the blender was not used to shake the protein coat off the bacteria. It is most likely in this case that the high concentration of the virus causes the shells to fall off the bacterial cell at a high rate as the bacteria can no longer take such a high infection.

Important Findings ● 75 -80% of the sulfur can be stripped away from the bacteria utilizing mechanical force. This number increases with increasing multiplicity of infection (without and mechanical force). ● Phosphorus is released in much smaller quantities than the sulfur. Phosphorus can be released without mechanical force, however, slightly more is released with mechanical force. The phosphorus release is due to damaged bacterial cells, which leak the internal DNA. ● Methods used do not inactive intracellular phage. ● Finally, the majority of the sulfur (protein) remains at the bacterial cell surface and does not aid in the multiplication of intracellular phage. The phosphorus (phage DNA) enters the bacterial cell soon after absorption of the phage and is protected from removal by the bacteria’s cell wall.

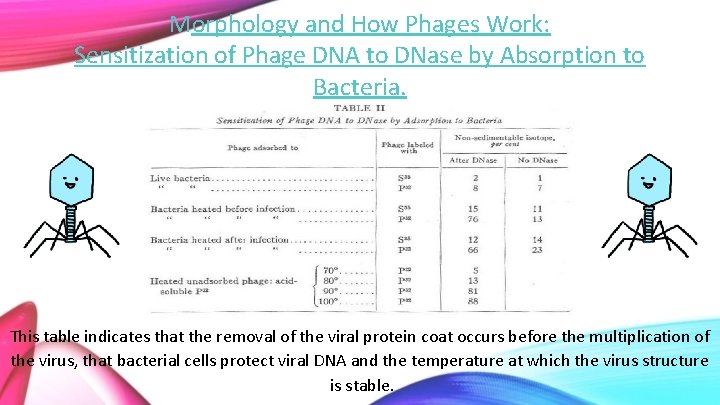
Morphology and How Phages Work: Sensitization of Phage DNA to DNase by Absorption to Bacteria. This table indicates that the removal of the viral protein coat occurs before the multiplication of the virus, that bacterial cells protect viral DNA and the temperature at which the virus structure is stable.

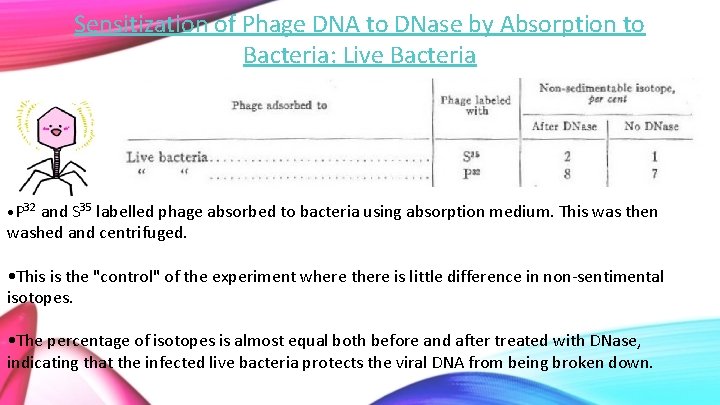
Sensitization of Phage DNA to DNase by Absorption to Bacteria: Live Bacteria • P 32 and S 35 labelled phage absorbed to bacteria using absorption medium. This was then washed and centrifuged. • This is the "control" of the experiment where there is little difference in non-sentimental isotopes. • The percentage of isotopes is almost equal both before and after treated with DNase, indicating that the infected live bacteria protects the viral DNA from being broken down.

Sensitization of Phage DNA to DNase by Absorption to Bacteria: Heated Before and After Infection • P 32 and S 35 labelled Phage absorbed to bacteria using absorption medium either before or after heating bacteria to 80°C. This was then washed and centrifuged. • Heated before: The infected phage DNA gets broken down by DNase (susceptible), indicating that the bacteria has been damaged by heat before infection, leaving the phage DNA open to breakdown by DNase. • Heated after: Results are similar to the heated before infection sample, indicating that the infected phage is relatively equally susceptible to DNase if the bacteria was heat killed before or after infection. • “No DNase” indicates non-sedimental DNA that is intact before DNase is added. • However, there should have been no change in S 35 compared to the live bacteria sample, indicating that there was some protein contamination—A crude experiment.

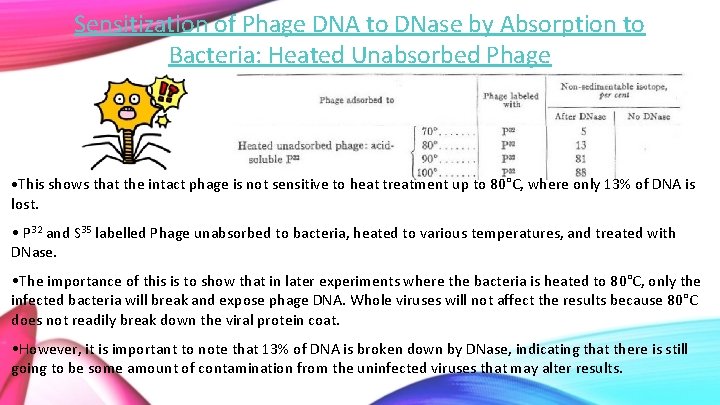
Sensitization of Phage DNA to DNase by Absorption to Bacteria: Heated Unabsorbed Phage • This shows that the intact phage is not sensitive to heat treatment up to 80°C, where only 13% of DNA is lost. • P 32 and S 35 labelled Phage unabsorbed to bacteria, heated to various temperatures, and treated with DNase. • The importance of this is to show that in later experiments where the bacteria is heated to 80°C, only the infected bacteria will break and expose phage DNA. Whole viruses will not affect the results because 80°C does not readily break down the viral protein coat. • However, it is important to note that 13% of DNA is broken down by DNase, indicating that there is still going to be some amount of contamination from the uninfected viruses that may alter results.

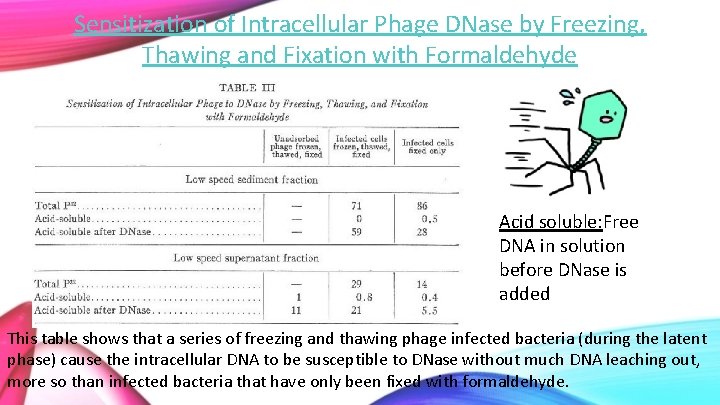
Sensitization of Intracellular Phage DNase by Freezing, Thawing and Fixation with Formaldehyde Acid soluble: Free DNA in solution before DNase is added This table shows that a series of freezing and thawing phage infected bacteria (during the latent phase) cause the intracellular DNA to be susceptible to DNase without much DNA leaching out, more so than infected bacteria that have only been fixed with formaldehyde.

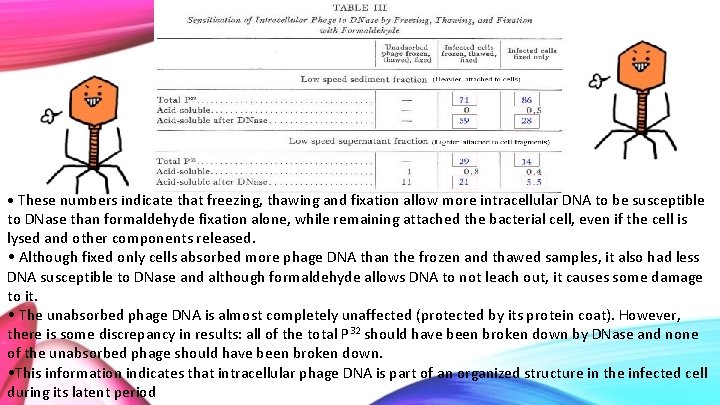
• These numbers indicate that freezing, thawing and fixation allow more intracellular DNA to be susceptible to DNase than formaldehyde fixation alone, while remaining attached the bacterial cell, even if the cell is lysed and other components released. • Although fixed only cells absorbed more phage DNA than the frozen and thawed samples, it also had less DNA susceptible to DNase and although formaldehyde allows DNA to not leach out, it causes some damage to it. • The unabsorbed phage DNA is almost completely unaffected (protected by its protein coat). However, there is some discrepancy in results: all of the total P 32 should have been broken down by DNase and none of the unabsorbed phage should have been broken down. • This information indicates that intracellular phage DNA is part of an organized structure in the infected cell during its latent period

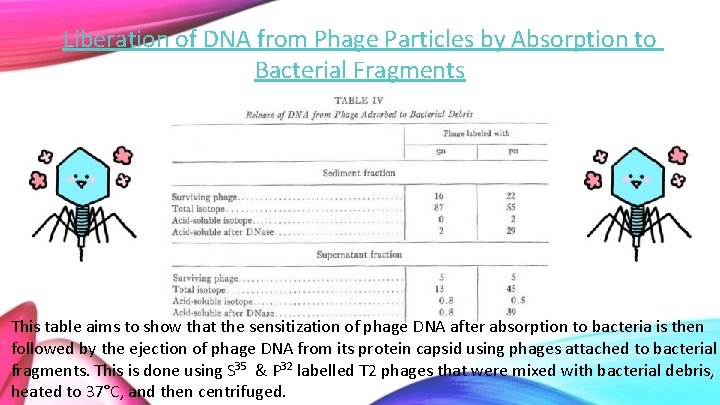
Liberation of DNA from Phage Particles by Absorption to Bacterial Fragments This table aims to show that the sensitization of phage DNA after absorption to bacteria is then followed by the ejection of phage DNA from its protein capsid using phages attached to bacterial fragments. This is done using S 35 & P 32 labelled T 2 phages that were mixed with bacterial debris, heated to 37°C, and then centrifuged.

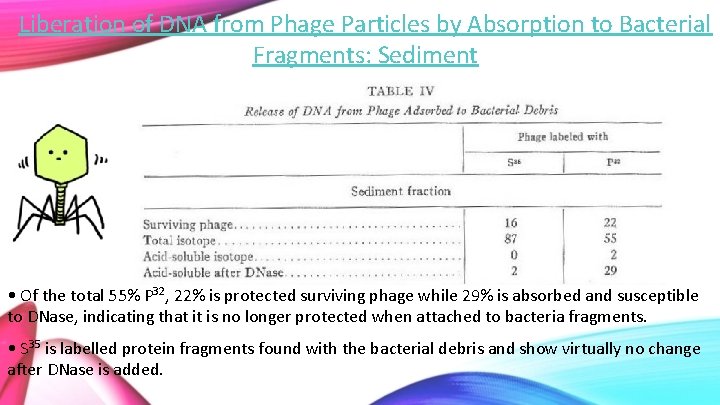
Liberation of DNA from Phage Particles by Absorption to Bacterial Fragments: Sediment • Of the total 55% P 32, 22% is protected surviving phage while 29% is absorbed and susceptible to DNase, indicating that it is no longer protected when attached to bacteria fragments. • S 35 is labelled protein fragments found with the bacterial debris and show virtually no change after DNase is added.

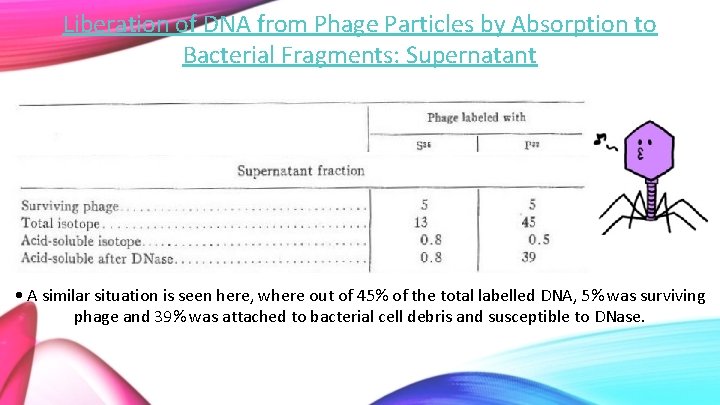
Liberation of DNA from Phage Particles by Absorption to Bacterial Fragments: Supernatant • A similar situation is seen here, where out of 45% of the total labelled DNA, 5% was surviving phage and 39% was attached to bacterial cell debris and susceptible to DNase.

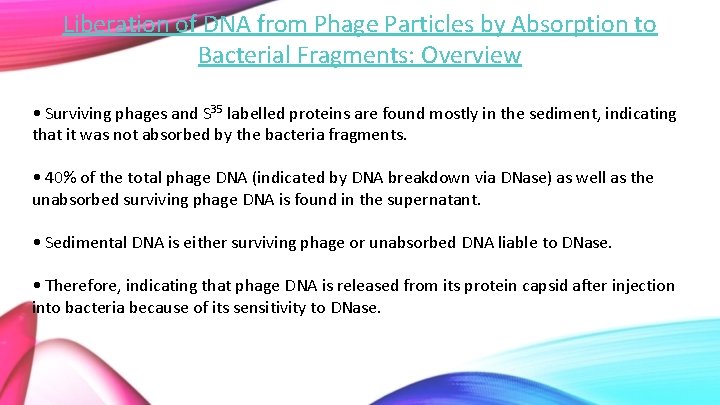
Liberation of DNA from Phage Particles by Absorption to Bacterial Fragments: Overview • Surviving phages and S 35 labelled proteins are found mostly in the sediment, indicating that it was not absorbed by the bacteria fragments. • 40% of the total phage DNA (indicated by DNA breakdown via DNase) as well as the unabsorbed surviving phage DNA is found in the supernatant. • Sedimental DNA is either surviving phage or unabsorbed DNA liable to DNase. • Therefore, indicating that phage DNA is released from its protein capsid after injection into bacteria because of its sensitivity to DNase.

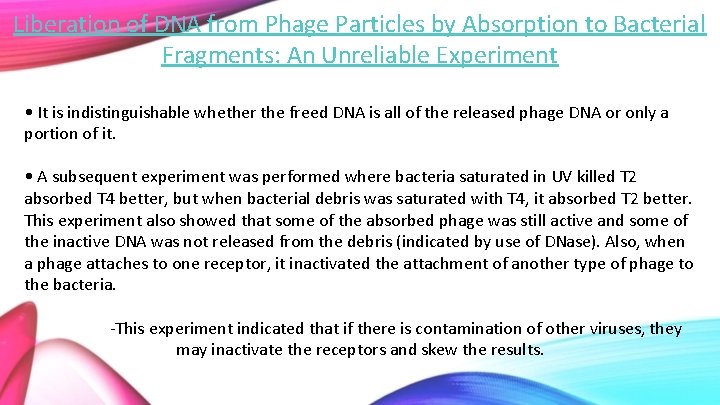
Liberation of DNA from Phage Particles by Absorption to Bacterial Fragments: An Unreliable Experiment • It is indistinguishable whether the freed DNA is all of the released phage DNA or only a portion of it. • A subsequent experiment was performed where bacteria saturated in UV killed T 2 absorbed T 4 better, but when bacterial debris was saturated with T 4, it absorbed T 2 better. This experiment also showed that some of the absorbed phage was still active and some of the inactive DNA was not released from the debris (indicated by use of DNase). Also, when a phage attaches to one receptor, it inactivated the attachment of another type of phage to the bacteria. -This experiment indicated that if there is contamination of other viruses, they may inactivate the receptors and skew the results.

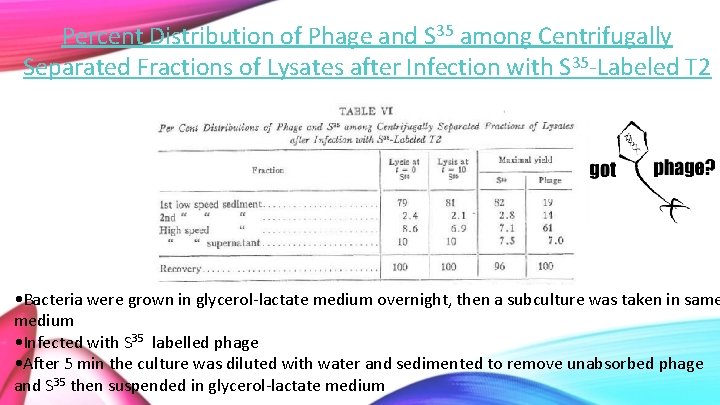
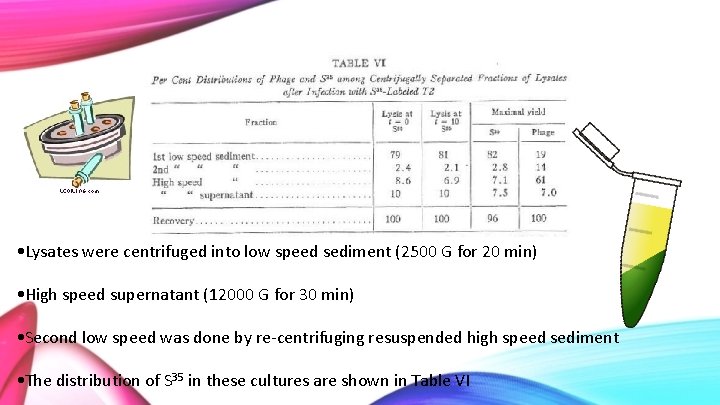
Percent Distribution of Phage and S 35 among Centrifugally Separated Fractions of Lysates after Infection with S 35 -Labeled T 2 • Bacteria were grown in glycerol-lactate medium overnight, then a subculture was taken in same medium • Infected with S 35 labelled phage • After 5 min the culture was diluted with water and sedimented to remove unabsorbed phage and S 35 then suspended in glycerol-lactate medium

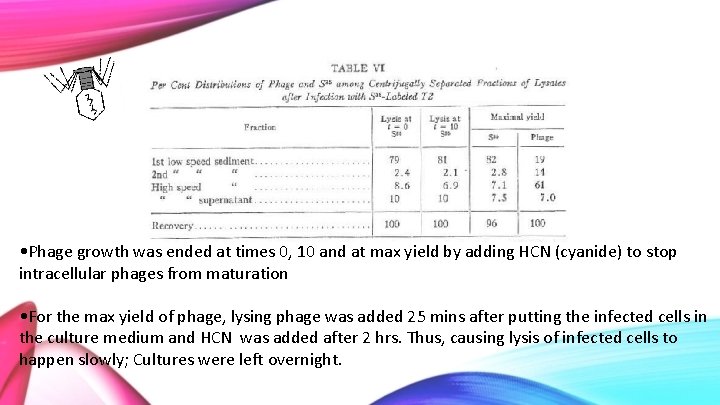
• Phage growth was ended at times 0, 10 and at max yield by adding HCN (cyanide) to stop intracellular phages from maturation • For the max yield of phage, lysing phage was added 25 mins after putting the infected cells in the culture medium and HCN was added after 2 hrs. Thus, causing lysis of infected cells to happen slowly; Cultures were left overnight.

• Lysates were centrifuged into low speed sediment (2500 G for 20 min) • High speed supernatant (12000 G for 30 min) • Second low speed was done by re-centrifuging resuspended high speed sediment • The distribution of S 35 in these cultures are shown in Table VI

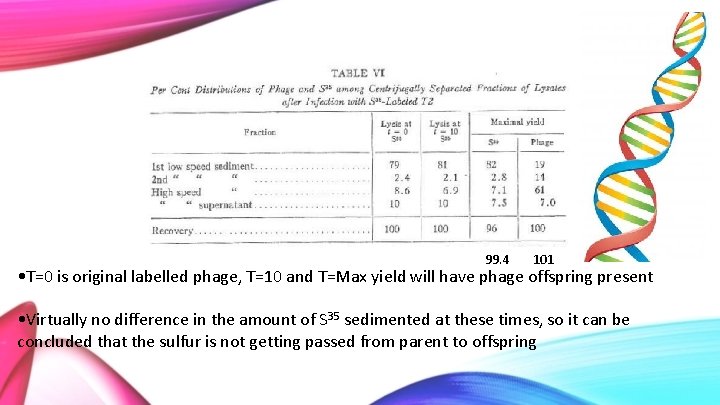
99. 4 101 • T=0 is original labelled phage, T=10 and T=Max yield will have phage offspring present • Virtually no difference in the amount of S 35 sedimented at these times, so it can be concluded that the sulfur is not getting passed from parent to offspring

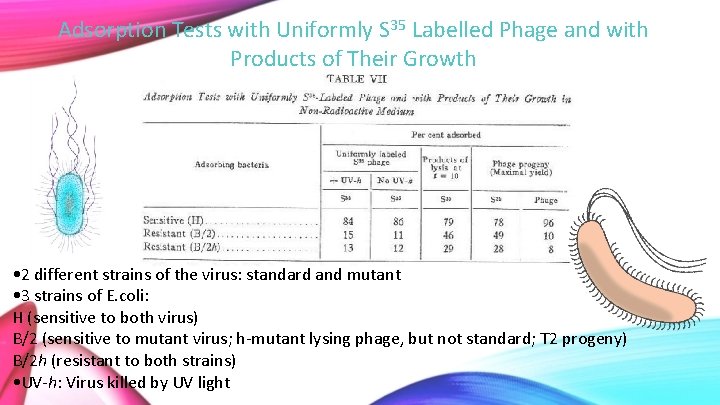
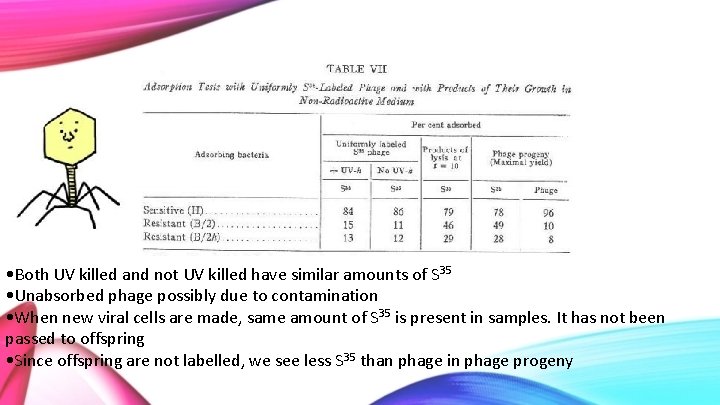
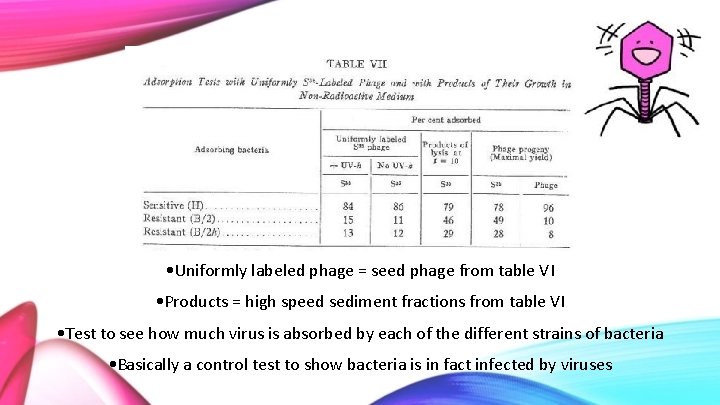
Adsorption Tests with Uniformly S 35 Labelled Phage and with Products of Their Growth • 2 different strains of the virus: standard and mutant • 3 strains of E. coli: H (sensitive to both virus) B/2 (sensitive to mutant virus; h-mutant lysing phage, but not standard; T 2 progeny) B/2 h (resistant to both strains) • UV-h: Virus killed by UV light

• Both UV killed and not UV killed have similar amounts of S 35 • Unabsorbed phage possibly due to contamination • When new viral cells are made, same amount of S 35 is present in samples. It has not been passed to offspring • Since offspring are not labelled, we see less S 35 than phage in phage progeny

• Uniformly labeled phage = seed phage from table VI • Products = high speed sediment fractions from table VI • Test to see how much virus is absorbed by each of the different strains of bacteria • Basically a control test to show bacteria is in fact infected by viruses

What about P 32? • The experiments that were conducted in tables VI and VII were also completed using P 32 labelled phage instead of S 35. The results were not collected in a table, but it is explained that P is shown to be transferred to offspring • About 30% of P 32 was found in the progeny • ”P 32 goes in, S 35 stays out”


Experiment 7: A Progeny of S 35 -Labeled Phage Nearly Free from the Parental Label

Methods – How’d they Complete it? • Infected bacteria with S 35 -labeled phage at a high ratio to ensure full infection achieved • Washed off any unabsorbed phage • Divided samples: Stripped and Non-stripped • Stripped samples were agitated in a Waring Blendor

Methods cont’ • Both samples were centrifuged to remove extracellular S 35 • Cells were then lysed and salt was added to cause S 35 to remain attached to the bacterial debris • This would then cause the sulfur to be in the sediment, not the supernatant.

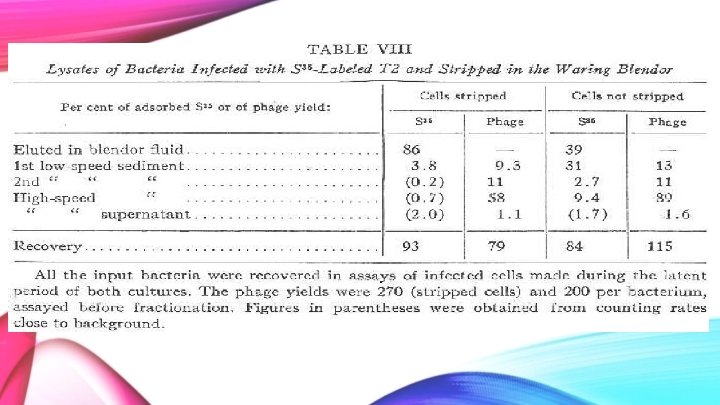
Cell Stripping Process • Stripping of the bacterium was achieved by using a Waring Blendor • This method removed phage stuck to the outside of the cell • In non-stripped sample, ghosts would still be adhered


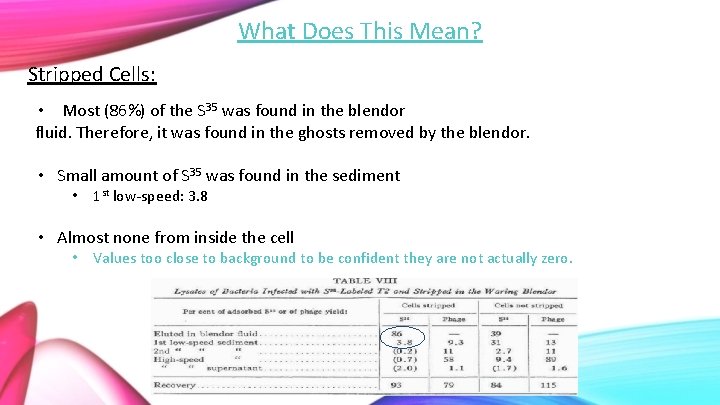
What Does This Mean? Stripped Cells: • Most (86%) of the S 35 was found in the blendor fluid. Therefore, it was found in the ghosts removed by the blendor. • Small amount of S 35 was found in the sediment • 1 st low-speed: 3. 8 • Almost none from inside the cell • Values too close to background to be confident they are not actually zero.

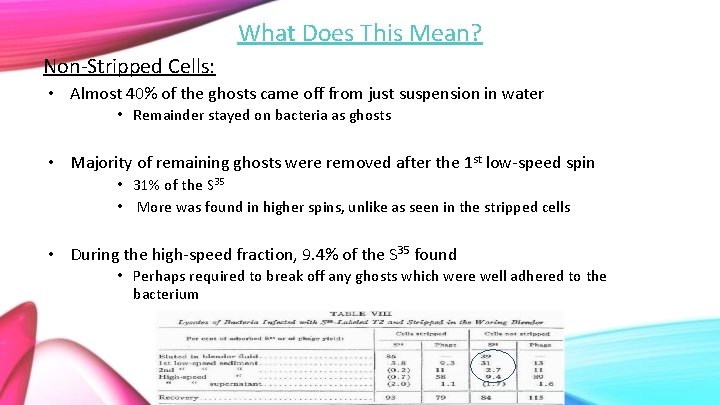
What Does This Mean? Non-Stripped Cells: • Almost 40% of the ghosts came off from just suspension in water • Remainder stayed on bacteria as ghosts • Majority of remaining ghosts were removed after the 1 st low-speed spin • 31% of the S 35 • More was found in higher spins, unlike as seen in the stripped cells • During the high-speed fraction, 9. 4% of the S 35 found • Perhaps required to break off any ghosts which were well adhered to the bacterium

What Does This Mean? • Majority of phage is recovered in the high-speed sediment • Therefore, the high-speed spin is required to release most of the phage progeny • NOTE: none of the numbers add to 100% • Take away: • there is very little S 35 present alongside the phage progeny!

Findings • Stripping reduces almost all of the S 35 -content of all fractions • With regards to the fraction containing most of the phage progeny, the S 35 -content is reduced to less than 1% (from nearly 10%) of the initial amount • High-speed sediment spin released most of the phage progeny • There is very little S 35 present alongside the phage progeny • Stripped Cells: Sediment did not contain labeled S 35 • The S 35 found in the lysate fractions was composed of the coats of the parental phage particles remains

Experiment 8: Properties of Phage Inactivated by Formaldehyde

Methods Treated virus with Formaldehyde: • Warmed Phage T 2 at 37°C for 1 hour in absorption medium • Contained 0. 1% commercial formalin • Dialyzed free from formaldehyde

What was the Outcome? • The fixation of DNA into phage with formaldehyde altered the phage replication process • Large reduction in plaque titer number • Decreased by 1000 fold!

Properties of Inactivated Phage 1) It is absorbed to sensitive bacteria • ~70% 2) The absorbed phage kills bacteria • ~35% effective 3) The DNA of the inactive particles is resistant to DNase • However, is sensitive to osmotic shock

Properties of Inactivated Phage 4) The DNA of the inactive particles is not sensitized to DNase by absorption to heat-killed bacteria • Also not released into solution by absorption to bacterial debris 5) Absorbed phage DNA can be detached • ~70% can be detached • Detached DNA is almost completely resistant to DNase.

What Do These Properties Show? T 2 Inactivated by Formaldehyde: • Cannot inject its DNA into cells to which it attaches • The behavior observed in this experiment (by inactivated T 2) supports previous experiments involving active phage

Discussion What Does All of this Suggest? • When T 2 attaches to bacteria cell: • A residue of 80% (or more) sulfur-containing protein stays at cell surface • Residue also composed of protective membrane material – Plays no further role in infection So what about the other 20% sulfur? !

Discussion What Does All of this Suggest? • 20% Sulfur in question - either does or does not enter cell: • Appears to remain extracellular • However, phosphorus and adenine (derived from DNA of infecting particle) are TRANSFERRED to phage progeny Showed us that sulfur-containing protein has no function in phage multiplication, yet DNA does.

Unanswered Questions 1) “Does any sulfur-free phage material other than DNA enter the cell? ” 2) “If so, is it transferred to the phage progeny? ” 3) “Is the transfer of phosphorus to progeny direct or indirect? ”

Discussion • Hershey and Chase did successfully show that: – Physical separation of bacteriophage T 2 into genetic and non-genetic parts is possible!

Summary • The sulfur-containing protein of the phage particle: • makes up the protective membrane and guards phage DNA from DNase • Principal antigenic material • Responsible for virus attaching to bacteria • Treating unabsorbed phage by heat-killing, heating or freezing and thawing have little or no sensitizing effect • Neither heating nor freezing/thawing releases phage DNA from infected cells • Thus, phage DNA forms part of intracellular structure.

Summary cont’ • Absorption of phage T 2 to bacterial debris • Part of the phage DNA appeared in solution, phage sulfur still attached to debris • The other half of phage DNA is attached to debris but separates due to action of DNase • Stripped cells release 75% of phage sulfur and 15% of phage phosphorus to solution due to shearing force • Cells still capable of phage progeny

Summary cont’ • Upon infection: • Most of phage sulfur remains at cell surface • Most of phage DNA enters cell • Bacteria infected with phage labeled radioactive sulfur yielded a phage progeny which contained less than 1% of the parental radioactivity • Those labeled with radioactive phosphorus contained 30% or more of the parental phosphorus

Summary cont’ • Inactivated phage (by formaldehyde) can absorb bacteria, but does not release its DNA to the cell. • Therefore, release of phage DNA from protective membrane depends on components of phage particle • Sulfur-containing protein of phage is confined to protective “coat” • • Responsible for absorption of bacteria Instrument of injection of phage DNA No function in growth of intracellular phage DNA has some unknown function

Simplified Conclusion • Absorbed phage: • Inserts DNA into cell • Sulfur-containing protein stays outside cell • Phage: • Release DNA into solution • Phage progeny: • Had parental P 32 • Little or no parental S 35 • DNA has “some function” while sulfur-containing protein did not have intracellular function

Other Experiments • The results of Hershey and Chase’s experiment sparked the interest of numerous scientists and convinced many that DNA was in fact the molecule of heredity • For example: Watson and Crick Resultantly, the works published by Hershey and Chase were essential in igniting the race to find the structure of DNA!

Conformation of Experiment In 1953 Watson and Crick won the race to find the structure of DNA! Their journal article “Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid” suggested the copying mechanism by which DNA acts as hereditary material and that DNA is synthesized by thousands of proteins found in cells.

The Nobel Prize In 1969 Hershey was awarded the Nobel Prize in Physiology or Medicine The prize was awarded jointly: 1/3 share for Alfred D. Hershey - 1/3 share for Max Delbrück - 1/3 share for Salvador E. Luria -

"for their discoveries concerning the replication mechanism and the genetic structure of viruses" • Nobel Prize Organization

Where are they Now? Alfred D. Hershey (1908 – 1997) • 1945 – Married Harriet Davidson • 1962 – Appointed Director of Genetics Research Unit at the Carnegie Institution of Washington in Cold Spring Harbor, New York • 1965 – Received the Kimber Genetics Award of the National Academy of Sciences • 1969 – Awarded Nobel Prize in Physiology or Medicine • 1970 – Honored with M. D. h. c. from Michigan State University

Where are they Now? Martha Chase (1927 – 2003) • 1950’s – Took part in meetings of the Phage Group of Biologists in Cold Spring Harbor, New York • 1964 – Received doctorate at the University of Southern California • Mid – Late 1960’s – Ended career in science due to personal problems

American Phage Group • What was is? • An informal network of biologists in the mid 20 th century based at the Cold Spring Harbor Laboratory in New York • What did they do? • Huge contributors to the world of bacterial genetics and molecular biology • Created course on phages that was taught every summer from the 1950’s till the 1960’s • Consisted of some of our favorite scientists! • Centered around Max Delbrück • Included Hershey, Watson, Luria, Benzer, Stent, Stahl, and Dulbecco

Where are we Today? • Genome mapping – 23 and. Me • Genetic engineering – Designer children • Disease prediction models – ex: Huntington’s disease

“I can only point out a curious fact. Year after year the Nobel Awards bring a moment of happiness not only to the recipients, not only to colleagues and friends of the recipients, but even to strangers” - Alfred Day Hershey

References Hershey, A. , & Chase, M. , (1952). Independent Functions of Viral Protein and Cold Spring Harbor, Long Island. Nucleic Acids in Growth of Bacteriophage. Department of Genetics, Carnegie Institution of Washington, "The Nobel Prize in Physiology or Medicine 1969". Nobelprize. org. Nobel Media AB 2014. Web. 26 Jan 2016. <http: //www. nobelprize. org/nobel_prizes/medicine/laureates/1969/> "Alfred D. Hershey - Biographical". Nobelprize. org. Nobel Media AB 2014. Web. 26 Jan 2016. <http: //www. nobelprize. org/nobel_prizes/medicine/laureates/1969/hershey-bio. html> "Chase, Martha Cowles (1927 - ). " World of Microbiology and Immunology. 2003. Encyclopedia. com. 26 Jan. 2016 <http: //www. encyclopedia. com Brainy. Quote, . "Alfred Day Hershey Quotes At Brainyquote". N. p. , 2016. Web. 27 Jan. 2016. Carr, Dr. Steve. "Hershey And Chase 1952". Bio 4241 - Advanced Topics in Genetics. N. p. , 2016. Web. 24 Jan. 2016. Carr, Dr. Steve. "Bio 4241 - Advanced Topics In Genetics". Mun. ca. N. p. , 2016. Web. 24 Jan. 2016. Encyclopedia. com, . "Chase, Martha Cowles (1927 - ) – FREE Chase, Martha Cowles (1927 - ) Information | Encyclopedia. Com: Find Chase, Martha Cowles (1927 - ) Research". N. p. , 2016. Web. 24 Jan. 2016 “Gender Bias in Science, Part IV: Martha Chase”. The Mad Science Blog. http: //www. themadscienceblog. com/2013/10/gender-bias-in-science-part-iv-martha. html. Oct. 28, 2013 Gutenberg, Project. "American Phage Group | Project Gutenberg Self-Publishing - Ebooks | Read Ebooks Online". Gutenberg. us. N. p. , 2016. Web. 24 Jan. 2016. Molecular Genetics. (2016). Retrieved January 26, 2016, from http: //biology. tutorvista. com/cell/molecular-genetics. ht
 Alfred hershey and martha chase
Alfred hershey and martha chase Martha chase e alfred hershey
Martha chase e alfred hershey Hershey and chase
Hershey and chase Plant viruses
Plant viruses Experimento de la licuadora de hershey y chase
Experimento de la licuadora de hershey y chase Hershey chase experiment definition
Hershey chase experiment definition Organis
Organis Blgen
Blgen Martha chase
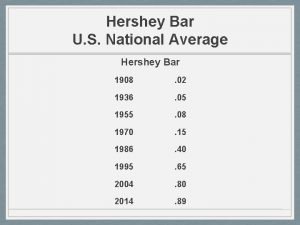
Martha chase Hershey bar picture
Hershey bar picture The hershey company history
The hershey company history Why did cheetos lip balm fail
Why did cheetos lip balm fail Hershey erp implementation
Hershey erp implementation Hershey
Hershey Hershey company swot analysis
Hershey company swot analysis It project failure case study
It project failure case study How many hershey’s kisses make up 1 mole?
How many hershey’s kisses make up 1 mole? Fanboys connectors
Fanboys connectors The mother of contemporary dance
The mother of contemporary dance Martha bailey economics
Martha bailey economics The crucible character traits
The crucible character traits Martha from senior year
Martha from senior year Martha rogers
Martha rogers Prix martha 2020
Prix martha 2020 Martha rogers concepts
Martha rogers concepts Carmen rogers
Carmen rogers Martha monroy
Martha monroy Voorplasing
Voorplasing Stilwell school of the arts
Stilwell school of the arts Martha organization
Martha organization Martha bernays
Martha bernays The crucible cast of characters
The crucible cast of characters Martha nussbaum l'intelligenza delle emozioni
Martha nussbaum l'intelligenza delle emozioni Providing improper or unprofessional treatment or care
Providing improper or unprofessional treatment or care Hyde as a frightening outsider
Hyde as a frightening outsider Dr martha thompson
Dr martha thompson Sigmund jansen
Sigmund jansen Muere lentamente quien se transforma en esclavo del hábito
Muere lentamente quien se transforma en esclavo del hábito Muere lentamente pablo neruda
Muere lentamente pablo neruda Muere lentamente quien no viaja quien no lee
Muere lentamente quien no viaja quien no lee Papai noel martha medeiros
Papai noel martha medeiros Martha stewart 1991
Martha stewart 1991 Martha medeiros muere lentamente
Martha medeiros muere lentamente Richard saitz md obituary
Richard saitz md obituary Morre lentamente martha medeiros
Morre lentamente martha medeiros Martha smith
Martha smith Nivel molecular
Nivel molecular Modelos de florence nightingale
Modelos de florence nightingale Martha leigh
Martha leigh Martha medeiros textos
Martha medeiros textos Hendersonová model
Hendersonová model Sdraot.world
Sdraot.world Martha daza
Martha daza Martha daza
Martha daza Biyo fizyolojik boyut
Biyo fizyolojik boyut Martha rogers
Martha rogers Mapa de riesgos
Mapa de riesgos La foglia di muriel
La foglia di muriel Texto martha medeiros
Texto martha medeiros Martha monroe
Martha monroe Martha fiennes
Martha fiennes Martha marrapese
Martha marrapese Dr martha st john
Dr martha st john Tamara halle
Tamara halle Martha burkle
Martha burkle Martha delgado peralta
Martha delgado peralta Martha kyrillidou
Martha kyrillidou Martha crago
Martha crago Martha delia sirvent cancino
Martha delia sirvent cancino Rueda de kolb
Rueda de kolb How to solve evaluating functions
How to solve evaluating functions Evaluating functions and operations on functions
Evaluating functions and operations on functions Chase and simon 1973
Chase and simon 1973 Chase strategy example
Chase strategy example Jones and chase issues management model
Jones and chase issues management model Advantages of chase strategy
Advantages of chase strategy Level chase and hybrid strategies
Level chase and hybrid strategies Who was alfred wegener and what did he theorize
Who was alfred wegener and what did he theorize Bourbon triumvirate pictures
Bourbon triumvirate pictures Piecewise functions absolute value
Piecewise functions absolute value I would rather eat potatoes than to eat rice.
I would rather eat potatoes than to eat rice. The great angle
The great angle How to write a check chase
How to write a check chase Stanton chase
Stanton chase Decay born chase
Decay born chase Richard vampire
Richard vampire O.j. simpson trial
O.j. simpson trial Bay chase cleanroom
Bay chase cleanroom