Actualizacin teraputica del cncer urotelial metastsico en 2018








- Slides: 48

Actualización terapéutica del cáncer urotelial metastásico en 2018 Joaquim Bellmunt, MD Ph. D Associate Professor of Medicine Harvard Medical School PSMAR-IMIM September 27 th, 2018

Disclosures • Advisory role: ◦ Genentech, Merck, Pfizer, GSK, BMS, Pierre-Fabre, Sanofi Aventis, Astellas, Onco. Genex, Janssen • Speaker role: ◦ Pfizer, Merck, GSK, Novartis, Pierre-Fabre, Astellas • Research funding: ◦ Takeda, Pfizer, Novartis, Sanofi Aventis

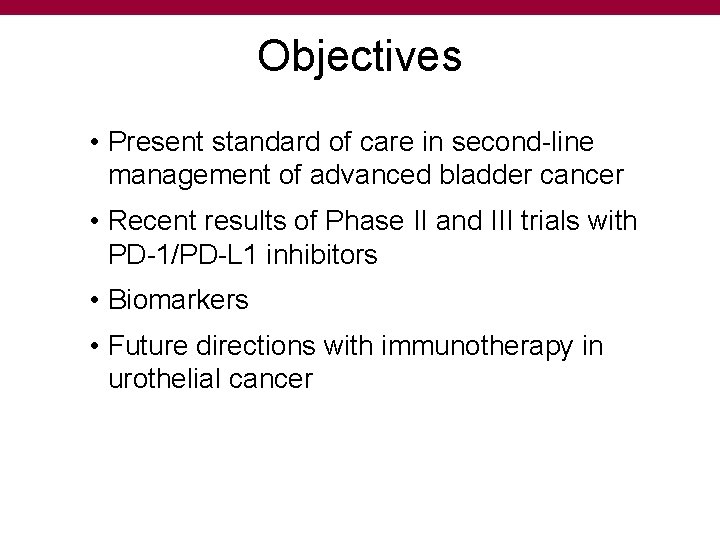
Objectives • Present standard of care in second-line management of advanced bladder cancer • Recent results of Phase II and III trials with PD-1/PD-L 1 inhibitors • Biomarkers • Future directions with immunotherapy in urothelial cancer

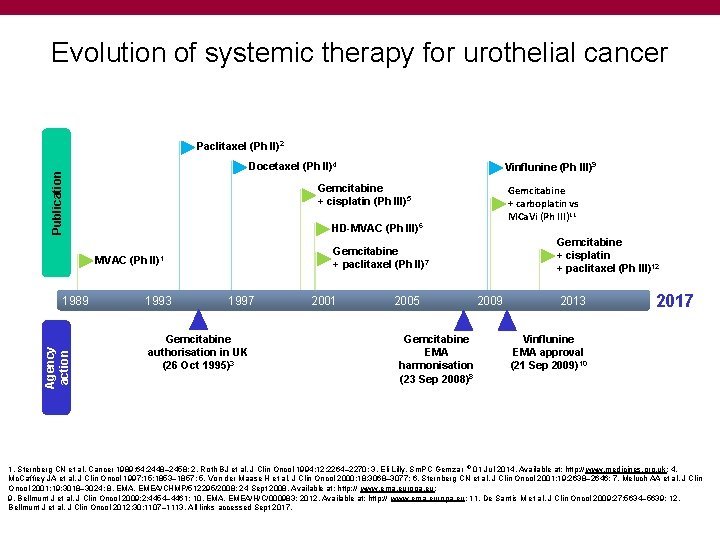
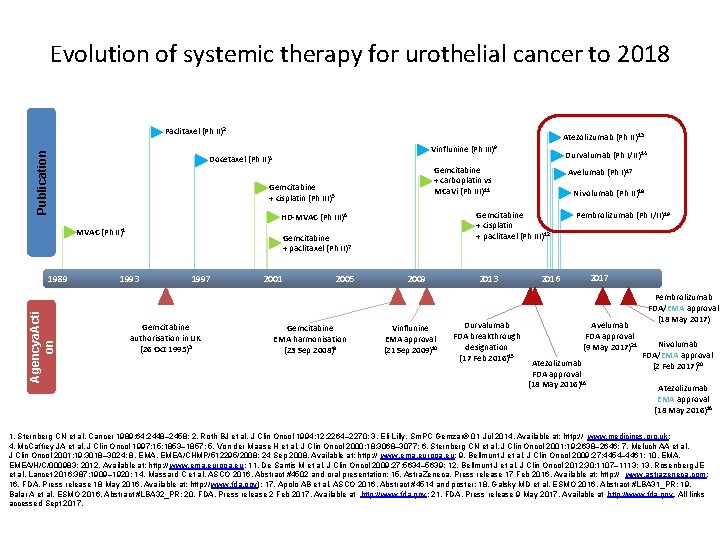
Evolution of systemic therapy for urothelial cancer Paclitaxel (Ph II)2 Publication Docetaxel (Ph II)4 Gemcitabine + cisplatin (Ph III)5 Agency action Gemcitabine + carboplatin vs MCa. Vi (Ph III)11 HD-MVAC (Ph III)6 1993 Gemcitabine + cisplatin + paclitaxel (Ph III)12 Gemcitabine + paclitaxel (Ph II)7 MVAC (Ph II)1 1989 Vinflunine (Ph III)9 1997 Gemcitabine authorisation in UK (26 Oct 1995)3 2001 2005 Gemcitabine EMA harmonisation (23 Sep 2008)8 2009 2013 2017 Vinflunine EMA approval (21 Sep 2009)10 1. Sternberg CN et al. Cancer 1989; 64: 2448– 2458; 2. Roth BJ et al. J Clin Oncol 1994; 12: 2264– 2270; 3. Eli Lilly. Sm. PC Gemzar ® 01 Jul 2014. Available at: http: // www. medicines. org. uk; 4. Mc. Caffrey JA et al. J Clin Oncol 1997; 15: 1853– 1857; 5. Von der Maase H et al. J Clin Oncol 2000; 18: 3068– 3077; 6. Sternberg CN et al. J Clin Oncol 2001; 19: 2638– 2646; 7. Meluch AA et al. J Clin Oncol 2001; 19: 3018– 3024; 8. EMA. EMEA/CHMP/512295/2008; 24 Sept 2008. Available at: http: // www. ema. europa. eu; 9. Bellmunt J et al. J Clin Oncol 2009; 2: 4454– 4461; 10. EMA. EMEA/H/C/000983; 2012. Available at: http: // www. ema. europa. eu; 11. De Santis M et al. J Clin Oncol 2009; 27: 5634– 5639; 12. Bellmunt J et al. J Clin Oncol 2012; 30: 1107– 1113. All links accessed Sept 2017.

Treatment options following platinum failure

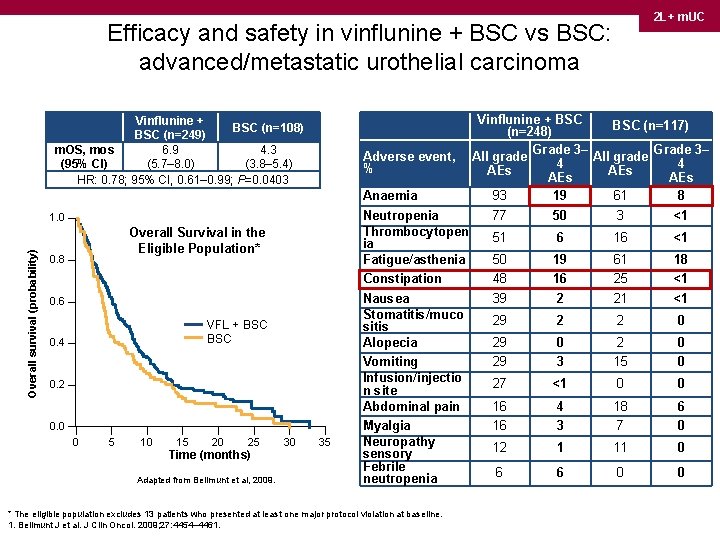
2 L+ m. UC Efficacy and safety in vinflunine + BSC vs BSC: advanced/metastatic urothelial carcinoma Vinflunine + BSC (n=108) BSC (n=249) m. OS, mos 6. 9 4. 3 (95% CI) (5. 7– 8. 0) (3. 8– 5. 4) HR: 0. 78; 95% CI, 0. 61– 0. 99; P=0. 0403 Adverse event, % Overall survival (probability) 1. 0 Overall Survival in the Eligible Population* 0. 8 0. 6 VFL + BSC 0. 4 0. 2 0. 0 0 5 10 15 20 25 Time (months) Adapted from Bellmunt et al, 2009. 30 35 Anaemia Neutropenia Thrombocytopen ia Fatigue/asthenia Constipation Nausea Stomatitis/muco sitis Alopecia Vomiting Infusion/injectio n site Abdominal pain Myalgia Neuropathy sensory Febrile neutropenia * The eligible population excludes 13 patients who presented at least one major protocol violation at baseline. 1. Bellmunt J et al. J Clin Oncol. 2009; 27: 4454– 4461. Vinflunine + BSC (n=117) (n=248) Grade 3– All grade 4 4 AEs AEs 93 19 61 8 77 50 3 <1 51 6 16 <1 50 48 39 19 16 2 61 25 21 18 <1 <1 29 2 2 0 29 29 0 3 2 15 0 0 27 <1 0 0 16 16 4 3 18 7 6 0 12 1 11 0 6 6 0 0

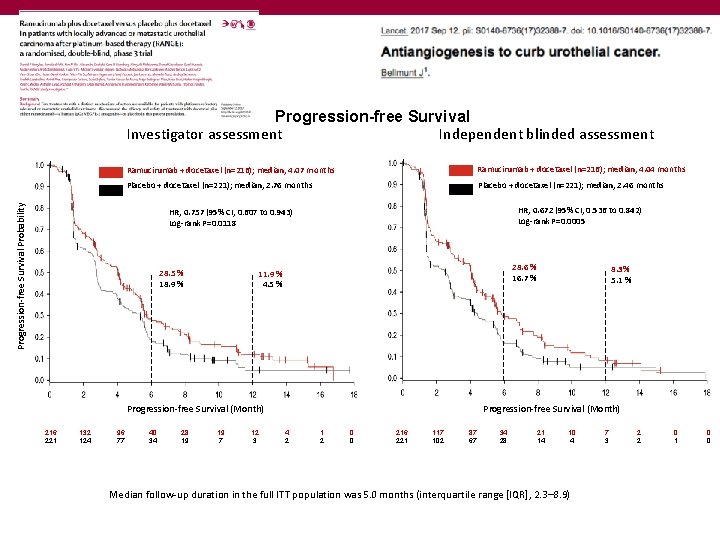
Progression-free Survival Independent blinded assessment Investigator assessment Ramucirumab + docetaxel (n=216); median, 4. 04 months Placebo + docetaxel (n=221); median, 2. 76 months Placebo + docetaxel (n=221); median, 2. 46 months Progression-free Survival Probability Ramucirumab + docetaxel (n=216); median, 4. 07 months HR, 0. 672 (95% CI, 0. 536 to 0. 842) Log-rank P=0. 0005 HR, 0. 757 (95% CI, 0. 607 to 0. 943) Log-rank P=0. 0118 28. 5 % 18. 9 % 28. 6 % 16. 7 % 11. 9 % 4. 5 % Progression-free Survival (Month) 216 221 132 124 96 77 40 34 28 19 19 7 12 3 8. 3% 5. 1 % 4 2 1 2 0 0 216 221 117 102 87 67 34 28 21 14 10 4 Median follow-up duration in the full ITT population was 5. 0 months (interquartile range [IQR], 2. 3– 8. 9) 7 3 2 2 0 1 0 0

Emerging Treatment Options in m. UC: Immunotherapy with Check-point Inhibitors

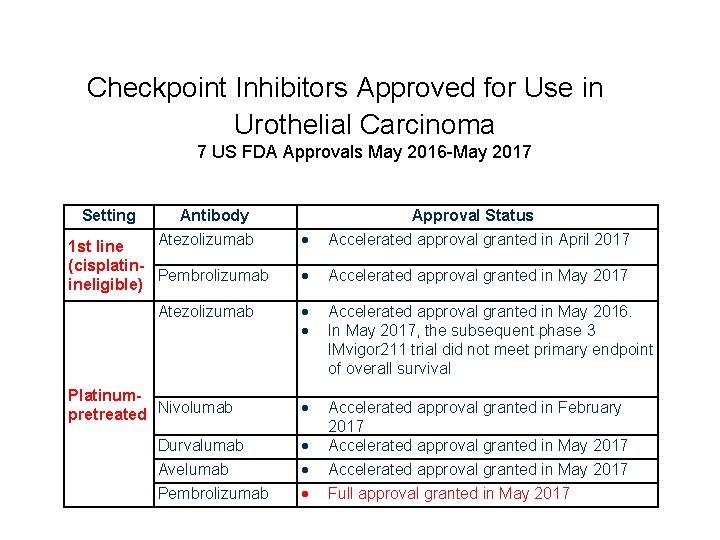
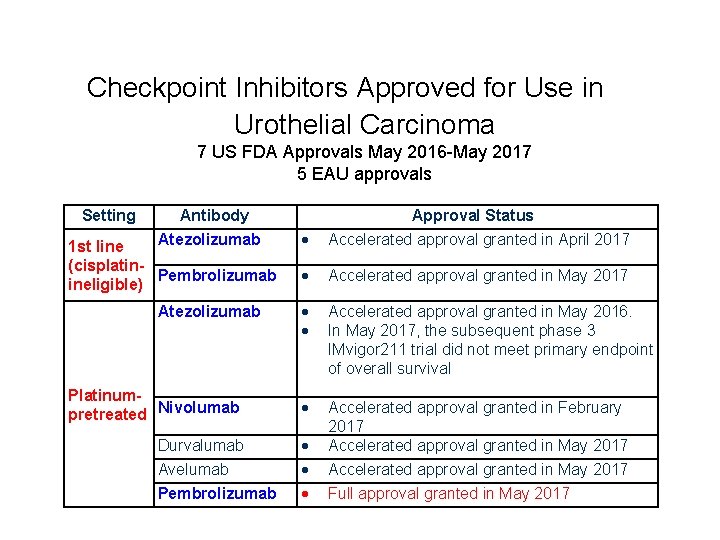
Checkpoint Inhibitors Approved for Use in Urothelial Carcinoma 7 US FDA Approvals May 2016 -May 2017 Setting Antibody Atezolizumab 1 st line (cisplatin. Pembrolizumab ineligible) Atezolizumab Platinumpretreated Nivolumab Durvalumab Avelumab Pembrolizumab Approval Status Accelerated approval granted in April 2017 Accelerated approval granted in May 2017 Accelerated approval granted in May 2016. In May 2017, the subsequent phase 3 IMvigor 211 trial did not meet primary endpoint of overall survival Accelerated approval granted in February 2017 Accelerated approval granted in May 2017 Full approval granted in May 2017

Checkpoint Inhibitors Approved for Use in Urothelial Carcinoma 7 US FDA Approvals May 2016 -May 2017 5 EAU approvals Setting Antibody Atezolizumab 1 st line (cisplatin. Pembrolizumab ineligible) Atezolizumab Platinumpretreated Nivolumab Durvalumab Avelumab Pembrolizumab Approval Status Accelerated approval granted in April 2017 Accelerated approval granted in May 2017 Accelerated approval granted in May 2016. In May 2017, the subsequent phase 3 IMvigor 211 trial did not meet primary endpoint of overall survival Accelerated approval granted in February 2017 Accelerated approval granted in May 2017 Full approval granted in May 2017

OVERVIEW OF LATEST DATA FOR CHECKPOINT INHIBITORS IN BLADDER CANCER

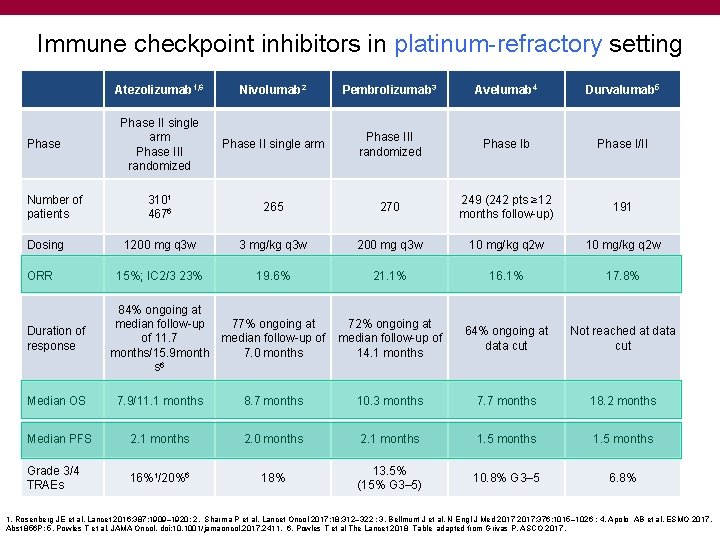
Immune checkpoint inhibitors in platinum-refractory setting Phase Number of patients Dosing ORR Duration of response Atezolizumab 1, 6 Nivolumab 2 Pembrolizumab 3 Avelumab 4 Durvalumab 5 Phase II single arm Phase III randomized Phase Ib Phase I/II 3101 4676 265 270 249 (242 pts ≥ 12 months follow-up) 191 1200 mg q 3 w 3 mg/kg q 3 w 200 mg q 3 w 10 mg/kg q 2 w 15%; IC 2/3 23% 19. 6% 21. 1% 16. 1% 17. 8% 72% ongoing at median follow-up of 14. 1 months 64% ongoing at data cut Not reached at data cut 84% ongoing at median follow-up 77% ongoing at of 11. 7 median follow-up of months/15. 9 month 7. 0 months s 6 Median OS 7. 9/11. 1 months 8. 7 months 10. 3 months 7. 7 months 18. 2 months Median PFS 2. 1 months 2. 0 months 2. 1 months 1. 5 months Grade 3/4 TRAEs 16%1/20%6 18% 13. 5% (15% G 3– 5) 10. 8% G 3‒ 5 6. 8% 1. Rosenberg JE et al. Lancet 2016; 387: 1909– 1920; 2. Sharma P et al. Lancet Oncol 2017; 18: 312– 322 ; 3. Bellmunt J et al. N Engl J Med 2017; 376: 1015– 1026 ; 4. Apolo AB et al. ESMO 2017. Abst 856 P; 5. Powles T et al. JAMA Oncol. doi: 10. 1001/jamaoncol. 2017. 2411. 6. Powles T et al The Lancet 2018 Table adapted from Grivas P. ASCO 2017.

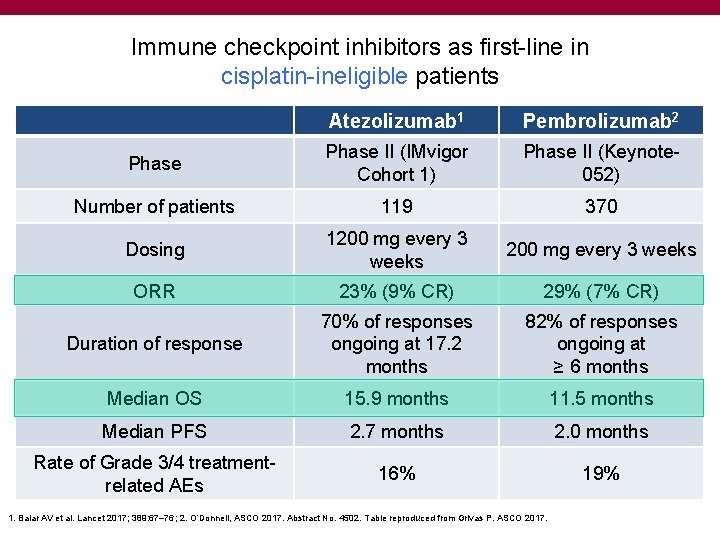
Immune checkpoint inhibitors as first-line in cisplatin-ineligible patients Atezolizumab 1 Pembrolizumab 2 Phase II (IMvigor Cohort 1) Phase II (Keynote 052) Number of patients 119 370 Dosing 1200 mg every 3 weeks ORR 23% (9% CR) 29% (7% CR) Duration of response 70% of responses ongoing at 17. 2 months 82% of responses ongoing at ≥ 6 months Median OS 15. 9 months 11. 5 months Median PFS 2. 7 months 2. 0 months Rate of Grade 3/4 treatmentrelated AEs 16% 19% 1. Balar AV et al. Lancet 2017; 389: 67– 76; 2. O’Donnell, ASCO 2017. Abstract No. 4502. Table reproduced from Grivas P. ASCO 2017.

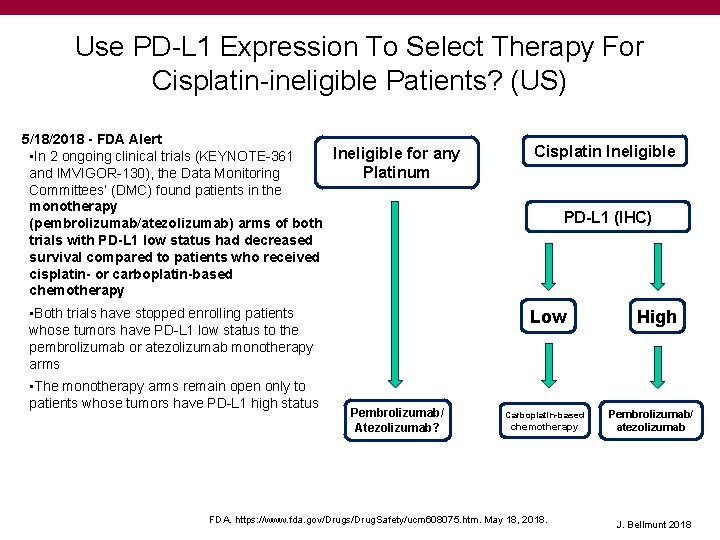
Use PD-L 1 Expression To Select Therapy For Cisplatin-ineligible Patients? (US) 5/18/2018 - FDA Alert Ineligible for any • In 2 ongoing clinical trials (KEYNOTE-361 and IMVIGOR-130), the Data Monitoring Platinum Committees’ (DMC) found patients in the monotherapy (pembrolizumab/atezolizumab) arms of both trials with PD-L 1 low status had decreased survival compared to patients who received cisplatin- or carboplatin-based chemotherapy • Both trials have stopped enrolling patients whose tumors have PD-L 1 low status to the pembrolizumab or atezolizumab monotherapy arms • The monotherapy arms remain open only to patients whose tumors have PD-L 1 high status Cisplatin Ineligible PD-L 1 (IHC) Low Pembrolizumab/ Atezolizumab? Carboplatin-based chemotherapy FDA. https: //www. fda. gov/Drugs/Drug. Safety/ucm 608075. htm. May 18, 2018. High Pembrolizumab/ atezolizumab J. Bellmunt 2018

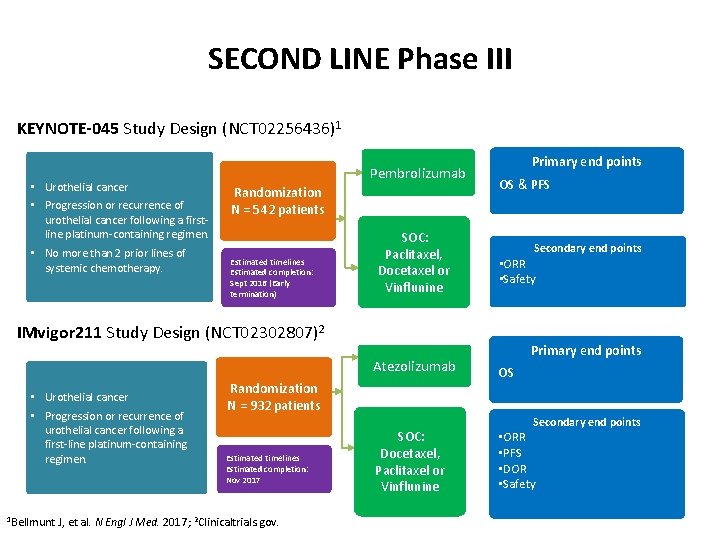
SECOND LINE Phase III KEYNOTE-045 Study Design (NCT 02256436)1 • Urothelial cancer • Progression or recurrence of urothelial cancer following a firstline platinum-containing regimen. • No more than 2 prior lines of systemic chemotherapy. Pembrolizumab Randomization N = 542 patients Estimated timelines Estimated completion: Sept 2016 (Early termination) SOC: Paclitaxel, Docetaxel or Vinflunine Primary end points OS & PFS Secondary end points • ORR • Safety IMvigor 211 Study Design (NCT 02302807)2 Atezolizumab • Urothelial cancer • Progression or recurrence of urothelial cancer following a first-line platinum-containing regimen. 1 Bellmunt Randomization N = 932 patients Estimated timelines Estimated completion: Nov 2017 J, et al. N Engl J Med. 2017; 2 Clinicaltrials. gov. SOC: Docetaxel, Paclitaxel or Vinflunine Primary end points OS Secondary end points • ORR • PFS • DOR • Safety

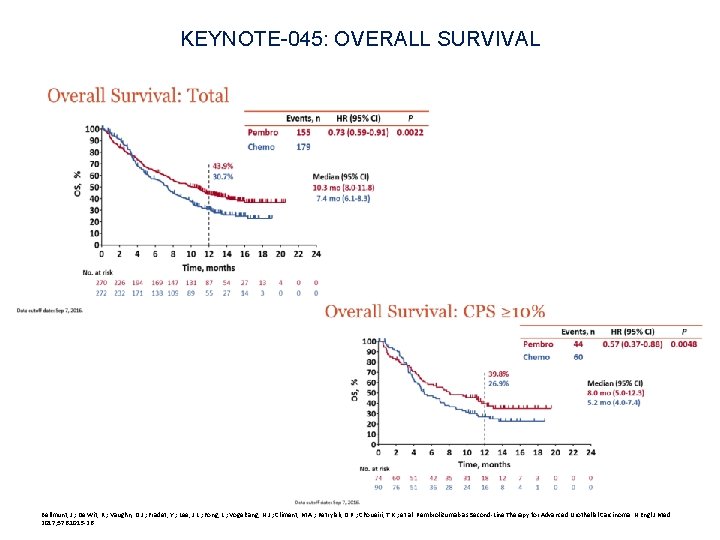
KEYNOTE-045: OVERALL SURVIVAL Bellmunt, J. ; De Wit, R. ; Vaughn, D. J. ; Fradet, Y. ; Lee, J. L. ; Fong, L. ; Vogelzang, N. J. ; Climent, M. A. ; Petrylak, D. P. ; Choueiri, T. K. ; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017; 376: 1015 -26.

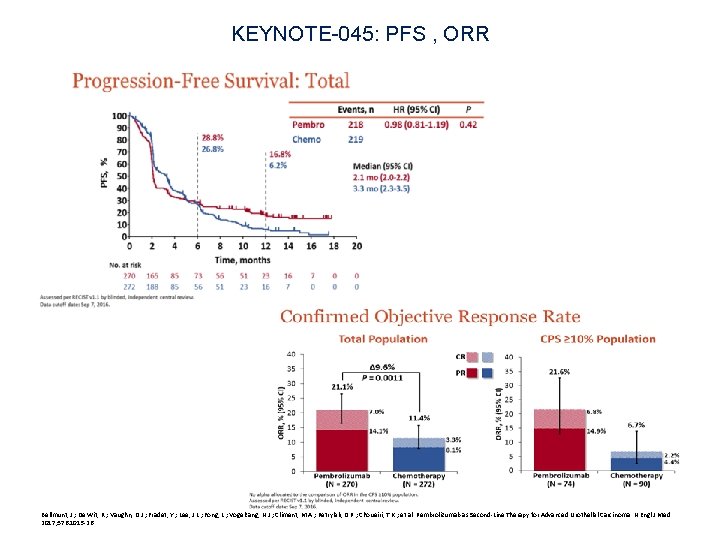
KEYNOTE-045: PFS , ORR Bellmunt, J. ; De Wit, R. ; Vaughn, D. J. ; Fradet, Y. ; Lee, J. L. ; Fong, L. ; Vogelzang, N. J. ; Climent, M. A. ; Petrylak, D. P. ; Choueiri, T. K. ; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017; 376: 1015 -26.

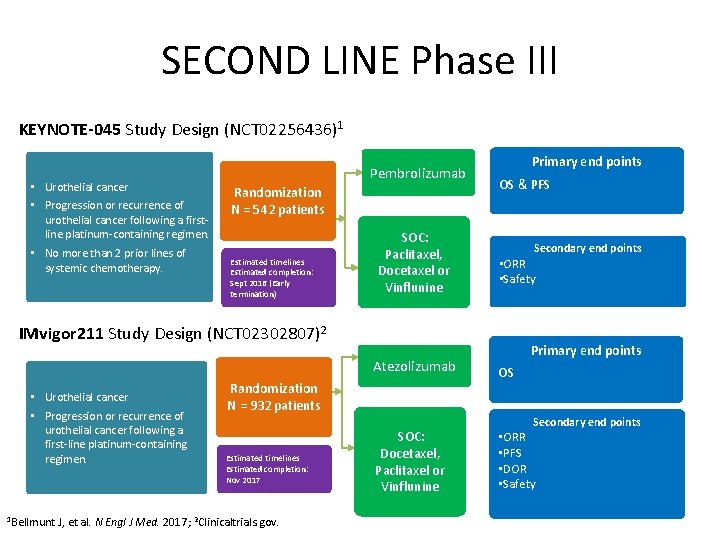
SECOND LINE Phase III KEYNOTE-045 Study Design (NCT 02256436)1 • Urothelial cancer • Progression or recurrence of urothelial cancer following a firstline platinum-containing regimen. • No more than 2 prior lines of systemic chemotherapy. Pembrolizumab Randomization N = 542 patients Estimated timelines Estimated completion: Sept 2016 (Early termination) SOC: Paclitaxel, Docetaxel or Vinflunine Primary end points OS & PFS Secondary end points • ORR • Safety IMvigor 211 Study Design (NCT 02302807)2 Atezolizumab • Urothelial cancer • Progression or recurrence of urothelial cancer following a first-line platinum-containing regimen. 1 Bellmunt Randomization N = 932 patients Estimated timelines Estimated completion: Nov 2017 J, et al. N Engl J Med. 2017; 2 Clinicaltrials. gov. SOC: Docetaxel, Paclitaxel or Vinflunine Primary end points OS Secondary end points • ORR • PFS • DOR • Safety

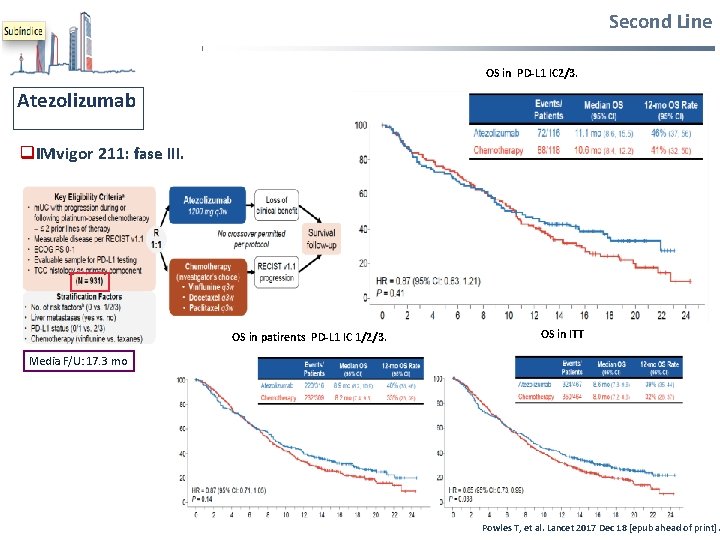
Second Line OS in PD-L 1 IC 2/3. Atezolizumab q. IMvigor 211: fase III. OS in patirents PD-L 1 IC 1/2/3. OS in ITT Media F/U: 17. 3 mo Powles T, et al. Lancet 2017 Dec 18 [epub ahead of print].

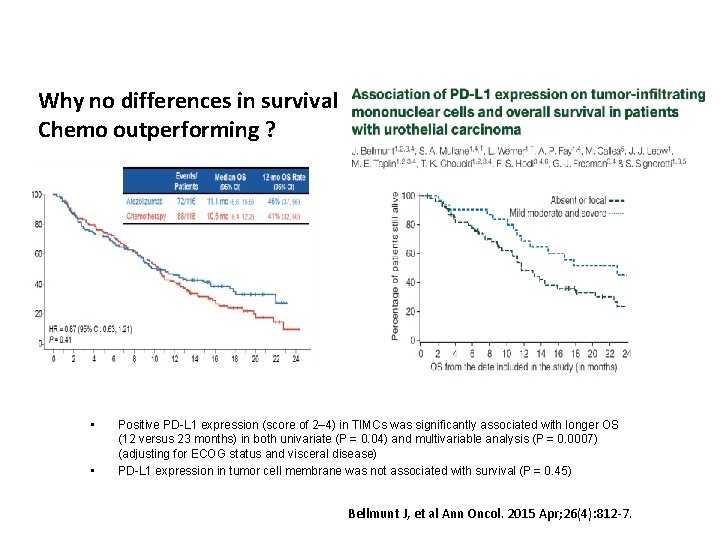
Why no differences in survival Chemo outperforming ? • • Positive PD-L 1 expression (score of 2– 4) in TIMCs was significantly associated with longer OS (12 versus 23 months) in both univariate (P = 0. 04) and multivariable analysis (P = 0. 0007) (adjusting for ECOG status and visceral disease) PD-L 1 expression in tumor cell membrane was not associated with survival (P = 0. 45) Bellmunt J, et al Ann Oncol. 2015 Apr; 26(4): 812 -7.

Atezolizumab beyond the evidence

Long term follow-up data • Phase I & II Atezolizumab • Phase II Nivolumab • Phase III Pembrolizumab

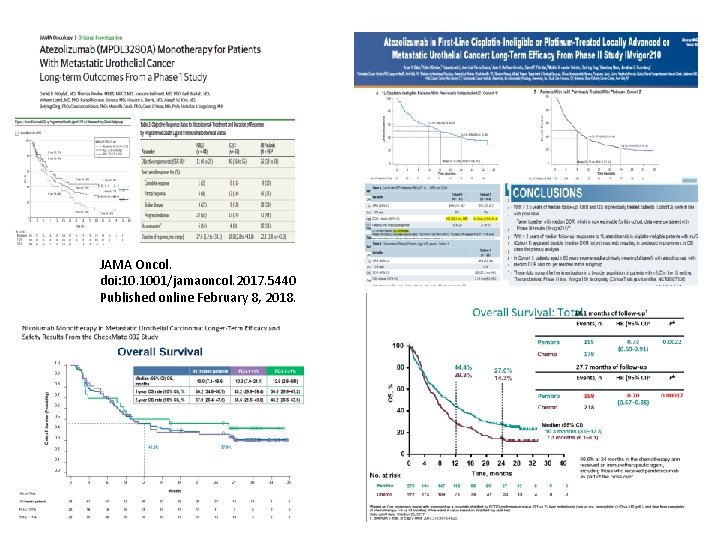
JAMA Oncol. doi: 10. 1001/jamaoncol. 2017. 5440 Published online February 8, 2018.

Evolution of systemic therapy for urothelial cancer to 2018 Publication Paclitaxel (Ph II)2 Docetaxel (Ph II)4 Agencya. Acti on Gemcitabine + paclitaxel (Ph II)7 1997 Gemcitabine authorisation in UK (26 Oct 1995)3 2001 2005 Gemcitabine EMA harmonisation (23 Sep 2008)8 Avelumab (Ph I)17 Nivolumab (Ph II)18 Gemcitabine + cisplatin + paclitaxel (Ph III)12 HD-MVAC (Ph III)6 1993 Durvalumab (Ph I/II)14 Gemcitabine + carboplatin vs MCa. Vi (Ph III)11 Gemcitabine + cisplatin (Ph III)5 MVAC (Ph II)1 1989 Atezolizumab (Ph II) 13 Vinflunine (Ph III)9 2009 Vinflunine EMA approval (21 Sep 2009)10 2013 Durvalumab FDA breakthrough designation (17 Feb 2016)15 Pembrolizumab (Ph I/II)19 2017 2016 Avelumab FDA approval (9 May 2017)21 Atezolizumab FDA approval (18 May 2016)16 Pembrolizumab FDA/EMA approval (18 May 2017) Nivolumab FDA/EMA approval (2 Feb 2017)20 Atezolizumab EMA approval (18 May 2016)16 1. Sternberg CN et al. Cancer 1989; 64: 2448– 2458; 2. Roth BJ et al. J Clin Oncol 1994; 12: 2264– 2270; 3. Eli Lilly. Sm. PC Gemzar® 01 Jul 2014. Available at: http: // www. medicines. org. uk; 4. Mc. Caffrey JA et al. J Clin Oncol 1997; 15: 1853– 1857; 5. Von der Maase H et al. J Clin Oncol 2000; 18: 3068– 3077; 6. Sternberg CN et al. J Clin Oncol 2001; 19: 2638– 2646; 7. Meluch AA et al. J Clin Oncol 2001; 19: 3018– 3024; 8. EMA. EMEA/CHMP/512295/2008; 24 Sep 2008. Available at: http: // www. ema. europa. eu; 9. Bellmunt J et al. J Clin Oncol 2009; 27: 4454– 4461; 10. EMA. EMEA/H/C/000983; 2012. Available at: http: // www. ema. europa. eu; 11. De Santis M et al. J Clin Oncol 2009; 27: 5634– 5639; 12. Bellmunt J et al. J Clin Oncol 2012; 30: 1107– 1113; 13. Rosenberg JE et al. Lancet 2016; 387: 1909– 1920; 14. Massard C et al. ASCO 2016. Abstract #4502 and oral presentation; 15. Astra. Zeneca. Press release 17 Feb 2016. Available at: http: // www. astrazeneca. com; 16. FDA. Press release 18 May 2016. Available at: http: // www. fda. gov); 17. Apolo AB et al. ASCO 2016. Abstract #4514 and poster; 18. Galsky MD et al. ESMO 2016. Abstract #LBA 31_PR; 19. Balar A et al. ESMO 2016. Abstract #LBA 32_PR; 20. FDA. Press release 2 Feb 2017. Available at http: //www. fda. gov; 21. FDA. Press release 9 May 2017. Available at http: //www. fda. gov. All links accessed Sept 2017.

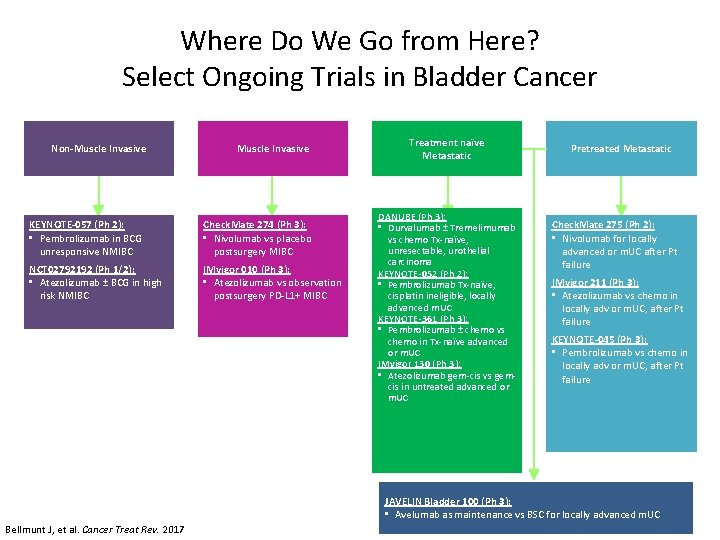
Where Do We Go from Here? Select Ongoing Trials in Bladder Cancer Non-Muscle Invasive KEYNOTE-057 (Ph 2): • Pembrolizumab in BCG unresponsive NMIBC Check. Mate 274 (Ph 3): • Nivolumab vs placebo postsurgery MIBC NCT 02792192 (Ph 1/2): • Atezolizumab ± BCG in high risk NMIBC IMvigor 010 (Ph 3): • Atezolizumab vs observation postsurgery PD-L 1+ MIBC Treatment naïve Metastatic DANUBE (Ph 3): • Durvalumab ± Tremelimumab vs chemo Tx-naïve, unresectable, urothelial carcinoma KEYNOTE-052 (Ph 2): • Pembrolizumab Tx-naïve, cisplatin ineligible, locally advanced m. UC KEYNOTE-361 (Ph 3): • Pembrolizumab ± chemo vs chemo in Tx-naïve advanced or m. UC IMvigor 130 (Ph 3): • Atezolizumab gem-cis vs gemcis in untreated advanced or m. UC Pretreated Metastatic Check. Mate 275 (Ph 2): • Nivolumab for locally advanced or m. UC after Pt failure IMvigor 211 (Ph 3): • Atezolizumab vs chemo in locally adv or m. UC, after Pt failure KEYNOTE-045 (Ph 3): • Pembrolizumab vs chemo in locally adv or m. UC, after Pt failure JAVELIN Bladder 100 (Ph 3): • Avelumab as maintenance vs BSC for locally advanced m. UC Bellmunt J, et al. Cancer Treat Rev. 2017

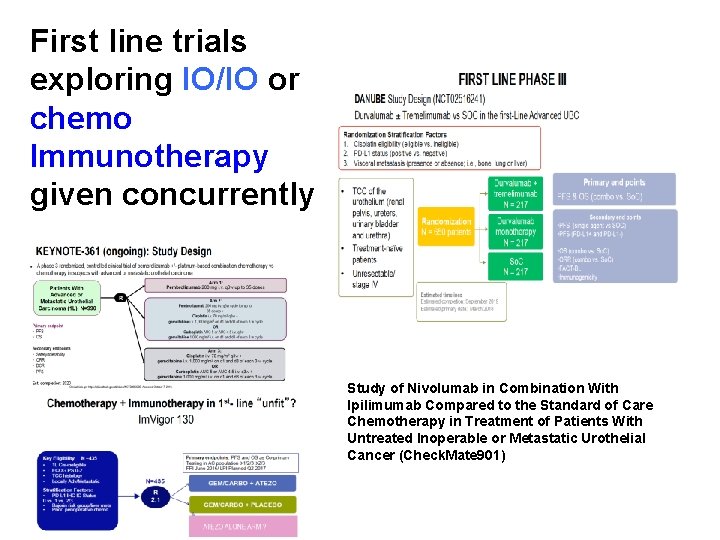
First line trials exploring IO/IO or chemo Immunotherapy given concurrently Study of Nivolumab in Combination With Ipilimumab Compared to the Standard of Care Chemotherapy in Treatment of Patients With Untreated Inoperable or Metastatic Urothelial Cancer (Check. Mate 901)

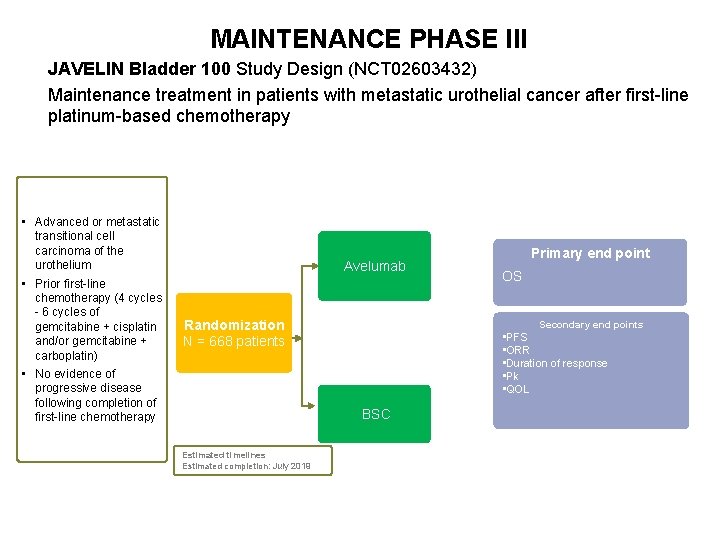
MAINTENANCE PHASE III JAVELIN Bladder 100 Study Design (NCT 02603432) Maintenance treatment in patients with metastatic urothelial cancer after first-line platinum-based chemotherapy • Advanced or metastatic transitional cell carcinoma of the urothelium • Prior first-line chemotherapy (4 cycles - 6 cycles of gemcitabine + cisplatin and/or gemcitabine + carboplatin) • No evidence of progressive disease following completion of first-line chemotherapy Avelumab Randomization N = 668 patients 71 OS Secondary end points • PFS • ORR • Duration of response • Pk • QOL BSC Estimated timelines Estimated completion: July 2019 Primary end point

1 st • Potential synergistic combos – IO/IO – IO/+ other immune approaches • ADC, BSA, Vaccines – IO/targeted therapies 1. - Exhausted Tumor infiltrating lymphocytes express multiple immunoinhibitory receptors: 2. - These are druggable targets for tumor immunotherapy

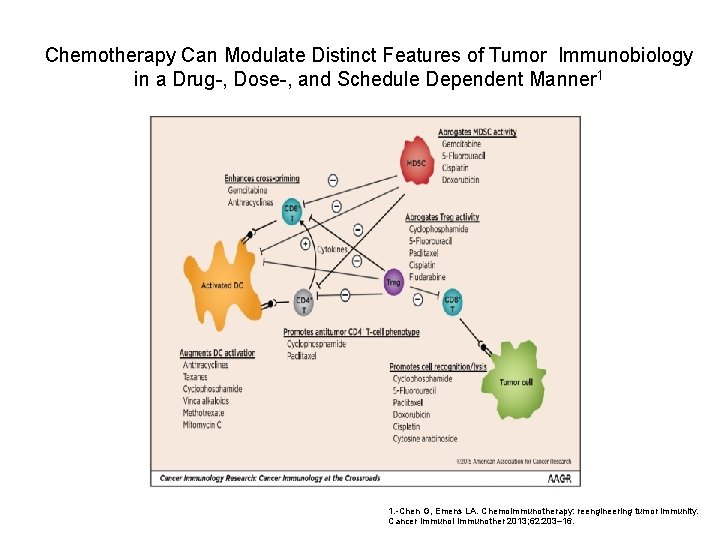
Chemotherapy Can Modulate Distinct Features of Tumor Immunobiology in a Drug-, Dose-, and Schedule Dependent Manner 1 1. -Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013; 62: 203– 16.

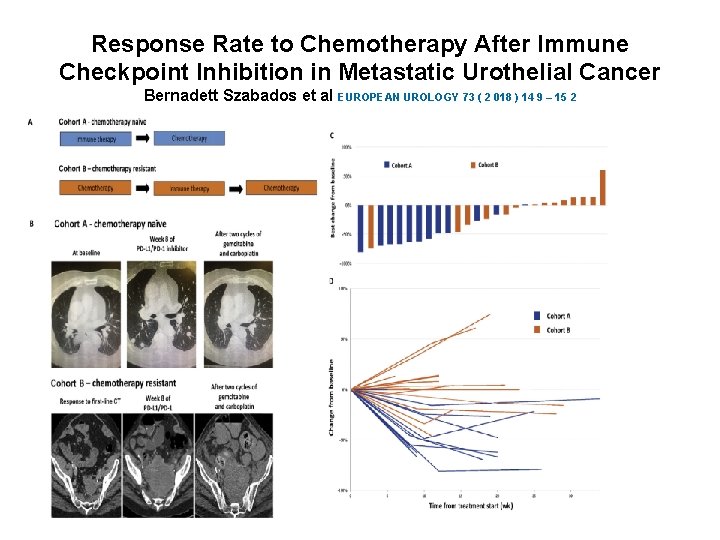
Response Rate to Chemotherapy After Immune Checkpoint Inhibition in Metastatic Urothelial Cancer Bernadett Szabados et al EUROPEAN UROLOGY 73 ( 2 018 ) 14 9 – 15 2

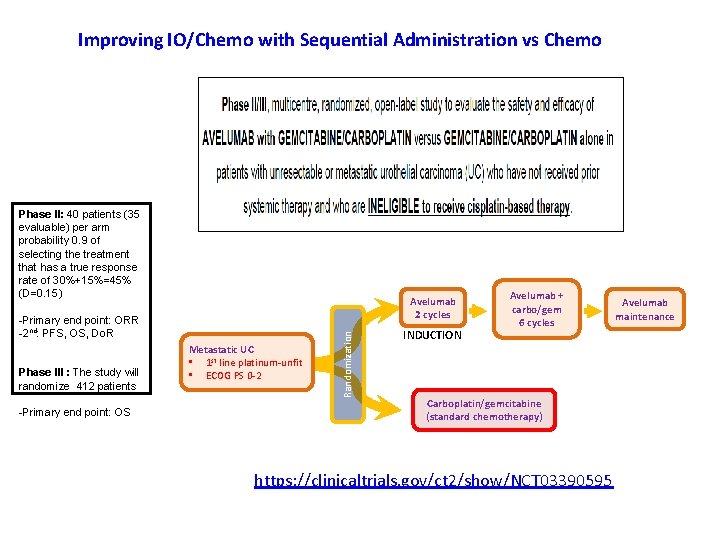
Improving IO/Chemo with Sequential Administration vs Chemo Phase II: 40 patients (35 evaluable) per arm probability 0. 9 of selecting the treatment that has a true response rate of 30%+15%=45% (D=0. 15) -Primary end point: ORR -2 nd: PFS, OS, Do. R Phase III : The study will randomize 412 patients -Primary end point: OS Metastatic UC • 1 st line platinum-unfit • ECOG PS 0 -2 Randomization Avelumab 2 cycles INDUCTION Avelumab + carbo/gem 6 cycles Carboplatin/gemcitabine (standard chemotherapy) https: //clinicaltrials. gov/ct 2/show/NCT 03390595 Avelumab maintenance

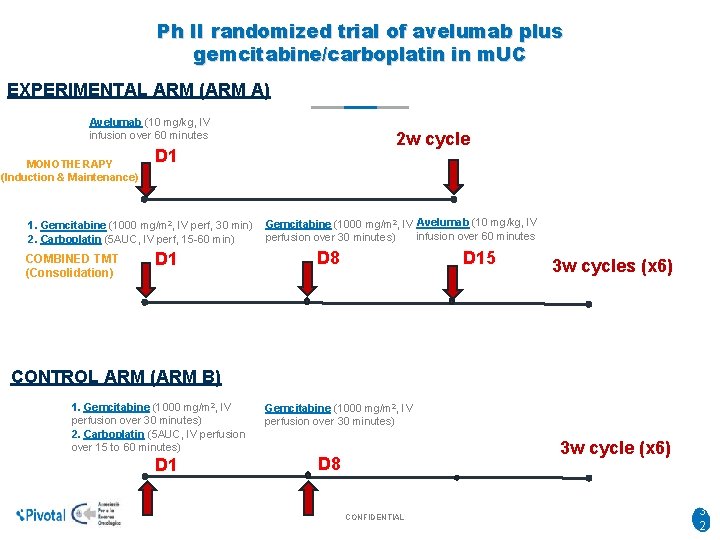
Ph II randomized trial of avelumab plus gemcitabine/carboplatin in m. UC EXPERIMENTAL ARM (ARM A) Avelumab (10 mg/kg, IV infusion over 60 minutes MONOTHERAPY (Induction & Maintenance) D 1 1. Gemcitabine (1000 mg/m 2, IV perf, 30 min) 2. Carboplatin (5 AUC, IV perf, 15 -60 min) COMBINED TMT (Consolidation) 2 w cycle D 1 Gemcitabine (1000 mg/m 2, IV Avelumab (10 mg/kg, IV infusion over 60 minutes perfusion over 30 minutes) D 8 D 15 3 w cycles (x 6) CONTROL ARM (ARM B) 1. Gemcitabine (1000 mg/m 2, IV perfusion over 30 minutes) 2. Carboplatin (5 AUC, IV perfusion over 15 to 60 minutes) D 1 Gemcitabine (1000 mg/m 2, IV perfusion over 30 minutes) 3 w cycle (x 6) D 8 CONFIDENTIAL 3 2

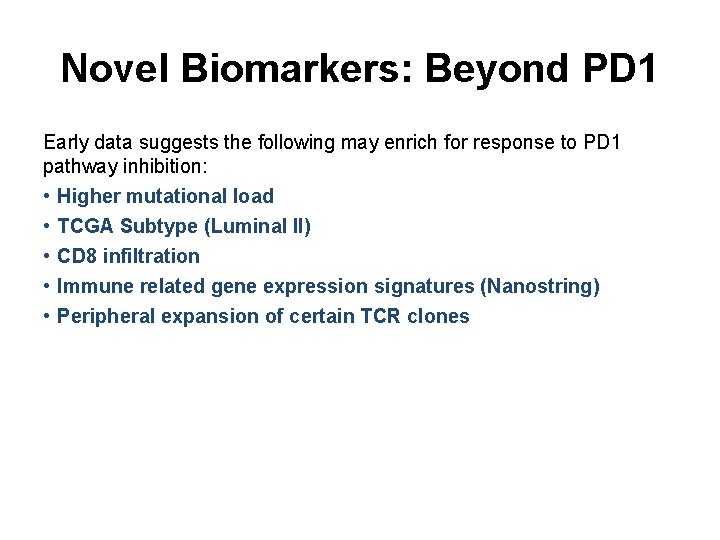
Novel Biomarkers: Beyond PD 1 Early data suggests the following may enrich for response to PD 1 pathway inhibition: • • • Higher mutational load TCGA Subtype (Luminal II) CD 8 infiltration Immune related gene expression signatures (Nanostring) Peripheral expansion of certain TCR clones

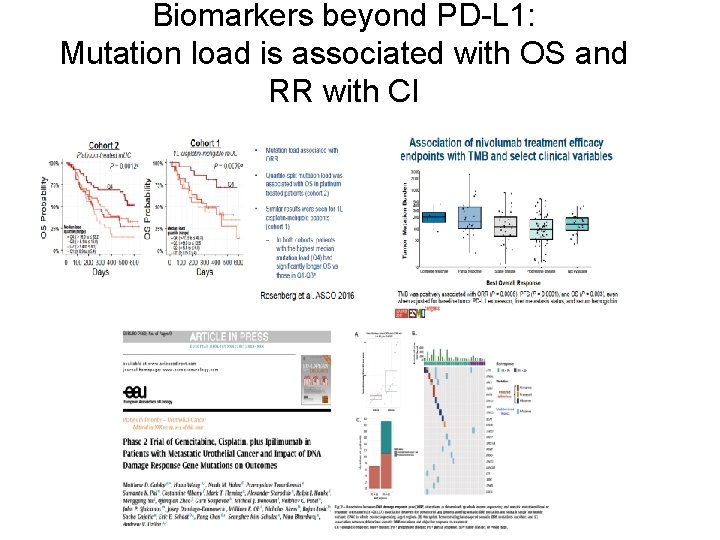
Biomarkers beyond PD-L 1: Mutation load is associated with OS and RR with CI

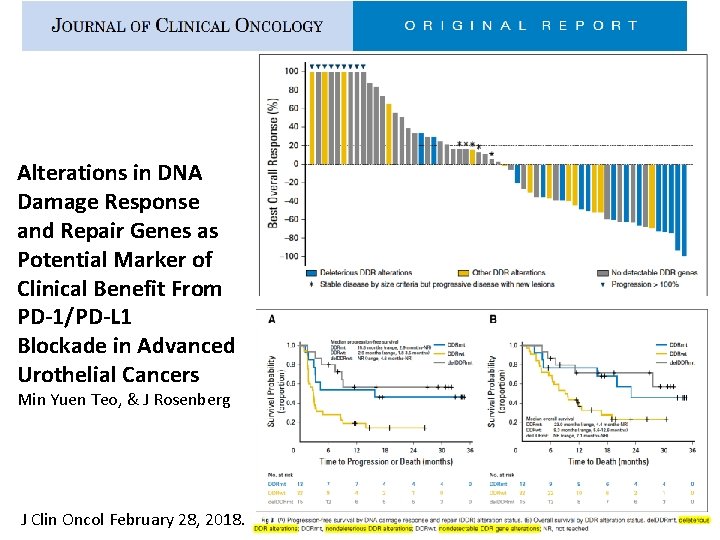
Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L 1 Blockade in Advanced Urothelial Cancers Min Yuen Teo, & J Rosenberg J Clin Oncol February 28, 2018.

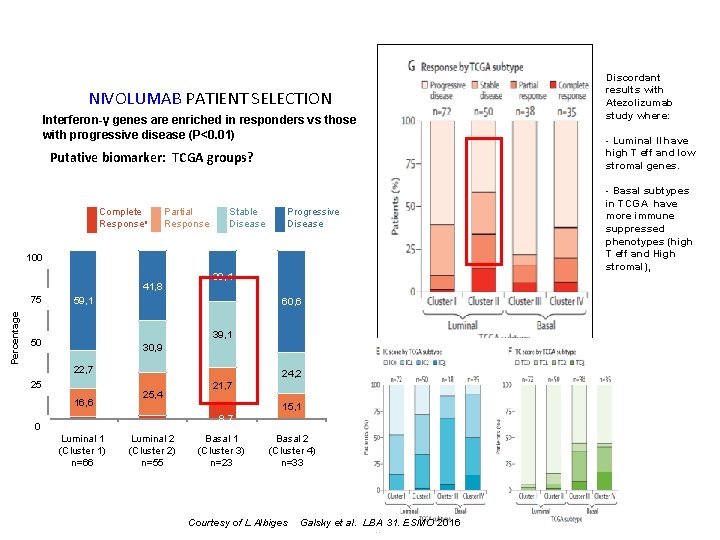
NIVOLUMAB PATIENT SELECTION Interferon-γ genes are enriched in responders vs those with progressive disease (P<0. 01) Putative biomarker: TCGA groups? Complete Responsea Partial Response Stable Disease Progressive Disease 100% 100 Percentage 75% 75 41, 8 59, 1 50% 50 25% 25 60, 6 39, 1 30, 9 22, 7 16, 6 0% 0 30, 4 Cluster 1 Luminal 11) (Luminal (Cluster n=66 1) n=66 24, 2 25, 4 Cluster 2 Luminal 2 (Luminal 2) (Cluster n=55 2) n=55 21, 7 15, 1 8. 7 Cluster 3 Cluster 4 Basal 1 Basal 2 (Basal 1) n=23 (Basal 2) n=33 (Cluster 3) (Cluster 4) n=23 n=33 Courtesy of L Albiges Galsky et al. LBA 31. ESMO 2016 Discordant results with Atezolizumab study where: - Luminal II have high T eff and low stromal genes. - Basal subtypes in TCGA have more immune suppressed phenotypes (high T eff and High stromal),

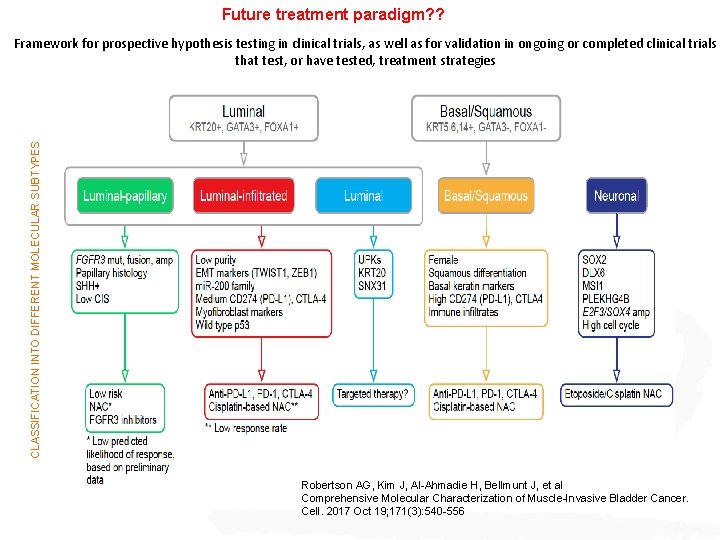
Future treatment paradigm? ? CLASSIFICATION INTO DIFFERENT MOLECULAR SUBTYPES Framework for prospective hypothesis testing in clinical trials, as well as for validation in ongoing or completed clinical trials that test, or have tested, treatment strategies Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, et al Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017 Oct 19; 171(3): 540 -556

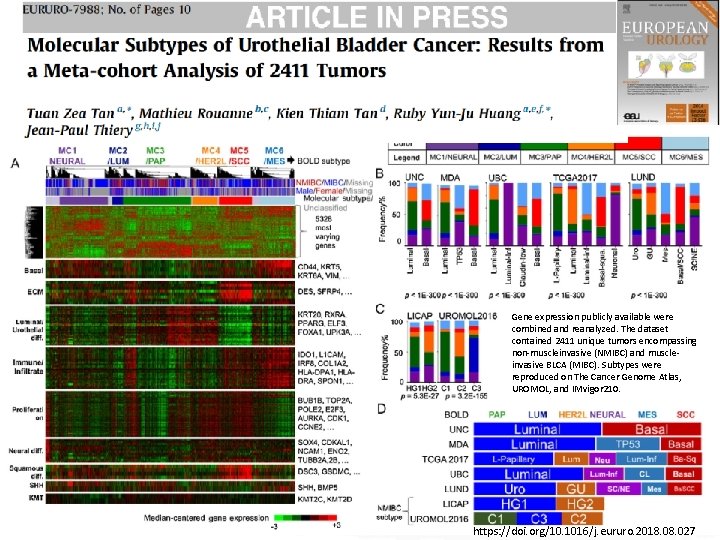
Gene expression publicly available were combined and reanalyzed. The dataset contained 2411 unique tumors encompassing non-muscleinvasive (NMIBC) and muscleinvasive BLCA (MIBC). Subtypes were reproduced on The Cancer Genome Atlas, UROMOL, and IMvigor 210. https: //doi. org/10. 1016/j. eururo. 2018. 027

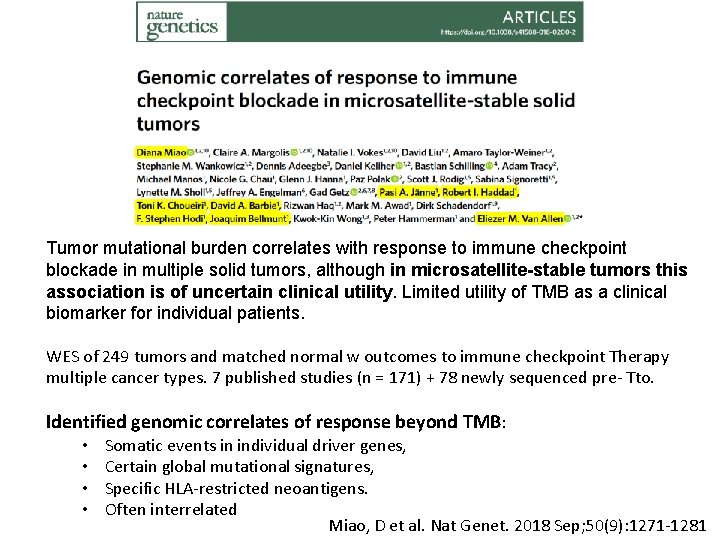
Tumor mutational burden correlates with response to immune checkpoint blockade in multiple solid tumors, although in microsatellite-stable tumors this association is of uncertain clinical utility. Limited utility of TMB as a clinical biomarker for individual patients. WES of 249 tumors and matched normal w outcomes to immune checkpoint Therapy multiple cancer types. 7 published studies (n = 171) + 78 newly sequenced pre- Tto. Identified genomic correlates of response beyond TMB: • • Somatic events in individual driver genes, Certain global mutational signatures, Specific HLA-restricted neoantigens. Often interrelated Miao, D et al. Nat Genet. 2018 Sep; 50(9): 1271 -1281

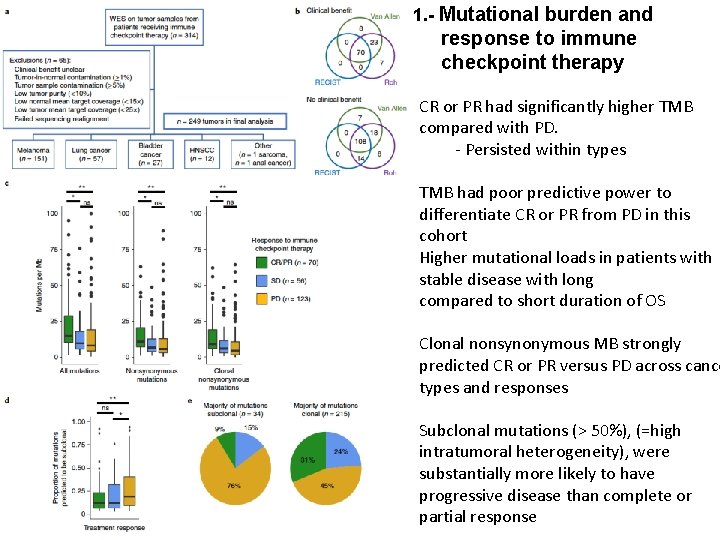
1. - Mutational burden and response to immune checkpoint therapy CR or PR had significantly higher TMB compared with PD. - Persisted within types TMB had poor predictive power to differentiate CR or PR from PD in this cohort Higher mutational loads in patients with stable disease with long compared to short duration of OS Clonal nonsynonymous MB strongly predicted CR or PR versus PD across cance types and responses Subclonal mutations (> 50%), (=high intratumoral heterogeneity), were substantially more likely to have progressive disease than complete or partial response

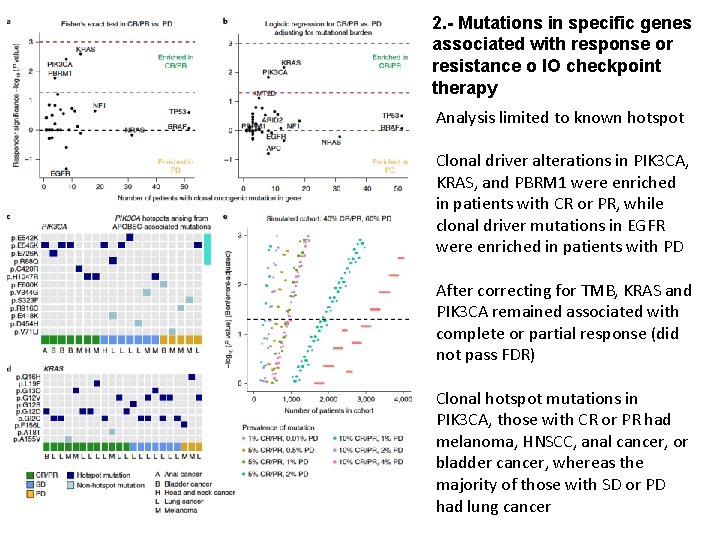
2. - Mutations in specific genes associated with response or resistance o IO checkpoint therapy Analysis limited to known hotspot Clonal driver alterations in PIK 3 CA, KRAS, and PBRM 1 were enriched in patients with CR or PR, while clonal driver mutations in EGFR were enriched in patients with PD After correcting for TMB, KRAS and PIK 3 CA remained associated with complete or partial response (did not pass FDR) Clonal hotspot mutations in PIK 3 CA, those with CR or PR had melanoma, HNSCC, anal cancer, or bladder cancer, whereas the majority of those with SD or PD had lung cancer

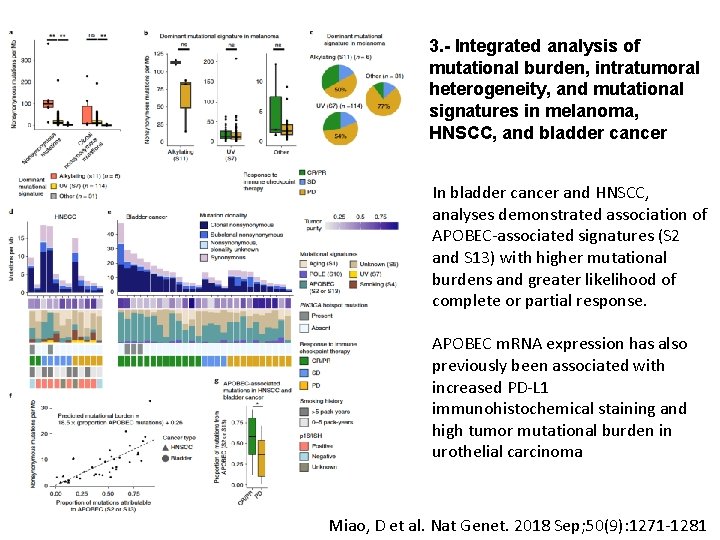
3. - Integrated analysis of mutational burden, intratumoral heterogeneity, and mutational signatures in melanoma, HNSCC, and bladder cancer In bladder cancer and HNSCC, analyses demonstrated association of APOBEC-associated signatures (S 2 and S 13) with higher mutational burdens and greater likelihood of complete or partial response. APOBEC m. RNA expression has also previously been associated with increased PD-L 1 immunohistochemical staining and high tumor mutational burden in urothelial carcinoma Miao, D et al. Nat Genet. 2018 Sep; 50(9): 1271 -1281

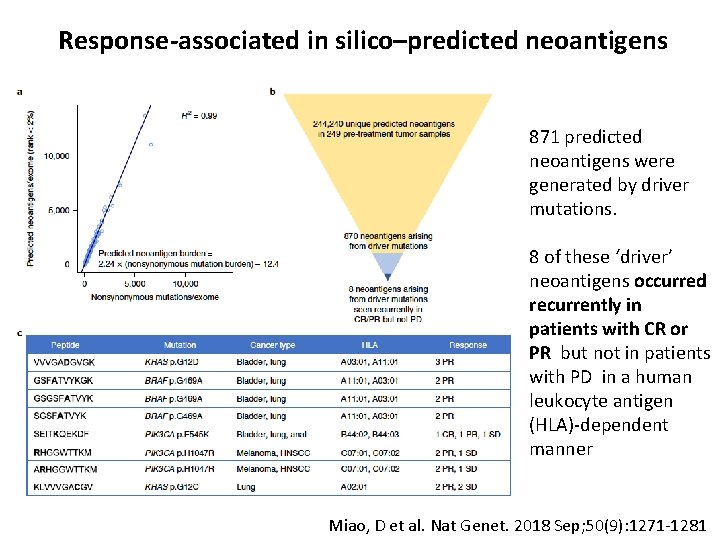
Response-associated in silico–predicted neoantigens 871 predicted neoantigens were generated by driver mutations. 8 of these ‘driver’ neoantigens occurred recurrently in patients with CR or PR but not in patients with PD in a human leukocyte antigen (HLA)-dependent manner Miao, D et al. Nat Genet. 2018 Sep; 50(9): 1271 -1281

TGFbeta papers Any new resistance mechanism ?


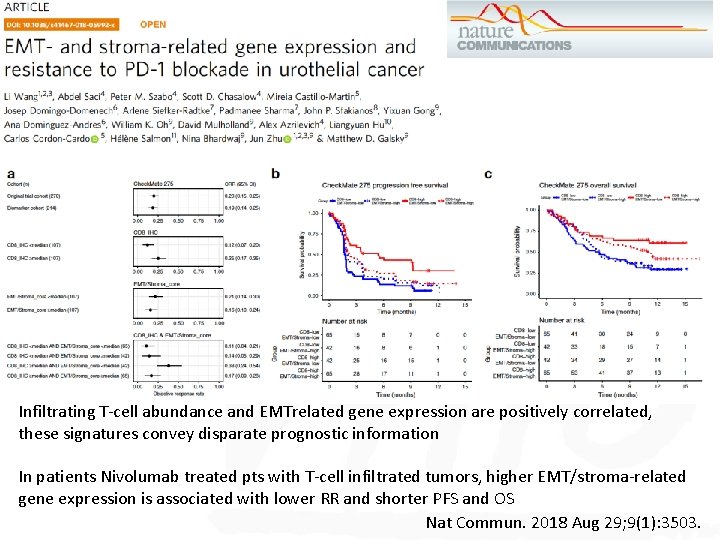
Infiltrating T-cell abundance and EMTrelated gene expression are positively correlated, these signatures convey disparate prognostic information In patients Nivolumab treated pts with T-cell infiltrated tumors, higher EMT/stroma-related gene expression is associated with lower RR and shorter PFS and OS Nat Commun. 2018 Aug 29; 9(1): 3503.

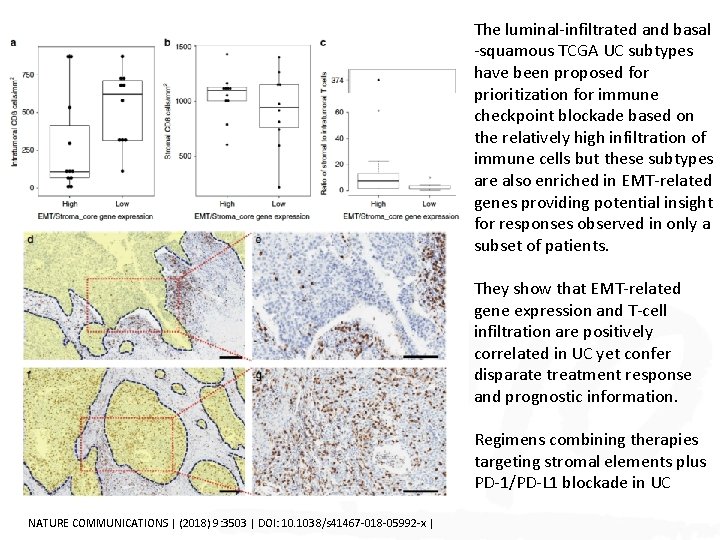
The luminal-infiltrated and basal -squamous TCGA UC subtypes have been proposed for prioritization for immune checkpoint blockade based on the relatively high infiltration of immune cells but these subtypes are also enriched in EMT-related genes providing potential insight for responses observed in only a subset of patients. They show that EMT-related gene expression and T-cell infiltration are positively correlated in UC yet confer disparate treatment response and prognostic information. Regimens combining therapies targeting stromal elements plus PD-1/PD-L 1 blockade in UC NATURE COMMUNICATIONS | (2018) 9: 3503 | DOI: 10. 1038/s 41467 -018 -05992 -x |

Take Home Messages • Immunotherapy in Urothelial Cancer is here to stay • New FDA approvals: • Pembrolizumab and atezolizumab in 1 st line cisplatinunfit (requires PD-L 1+) • First FDA approval in m. UC in the last 30 years • Does PD-L 1 expression guide treatment selection? • Several ongoing phase III trials with chemo-IO or IO-IO combinations for platinum-fit or unfit patients • Promising biomarker results…but still very preliminary
 Cncer
Cncer Investigacionales
Investigacionales Teraputica
Teraputica Teraputica
Teraputica Teraputica
Teraputica Cinetapride
Cinetapride Teraputica
Teraputica Geavlete bogdan
Geavlete bogdan Que letra continua m v t m j
Que letra continua m v t m j D.m. n. 769 del 2018
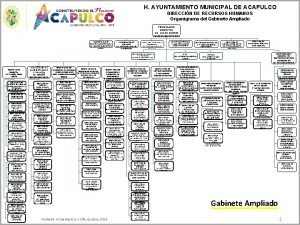
D.m. n. 769 del 2018 Organigrama ayuntamiento de acapulco
Organigrama ayuntamiento de acapulco Zpg biologie
Zpg biologie Ydelsesbeskrivelse 2012
Ydelsesbeskrivelse 2012 Working together to safeguard children’ 2018 summary
Working together to safeguard children’ 2018 summary Astroquiz round 1
Astroquiz round 1 Saasta astro quiz 2018 questions and answers
Saasta astro quiz 2018 questions and answers Astro quiz 2018 questions and answers
Astro quiz 2018 questions and answers Axug 2018
Axug 2018 Data governance forrester
Data governance forrester Tanzania national nutrition survey 2018
Tanzania national nutrition survey 2018 Tanzania national nutrition survey 2020
Tanzania national nutrition survey 2020 Tungsten fabric openstack
Tungsten fabric openstack Global ev outlook 2018
Global ev outlook 2018 Spsp 2018
Spsp 2018 Flacs checkpoint b spanish exam june 2018 answers
Flacs checkpoint b spanish exam june 2018 answers Legea 270 din 2018 cu modificari
Legea 270 din 2018 cu modificari Cohen manion and morrison 2018
Cohen manion and morrison 2018 Informe técnico 1005-2018-servir-gpgsc
Informe técnico 1005-2018-servir-gpgsc Resolución 583 de 2018
Resolución 583 de 2018 Pnp mc 2018-050
Pnp mc 2018-050 Dte lsp program number
Dte lsp program number Prove invalsi oxford
Prove invalsi oxford Persyaratan teknis minimal pupuk organik
Persyaratan teknis minimal pupuk organik Perlem 9 tahun 2018
Perlem 9 tahun 2018 Tsds peims 2017 2018
Tsds peims 2017 2018 Dsr 2019 vol 1
Dsr 2019 vol 1 Osslt format
Osslt format Omai 62 din 2018
Omai 62 din 2018 Nts n° 144-minsa/2018/digesa
Nts n° 144-minsa/2018/digesa Classe d'uso ntc 2018
Classe d'uso ntc 2018 Opanaf 1825/2018
Opanaf 1825/2018 Nom 005-ssa3-2018
Nom 005-ssa3-2018 2018 nys math test
2018 nys math test Techchecklessons 2018
Techchecklessons 2018 Nbr 6023:2018
Nbr 6023:2018 2018 nds
2018 nds Gamsat duration
Gamsat duration Advanced group policy management
Advanced group policy management Program kerja omk
Program kerja omk