FCN 772 Legal and Regulatory Aspects May 5

















- Slides: 23

FCN 772 Legal and Regulatory Aspects May 5, 2008 Martha Marrapese Partner Keller and Heckman LLP 1001 G St, NW Suite 500 Washington, DC 20001 202 -434 -4123 marrapese@khlaw. com www. khlaw. com Washington, D. C. www. khlaw. com ● Brussels ● San Francisco ● Shanghai Copyright © 2007

Legal Framework • A food additive is defined as a substance that is “reasonably expected to become a component of food under the intended conditions of use” • Considered “unsafe” unless it is used in accordance with an applicable food additive regulation or clearance • Uncleared food additives are automatically unsafe and adulterated 2 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

A UV/EB Coating, Adhesive, Or Ink Complies With The Act If : 1. 2. 3. 4. Its use complies with an existing food additive regulation, e. g. , 21 CFR § 176. 170, 21 CFR § 178. 3297 or FCN; Its components are the subject of a prior FDA or United States Department of Agriculture (USDA) sanction or approval prior to 1958; Its components are deemed GRAS; or It is not reasonably expected to become a component of food, based on appropriate extraction studies or equivalent data 3 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

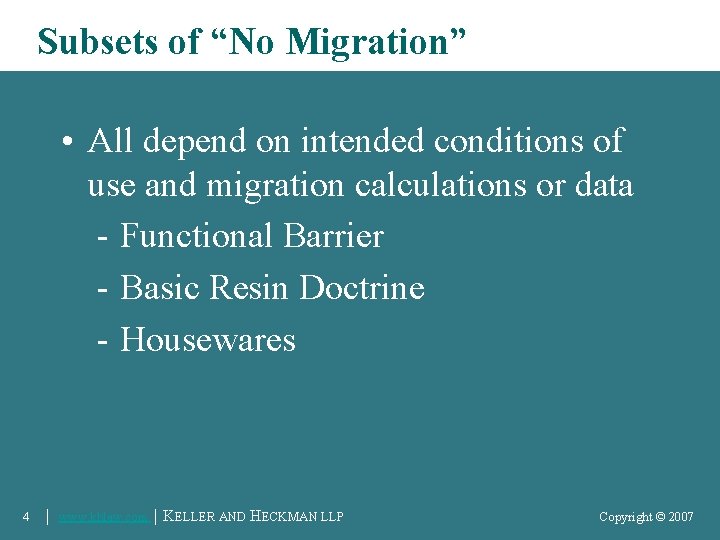
Subsets of “No Migration” • All depend on intended conditions of use and migration calculations or data - Functional Barrier - Basic Resin Doctrine - Housewares 4 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Legal Framework, cont. • Food Contact Notification (FCN) ØPre-market notification to FDA ØInformation supporting the conclusion that the substance is safe for the intended use(s) ØIf no objection within 120 days, the submitter may market the product 5 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

FCNs, cont. • FCNs are proprietary to notifier identified in filing • Customers may rely on supplier’s notification • FDA issues a letter to the notifier stating that the notification is effective • Notifications will not appear in CFR or Federal Register; FDA lists effective FCNs on its web site 6 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Food Packaging Alliance In Plain Terms A formulator may combine the Alliance FCN materials with already FDA sanctioned components to offer inks, coatings, and adhesives suitable for food packaging, in many cases without having to obtain additional clearances. 7 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Examples of Useful Clearances • Can coatings Ø 21 CFR § 175. 300 (pertaining to metal substrates or repeated use applications) • Paper coatings Ø 21 CFR § 176. 170 and/or 21 CFR § 176. 180 • Films Ø Polyolefins described in 21 CFR § 177. 1520(c)2. 2 or 3. 2 a may be used in cooking Ø Polyolefins in 21 CFR 177. 1520(c)2. 1 or (c)3. 1 a are only permitted for non-cooking applications Ø 21 CFR § 175. 320 lists cleared resinous and polymeric coatings for polyolefin films • Inks Ø Many ink components can be found in 21 CFR § 178. 3297 (Colorants for polymers) Ø Or, if used in paper applications, under 21 CFR § 76. 170 8 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

What is the Food Contact Substance? • A mixture of one or more of ØTPGDA ØTMPTA ØTMPEOTA ØBADGEDA Øoptionally containing ESACURE ONE • Cleared effective March 7, 2008 9 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

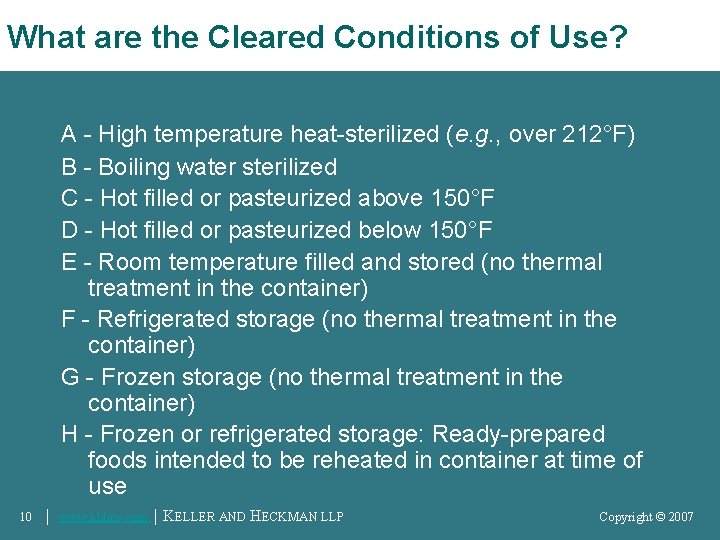
What are the Cleared Conditions of Use? A - High temperature heat-sterilized (e. g. , over 212°F) B - Boiling water sterilized C - Hot filled or pasteurized above 150°F D - Hot filled or pasteurized below 150°F E - Room temperature filled and stored (no thermal treatment in the container) F - Refrigerated storage (no thermal treatment in the container) G - Frozen storage (no thermal treatment in the container) H - Frozen or refrigerated storage: Ready-prepared foods intended to be reheated in container at time of use 10 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

What types of packaging? • Food contact layers and non-food contact layers • To be used as coatings (including inks) or components of coatings (including inks) on ØPolymeric substrates ØPaper and paperboard ØMetal substrates, or Øas a component in Adhesives. 11 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Who can use the Clearance? • Only companies listed in FCN 772 as the notifiers and their customers may rely on the clearance to manufacture food packaging materials • The acrylates and photoinitiator must be supplied by an FCN-listed company • Competitors must file their own FCNs 12 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

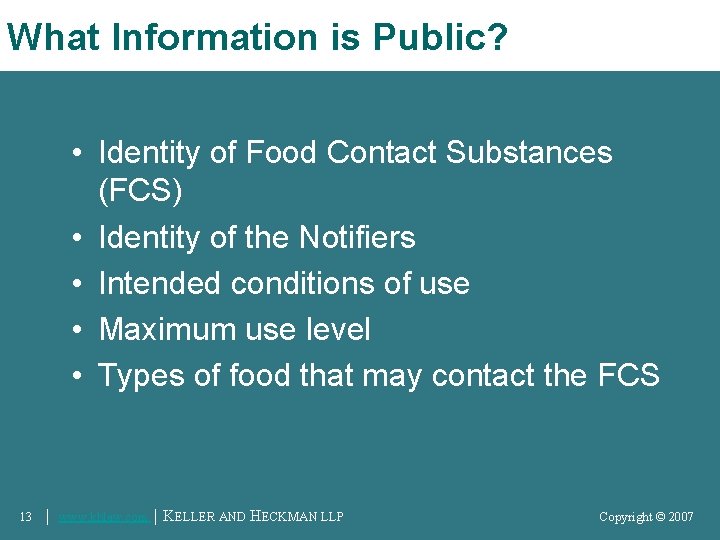
What Information is Public? • Identity of Food Contact Substances (FCS) • Identity of the Notifiers • Intended conditions of use • Maximum use level • Types of food that may contact the FCS 13 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

What to Tell Customers? • Some consumer product end users insist on seeing an FDA listing or regulation • Reference FCN 772: Ø“This product is cleared for broad use in food packaging by FCN 772, with a maximum extraction level of 10 μg/in 2 for the substance and 10 μg/in 2 for total extractables (TE). See http: //www. cfsan. fda. gov/~dms/opa-fcn. html” 14 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

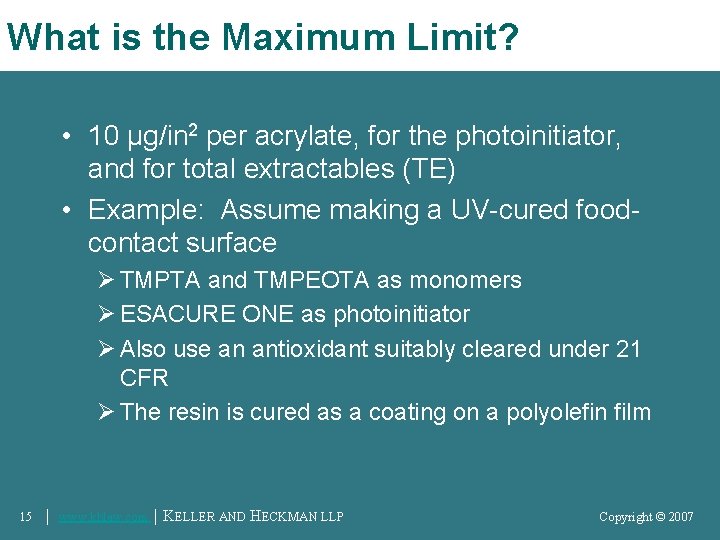
What is the Maximum Limit? • 10 μg/in 2 per acrylate, for the photoinitiator, and for total extractables (TE) • Example: Assume making a UV-cured foodcontact surface Ø TMPTA and TMPEOTA as monomers Ø ESACURE ONE as photoinitiator Ø Also use an antioxidant suitably cleared under 21 CFR Ø The resin is cured as a coating on a polyolefin film 15 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

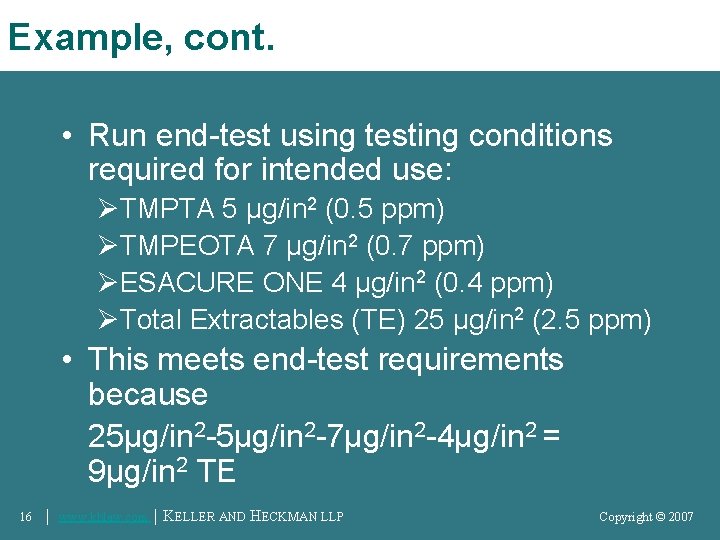
Example, cont. • Run end-test using testing conditions required for intended use: ØTMPTA 5 μg/in 2 (0. 5 ppm) ØTMPEOTA 7 μg/in 2 (0. 7 ppm) ØESACURE ONE 4 μg/in 2 (0. 4 ppm) ØTotal Extractables (TE) 25 μg/in 2 (2. 5 ppm) • This meets end-test requirements because 25μg/in 2 -7μg/in 2 -4μg/in 2 = 9μg/in 2 TE 16 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

What Verification is Needed? • Extraction test and analysis in FCN ØProtocol is proprietary Rad. Tech Alliance Members and customers • Can another method be used? ØYes, if equivalent • How often do you have to test? ØAs often as necessary. E. g. , initially, six months, any process changes • Who tests? ØThe company that is using the product 17 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

When Is a New FCN Required? • If substance made by different manufacturer/notifier than identified in FCN • New conditions or levels of use (NEW FCN, not amendment of old) • When specifications of FCS substantively change • When changes made in manufacture that substantively change: - Product identity - Purity profile - Good Manufacturing Practices (GMP) changes 18 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

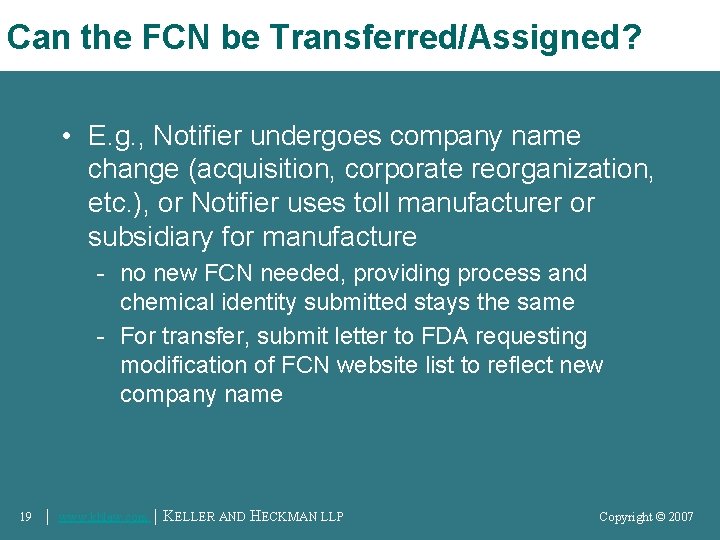
Can the FCN be Transferred/Assigned? • E. g. , Notifier undergoes company name change (acquisition, corporate reorganization, etc. ), or Notifier uses toll manufacturer or subsidiary for manufacture - no new FCN needed, providing process and chemical identity submitted stays the same - For transfer, submit letter to FDA requesting modification of FCN website list to reflect new company name 19 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

What to do When you Suspect a Non-FCN Supplied Material? • Uncleared food additives are automatically unsafe and adulterated • After food has been packaged, can lead to claims for direct damages (the value of the packaged food) far out of proportion to the value of the packaging materials themselves. Ø Also additional consequential or incidental damages such as loss of future sales from damage to a brand's reputation with consumers. • Bottom line: if there is a problem, the damages can add up fast 20 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Enforcement, cont. • FDA’s actions can include issuing a warning letter, requesting a recall of affected products, requiring a recall if potential exposure is harmful enough, and issuing a fine. • What action FDA will take depends upon the safety implications raised by the uncleared additive. • The person may inform the company of its suspicion to seek clarification for the purpose of determining if additional action is warranted, such as informing FDA of the suspected activity. 21 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

How to Build on FCN 772? • Alliance members and their customers can reference the Alliance FCN in new FCN filings • Less additional effort to obtain clearance for an uncleared component in a new proprietary formulation 22 │ www. khlaw. com │ KELLER AND HECKMAN LLP Copyright © 2006 Copyright © 2007

Thank you! Martha Marrapese Partner Keller and Heckman LLP 1001 G St, NW Suite 500 Washington, DC 20001 202 -434 -4123 marrapese@khlaw. com www. khlaw. com Washington, D. C. www. khlaw. com ● Brussels ● San Francisco ● Shanghai Copyright © 2007
 Suliranin sa industriya
Suliranin sa industriya Wheeler de witt
Wheeler de witt Legal regulatory and political issues
Legal regulatory and political issues Legal and regulatory framework of microfinance in india
Legal and regulatory framework of microfinance in india Chapter 3 legal and ethical issues
Chapter 3 legal and ethical issues Law and ethics in information security
Law and ethics in information security Legal aspects of catering premises
Legal aspects of catering premises Mario full screen
Mario full screen Legal aspects of doing business in canada
Legal aspects of doing business in canada Irving isd v tatro
Irving isd v tatro Legal aspects of advertising
Legal aspects of advertising Aspek manajemen proyek
Aspek manajemen proyek Legal issues in community health nursing
Legal issues in community health nursing Scbon
Scbon Nursing legal terms
Nursing legal terms Legal aspects definition
Legal aspects definition Chapter 22 regulatory and advisory agencies
Chapter 22 regulatory and advisory agencies Dea number verification
Dea number verification Managing diversity and regulatory challenges
Managing diversity and regulatory challenges Warehousing development and regulatory authority
Warehousing development and regulatory authority Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Lara renew nursing license
Lara renew nursing license Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress













































