Continuum of care en cncer de colon metastsico




- Slides: 52

“Continuum of care” en cáncer de colon metastásico no curable Mauricio Lema Medina MD Clínica de Oncología Astorga – Clínica SOMA – Medicáncer Medellín, Colombia

Quimioterapia A qué llegamos?

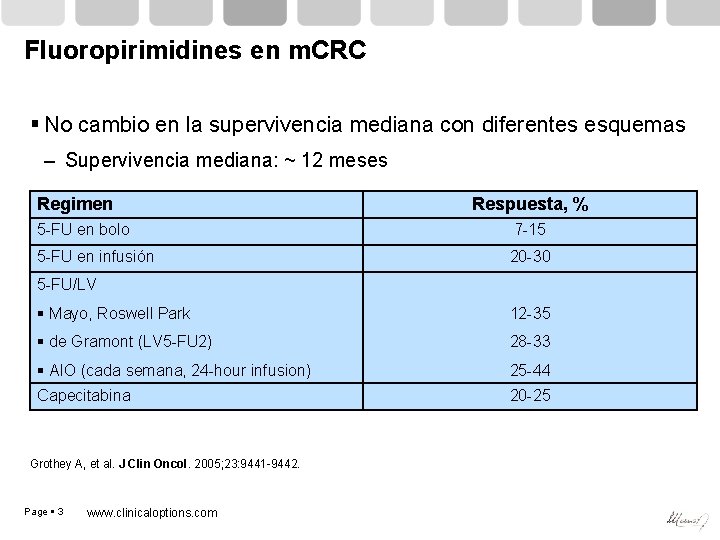
Fluoropirimidines en m. CRC No cambio en la supervivencia mediana con diferentes esquemas – Supervivencia mediana: ~ 12 meses Regimen Respuesta, % 5 -FU en bolo 7 -15 5 -FU en infusión 20 -30 5 -FU/LV Mayo, Roswell Park 12 -35 de Gramont (LV 5 -FU 2) 28 -33 AIO (cada semana, 24 -hour infusion) Capecitabina 25 -44 Grothey A, et al. J Clin Oncol. 2005; 23: 9441 -9442. Page 3 www. clinicaloptions. com 20 -25

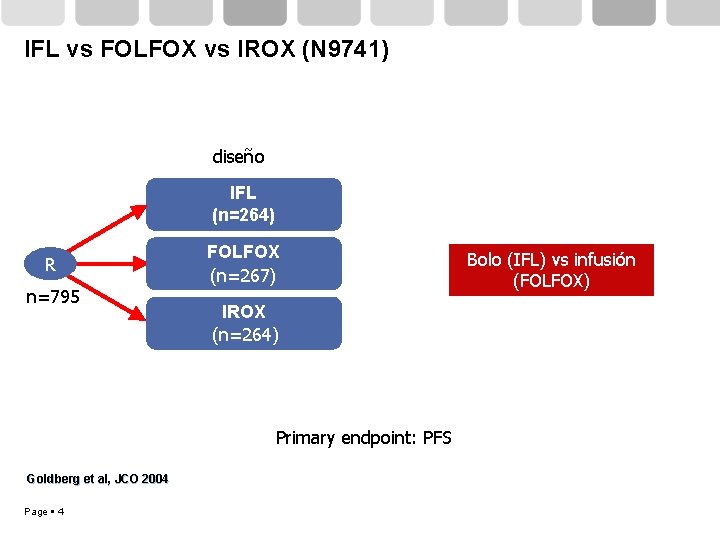
IFL vs FOLFOX vs IROX (N 9741) diseño IFL (n=264) R n=795 FOLFOX (n=267) IROX (n=264) Primary endpoint: PFS Goldberg et al, JCO 2004 Page 4 Bolo (IFL) vs infusión (FOLFOX)

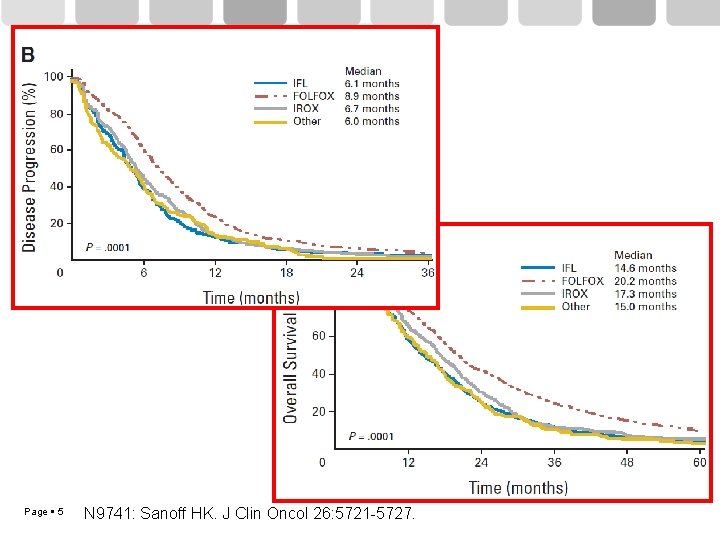
Page 5 N 9741: Sanoff HK. J Clin Oncol 26: 5721 -5727.

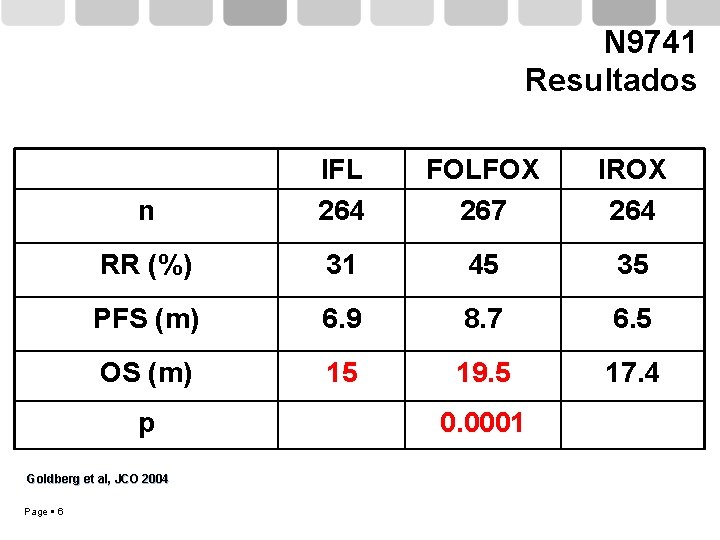
N 9741 Resultados n IFL 264 FOLFOX 267 IROX 264 RR (%) 31 45 35 PFS (m) 6. 9 8. 7 6. 5 OS (m) 15 19. 5 17. 4 p Goldberg et al, JCO 2004 Page 6 0. 0001

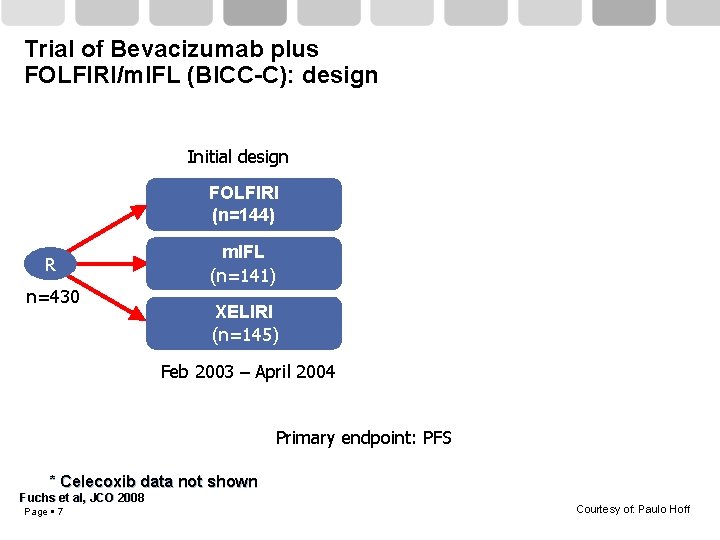
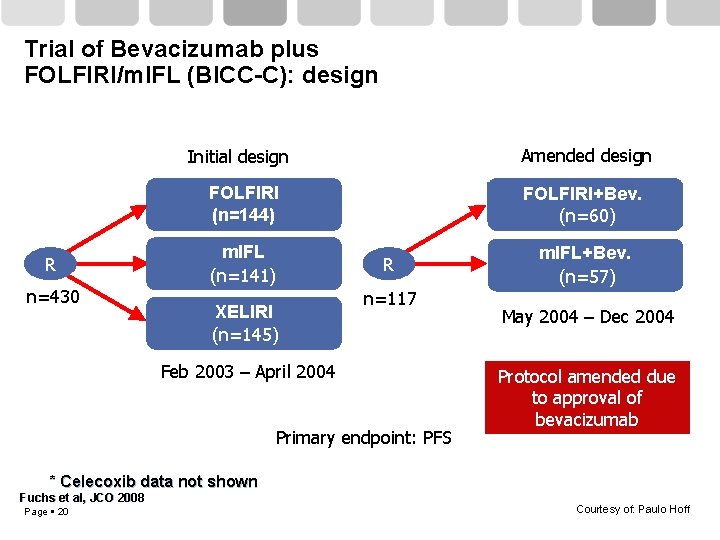
Trial of Bevacizumab plus FOLFIRI/m. IFL (BICC-C): design Initial design FOLFIRI (n=144) R n=430 m. IFL (n=141) XELIRI (n=145) Feb 2003 – April 2004 Primary endpoint: PFS * Celecoxib data not shown Fuchs et al, JCO 2008 Page 7 Courtesy of: Paulo Hoff

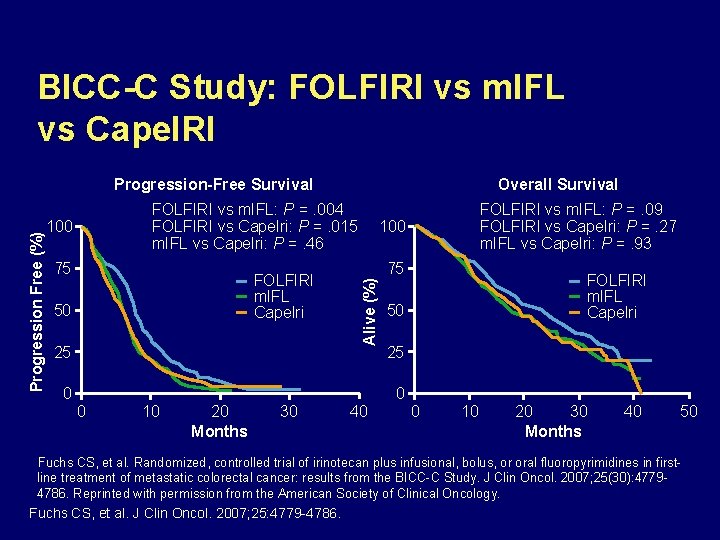
BICC-C Study: FOLFIRI vs m. IFL vs Cape. IRI FOLFIRI vs m. IFL: P =. 004 FOLFIRI vs Capelri: P =. 015 m. IFL vs Capelri: P =. 46 100 75 FOLFIRI m. IFL Capelri 50 25 0 Overall Survival 0 10 20 Months 30 FOLFIRI vs m. IFL: P =. 09 FOLFIRI vs Capelri: P =. 27 m. IFL vs Capelri: P =. 93 100 75 Alive (%) Progression Free (%) Progression-Free Survival 40 FOLFIRI m. IFL Capelri 50 25 0 0 10 20 30 Months 40 50 Fuchs CS, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in firstline treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007; 25(30): 47794786. Reprinted with permission from the American Society of Clinical Oncology. Fuchs CS, et al. J Clin Oncol. 2007; 25: 4779 -4786.

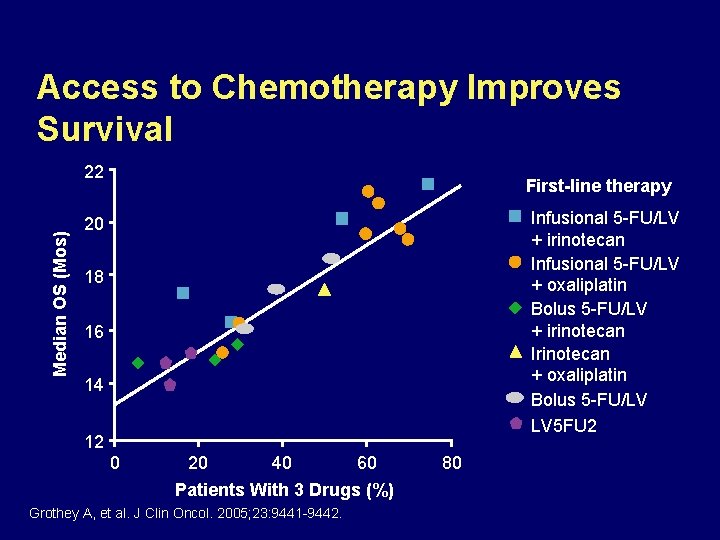
Access to Chemotherapy Improves Survival Median OS (Mos) 22 First-line therapy Infusional 5 -FU/LV + irinotecan Infusional 5 -FU/LV + oxaliplatin Bolus 5 -FU/LV + irinotecan Irinotecan + oxaliplatin Bolus 5 -FU/LV LV 5 FU 2 20 18 16 14 12 0 20 40 60 Patients With 3 Drugs (%) Grothey A, et al. J Clin Oncol. 2005; 23: 9441 -9442. 80

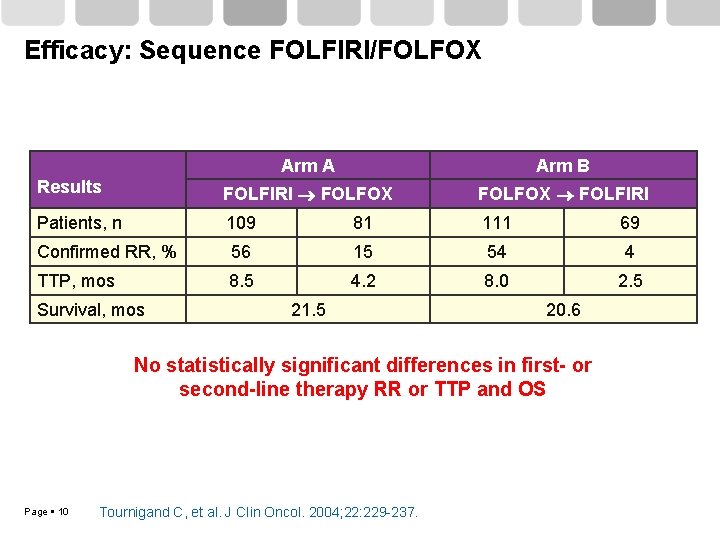
Efficacy: Sequence FOLFIRI/FOLFOX Arm A Arm B Results FOLFIRI FOLFOX FOLFIRI Patients, n 109 81 111 69 Confirmed RR, % 56 15 54 4 TTP, mos 8. 5 4. 2 8. 0 2. 5 Survival, mos 21. 5 20. 6 No statistically significant differences in first- or second-line therapy RR or TTP and OS Page 10 Tournigand C, et al. J Clin Oncol. 2004; 22: 229 -237.

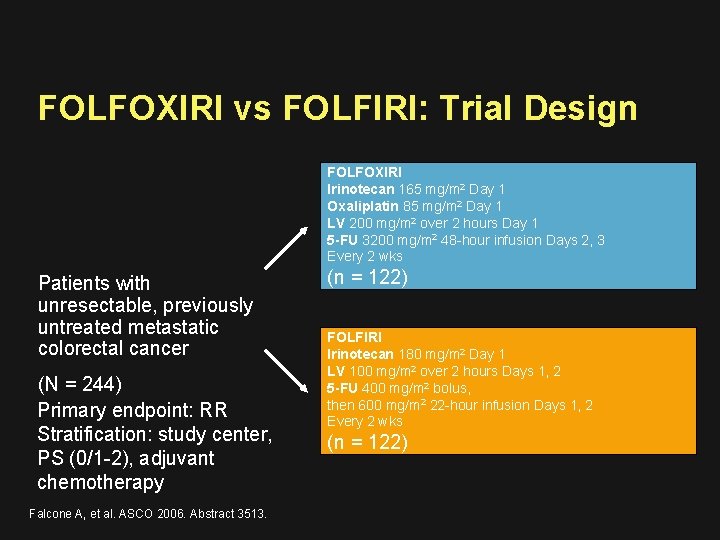
FOLFOXIRI vs FOLFIRI: Trial Design FOLFOXIRI Irinotecan 165 mg/m 2 Day 1 Oxaliplatin 85 mg/m 2 Day 1 LV 200 mg/m 2 over 2 hours Day 1 5 -FU 3200 mg/m 2 48 -hour infusion Days 2, 3 Every 2 wks Patients with unresectable, previously untreated metastatic colorectal cancer (N = 244) Primary endpoint: RR Stratification: study center, PS (0/1 -2), adjuvant chemotherapy Falcone A, et al. ASCO 2006. Abstract 3513. (n = 122) FOLFIRI Irinotecan 180 mg/m 2 Day 1 LV 100 mg/m 2 over 2 hours Days 1, 2 5 -FU 400 mg/m 2 bolus, then 600 mg/m 2 22 -hour infusion Days 1, 2 Every 2 wks (n = 122)

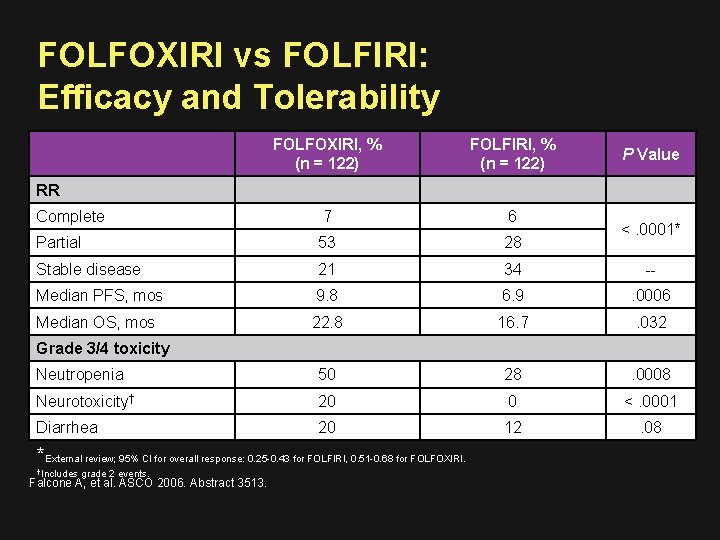
FOLFOXIRI vs FOLFIRI: Efficacy and Tolerability FOLFOXIRI, % (n = 122) FOLFIRI, % (n = 122) Complete 7 6 Partial 53 28 Stable disease 21 34 -- Median PFS, mos 9. 8 6. 9 . 0006 Median OS, mos 22. 8 16. 7 . 032 Neutropenia 50 28 . 0008 Neurotoxicity† 20 0 <. 0001 Diarrhea 20 12 . 08 P Value RR <. 0001* Grade 3/4 toxicity *External review; 95% CI for overall response: 0. 25 -0. 43 for FOLFIRI, 0. 51 -0. 68 for FOLFOXIRI. †Includes grade 2 events. Falcone A, et al. ASCO 2006. Abstract 3513.

Quimioterapia más Bevacizumab

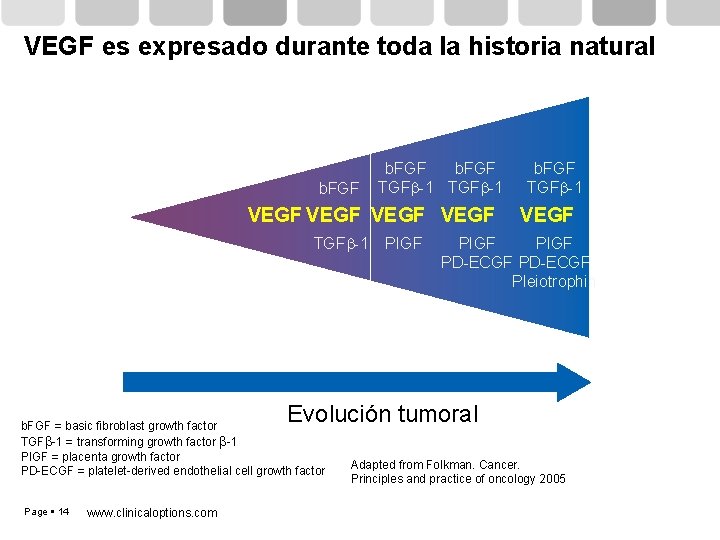
VEGF es expresado durante toda la historia natural b. FGF TGFb-1 VEGF TGFb-1 PIGF b. FGF TGFb-1 VEGF PIGF PD-ECGF Pleiotrophin Evolución tumoral b. FGF = basic fibroblast growth factor TGFb-1 = transforming growth factor b-1 PIGF = placenta growth factor PD-ECGF = platelet-derived endothelial cell growth factor Page 14 www. clinicaloptions. com Adapted from Folkman. Cancer. Principles and practice of oncology 2005

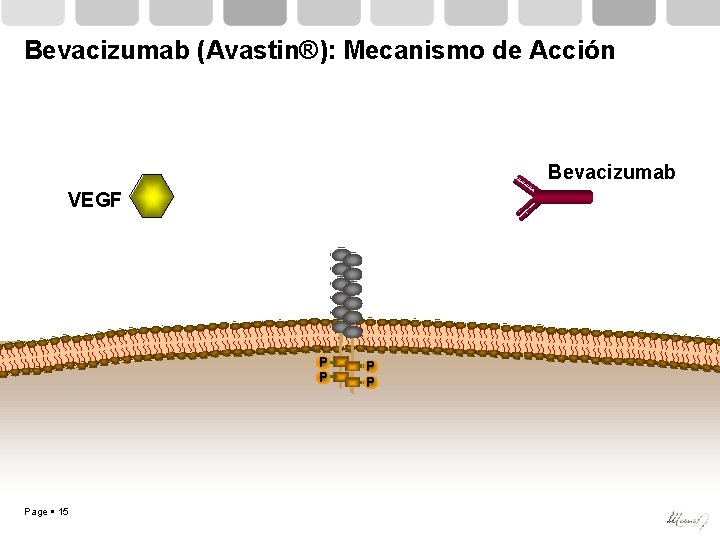
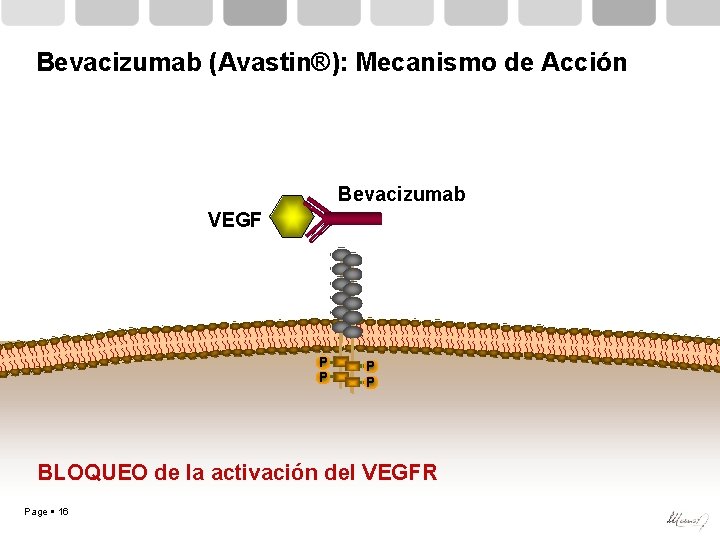
Bevacizumab (Avastin®): Mecanismo de Acción Bevacizumab VEGF P P Page 15 P P

Bevacizumab (Avastin®): Mecanismo de Acción Bevacizumab VEGF P P BLOQUEO de la activación del VEGFR Page 16

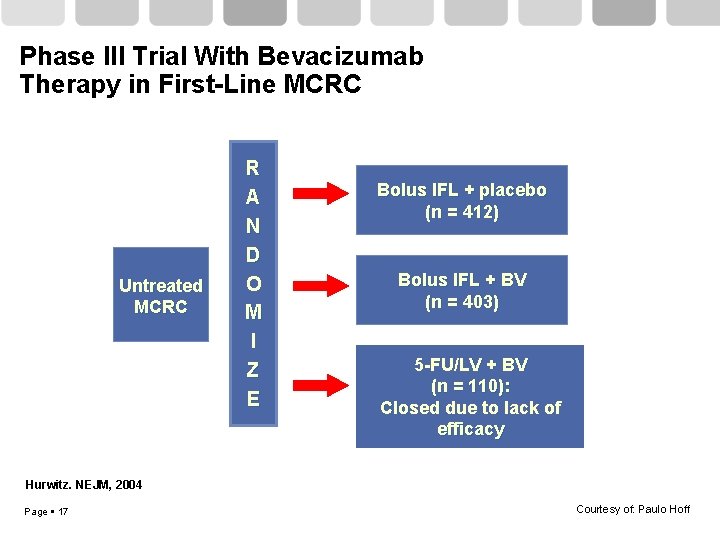
Phase III Trial With Bevacizumab Therapy in First-Line MCRC Untreated MCRC R A N D O M I Z E Bolus IFL + placebo (n = 412) Bolus IFL + BV (n = 403) 5 -FU/LV + BV (n = 110): Closed due to lack of efficacy Hurwitz. NEJM, 2004 Page 17 Courtesy of: Paulo Hoff

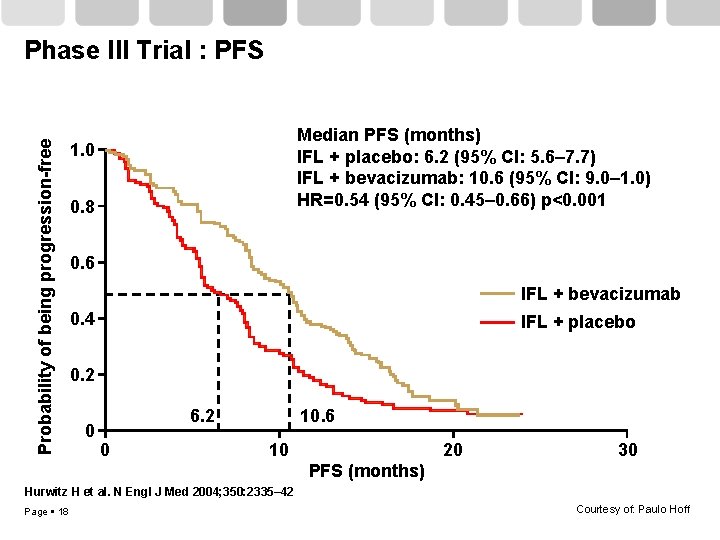
Probability of being progression-free Phase III Trial : PFS Median PFS (months) IFL + placebo: 6. 2 (95% CI: 5. 6– 7. 7) IFL + bevacizumab: 10. 6 (95% CI: 9. 0– 1. 0) HR=0. 54 (95% CI: 0. 45– 0. 66) p<0. 001 1. 0 0. 8 0. 6 IFL + bevacizumab 0. 4 IFL + placebo 0. 2 0 6. 2 0 10. 6 10 20 30 PFS (months) Hurwitz H et al. N Engl J Med 2004; 350: 2335– 42 Page 18 Courtesy of: Paulo Hoff

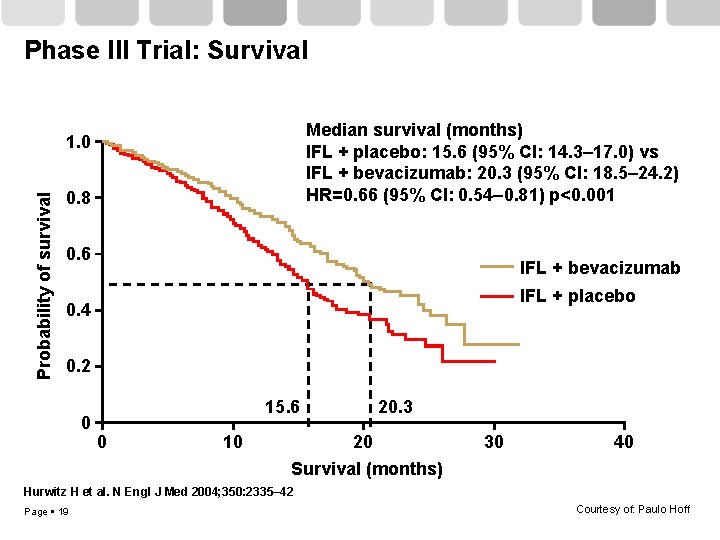
Phase III Trial: Survival Median survival (months) IFL + placebo: 15. 6 (95% CI: 14. 3– 17. 0) vs IFL + bevacizumab: 20. 3 (95% CI: 18. 5– 24. 2) HR=0. 66 (95% CI: 0. 54– 0. 81) p<0. 001 Probability of survival 1. 0 0. 8 0. 6 IFL + bevacizumab IFL + placebo 0. 4 0. 2 0 15. 6 0 10 20. 3 20 Survival (months) 30 40 Hurwitz H et al. N Engl J Med 2004; 350: 2335– 42 Page 19 Courtesy of: Paulo Hoff

Trial of Bevacizumab plus FOLFIRI/m. IFL (BICC-C): design R n=430 Initial design Amended design FOLFIRI (n=144) FOLFIRI+Bev. (n=60) m. IFL (n=141) XELIRI (n=145) R n=117 Feb 2003 – April 2004 Primary endpoint: PFS m. IFL+Bev. (n=57) May 2004 – Dec 2004 Protocol amended due to approval of bevacizumab * Celecoxib data not shown Fuchs et al, JCO 2008 Page 20 Courtesy of: Paulo Hoff

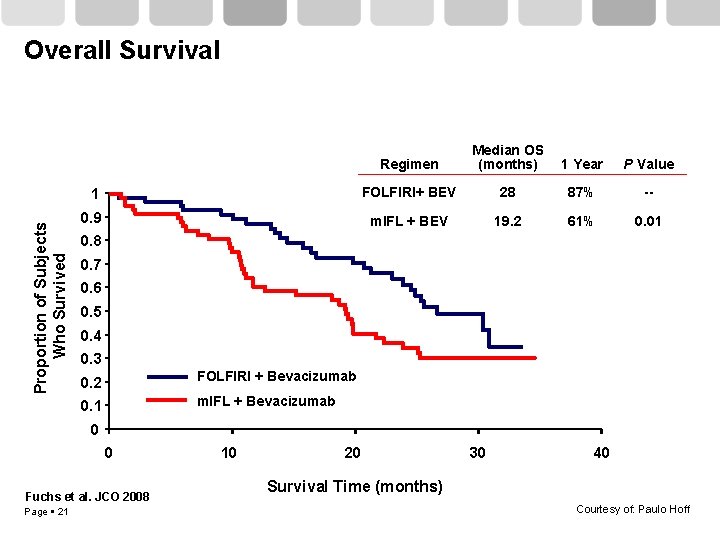
Proportion of Subjects Who Survived Overall Survival Regimen Median OS (months) 1 Year P Value 1 FOLFIRI+ BEV 28 87% -- 0. 9 m. IFL + BEV 19. 2 61% 0. 01 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 FOLFIRI + Bevacizumab 0. 1 m. IFL + Bevacizumab 0 0 Fuchs et al. JCO 2008 Page 21 10 20 30 40 Survival Time (months) Courtesy of: Paulo Hoff

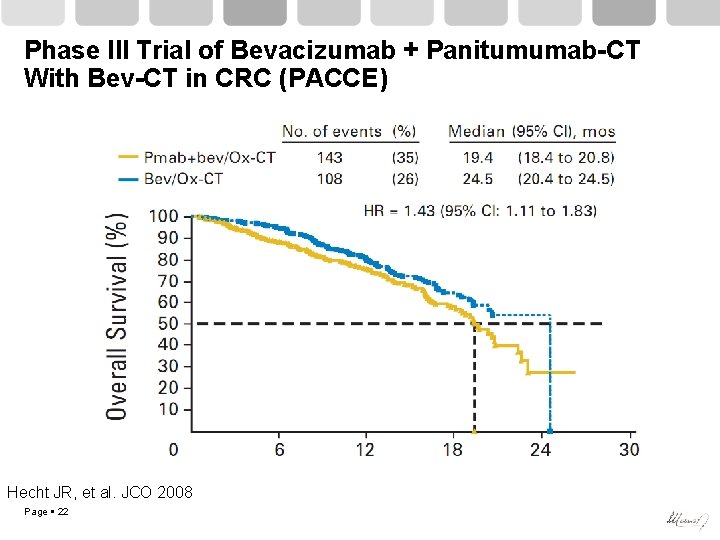
Phase III Trial of Bevacizumab + Panitumumab-CT With Bev-CT in CRC (PACCE) Hecht JR, et al. JCO 2008 Page 22

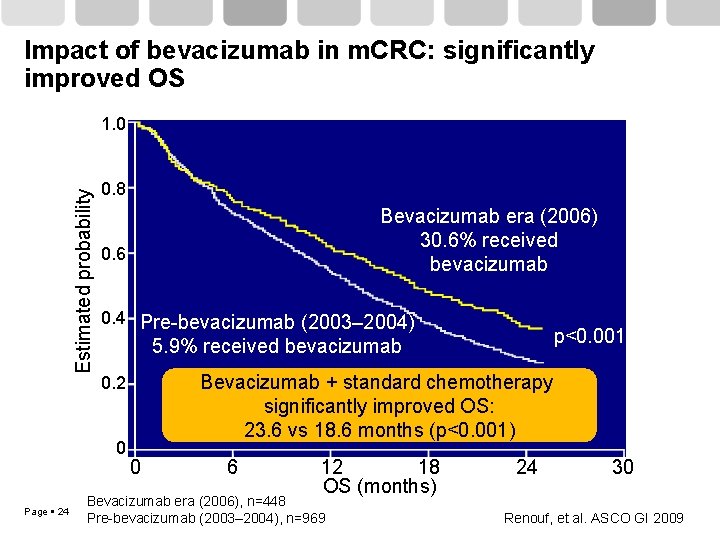
Impact of bevacizumab on OS in m. CRC: a population-based study Patients with m. CRC (n=1, 417): 2003– 2004 (pre-bevacizumab) versus 2006 (post-bevacizumab) Proportion of patients receiving Irinotecan or oxaliplatin and 5 -FU: no change (p=0. 68) Anti-EGFR therapy: no change (p=0. 63) Bevacizumab therapy: increased 5. 9% vs 30. 6% (p<0. 001) Addition of bevacizumab to systemic chemotherapy significantly improved OS: 23. 6 vs 18. 6 months (p<0. 001) Page 23 Renouf, et al. ASCO GI 2009

Impact of bevacizumab in m. CRC: significantly improved OS Estimated probability 1. 0 0. 8 0. 6 0. 4 Pre-bevacizumab (2003– 2004) p<0. 001 5. 9% received bevacizumab Bevacizumab + standard chemotherapy significantly improved OS: 23. 6 vs 18. 6 months (p<0. 001) 0. 2 0 Page 24 Bevacizumab era (2006) 30. 6% received bevacizumab 0 6 12 18 OS (months) Bevacizumab era (2006), n=448 Pre-bevacizumab (2003– 2004), n=969 24 30 Renouf, et al. ASCO GI 2009

Phase IV BRi. TE Therapy in First-Line MCRC Untreated MCRC Page 25 E N R O L L Bev + CT Grothey, et al. JCO 2008

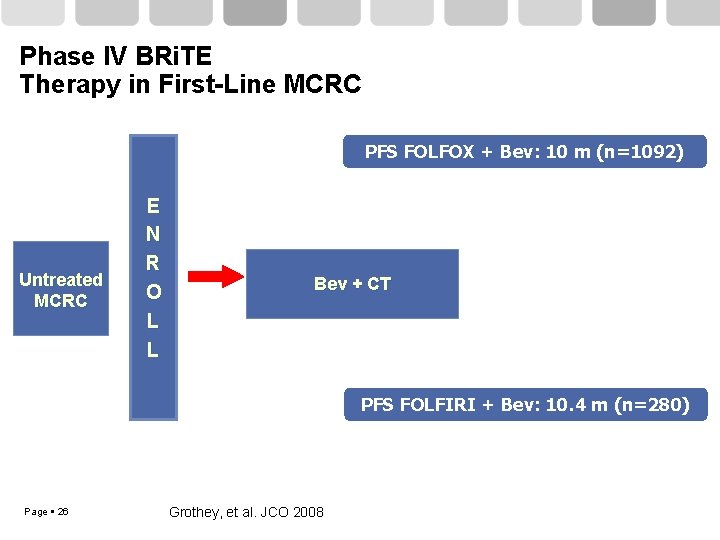
Phase IV BRi. TE Therapy in First-Line MCRC PFS FOLFOX + Bev: 10 m (n=1092) Untreated MCRC E N R O L L Bev + CT PFS FOLFIRI + Bev: 10. 4 m (n=280) Page 26 Grothey, et al. JCO 2008

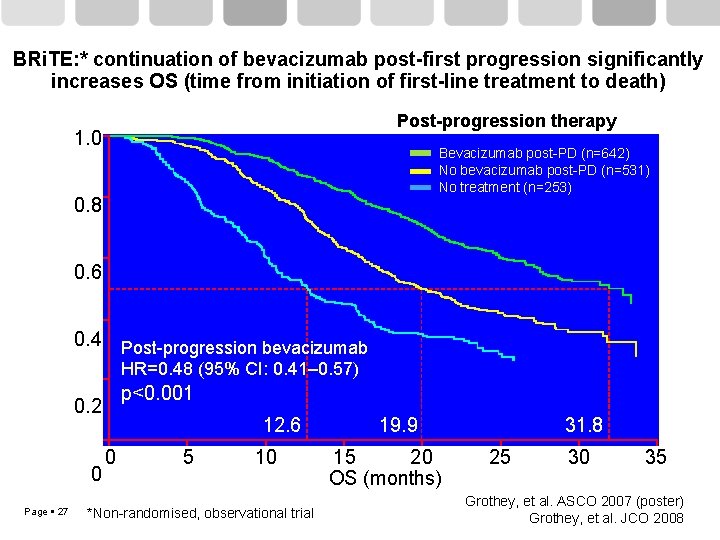
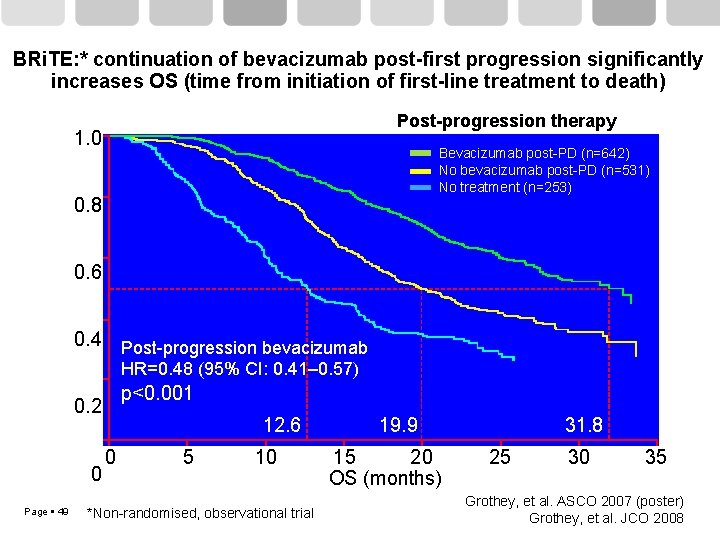
BRi. TE: * continuation of bevacizumab post-first progression significantly increases OS (time from initiation of first-line treatment to death) Post-progression therapy Estimated probability 1. 0 Bevacizumab post-PD (n=642) No bevacizumab post-PD (n=531) No treatment (n=253) 0. 8 0. 6 0. 4 Post-progression bevacizumab HR=0. 48 (95% CI: 0. 41– 0. 57) p<0. 001 0. 2 0 Page 27 12. 6 0 5 10 *Non-randomised, observational trial 19. 9 15 20 OS (months) 31. 8 25 30 35 Grothey, et al. ASCO 2007 (poster) Grothey, et al. JCO 2008

Quimioterapia más cetuximab

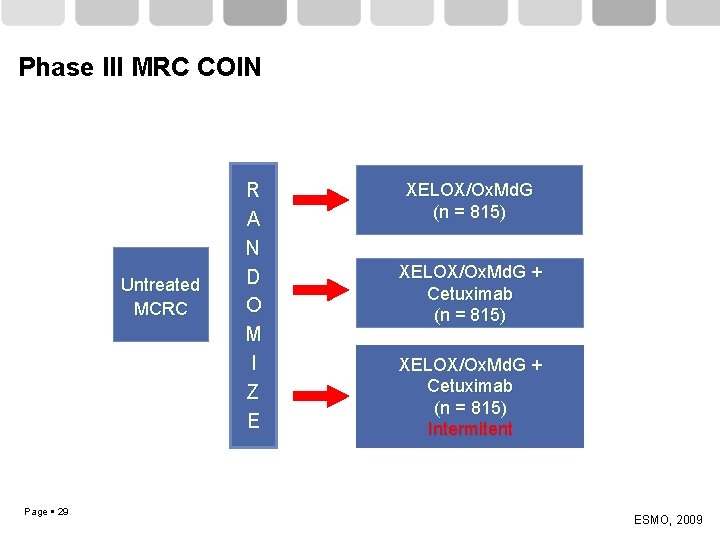
Phase III MRC COIN Untreated MCRC Page 29 R A N D O M I Z E XELOX/Ox. Md. G (n = 815) XELOX/Ox. Md. G + Cetuximab (n = 815) Intermitent ESMO, 2009

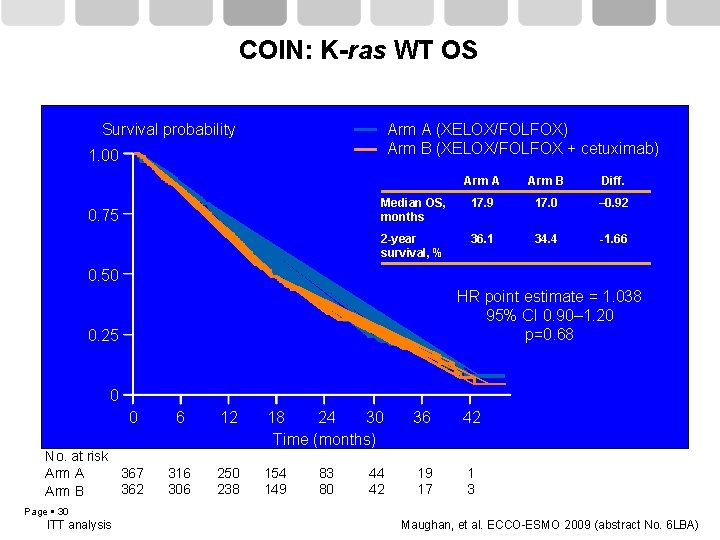
COIN: K-ras WT OS Survival probability Arm A (XELOX/FOLFOX) Arm B (XELOX/FOLFOX + cetuximab) 1. 00 0. 75 Arm A Arm B Diff. Median OS, months 17. 9 17. 0 – 0. 92 2 -year survival, % 36. 1 34. 4 -1. 66 0. 50 HR point estimate = 1. 038 95% CI 0. 90– 1. 20 p=0. 68 0. 25 0 0 No. at risk 367 Arm A 362 Arm B 6 12 18 24 30 Time (months) 36 42 316 306 250 238 154 149 19 17 1 3 83 80 44 42 Page 30 ITT analysis Maughan, et al. ECCO-ESMO 2009 (abstract No. 6 LBA)

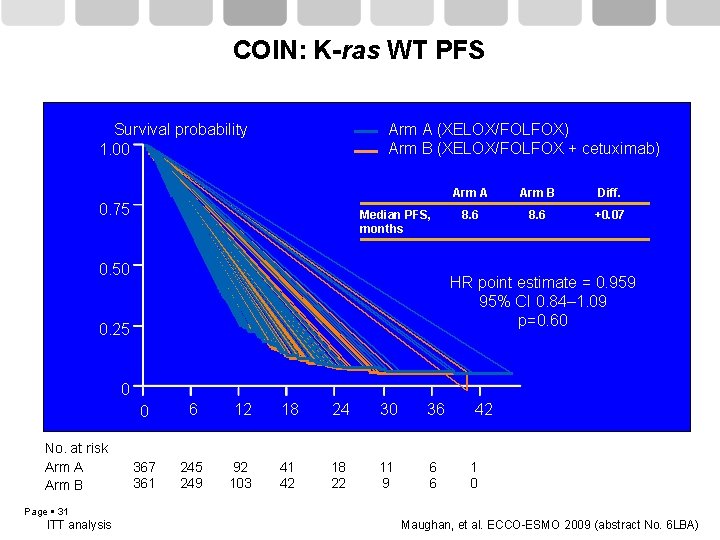
COIN: K-ras WT PFS Arm A (XELOX/FOLFOX) Arm B (XELOX/FOLFOX + cetuximab) Survival probability 1. 00 0. 75 Median PFS, months 0. 50 Arm A Arm B Diff. 8. 6 +0. 07 HR point estimate = 0. 959 95% CI 0. 84– 1. 09 p=0. 60 0. 25 0 No. at risk Arm A Arm B 0 6 12 18 24 30 36 367 361 245 249 92 103 41 42 18 22 11 9 6 6 42 1 0 Page 31 ITT analysis Maughan, et al. ECCO-ESMO 2009 (abstract No. 6 LBA)

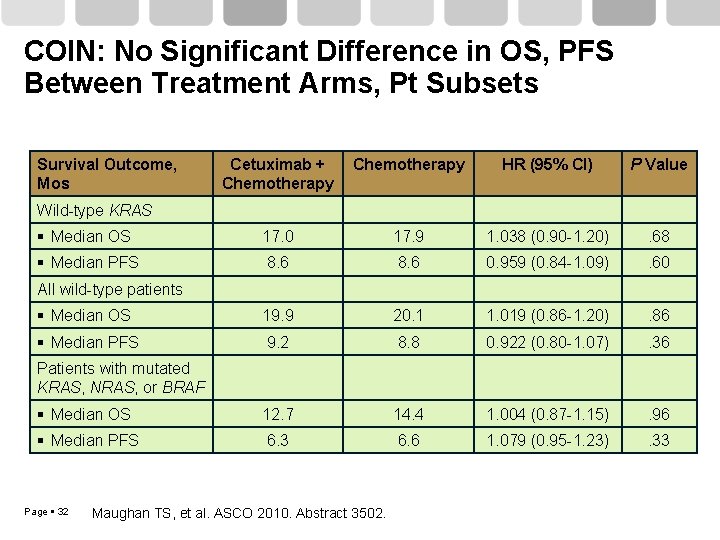
COIN: No Significant Difference in OS, PFS Between Treatment Arms, Pt Subsets Survival Outcome, Mos Cetuximab + Chemotherapy HR (95% CI) P Value Median OS 17. 0 17. 9 1. 038 (0. 90 -1. 20) . 68 Median PFS 8. 6 0. 959 (0. 84 -1. 09) . 60 Median OS 19. 9 20. 1 1. 019 (0. 86 -1. 20) . 86 Median PFS 9. 2 8. 8 0. 922 (0. 80 -1. 07) . 36 Median OS 12. 7 14. 4 1. 004 (0. 87 -1. 15) . 96 Median PFS 6. 3 6. 6 1. 079 (0. 95 -1. 23) . 33 Wild-type KRAS All wild-type patients Patients with mutated KRAS, NRAS, or BRAF Page 32 Maughan TS, et al. ASCO 2010. Abstract 3502.

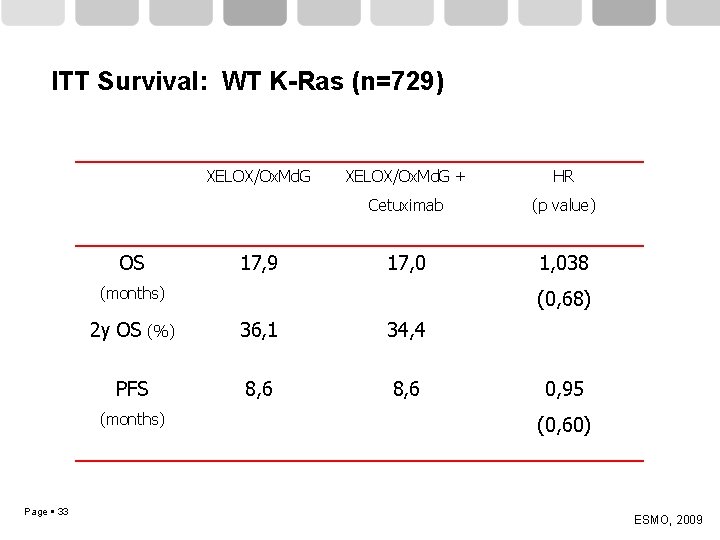
ITT Survival: WT K-Ras (n=729) XELOX/Ox. Md. G OS 17, 9 XELOX/Ox. Md. G + HR Cetuximab (p value) 17, 0 1, 038 (months) 2 y OS (%) 36, 1 34, 4 PFS 8, 6 (months) Page 33 (0, 68) 0, 95 (0, 60) ESMO, 2009

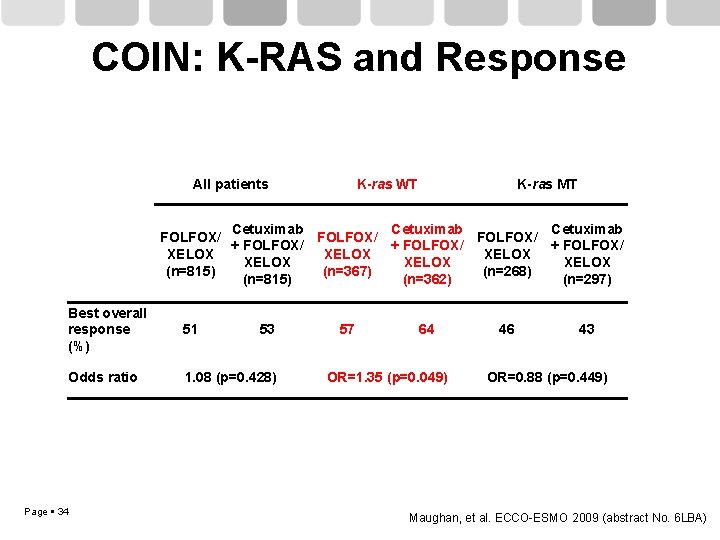
COIN: K-RAS and Response All patients K-ras WT K-ras MT Cetuximab FOLFOX/ + FOLFOX/ XELOX XELOX (n=815) (n=367) (n=268) (n=815) (n=362) (n=297) Best overall response (%) 51 Odds ratio 1. 08 (p=0. 428) Page 34 53 57 64 OR=1. 35 (p=0. 049) 46 43 OR=0. 88 (p=0. 449) Maughan, et al. ECCO-ESMO 2009 (abstract No. 6 LBA)

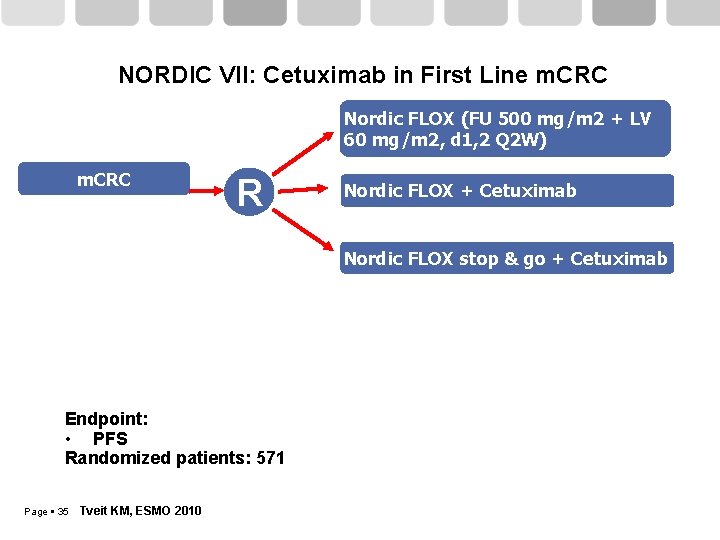
NORDIC VII: Cetuximab in First Line m. CRC Nordic FLOX (FU 500 mg/m 2 + LV 60 mg/m 2, d 1, 2 Q 2 W) m. CRC R Nordic FLOX + Cetuximab Nordic FLOX stop & go + Cetuximab Endpoint: • PFS Randomized patients: 571 Page 35 Tveit KM, ESMO 2010

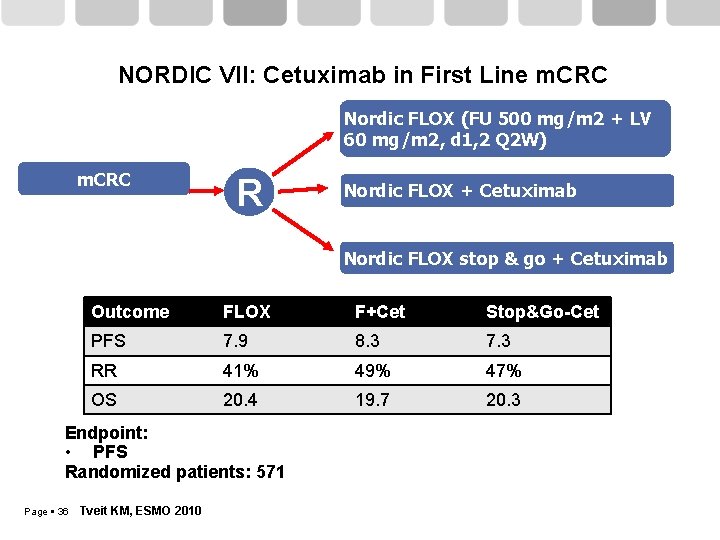
NORDIC VII: Cetuximab in First Line m. CRC Nordic FLOX (FU 500 mg/m 2 + LV 60 mg/m 2, d 1, 2 Q 2 W) m. CRC R Nordic FLOX + Cetuximab Nordic FLOX stop & go + Cetuximab Outcome FLOX F+Cet Stop&Go-Cet PFS 7. 9 8. 3 7. 3 RR 41% 49% 47% OS 20. 4 19. 7 20. 3 Endpoint: • PFS Randomized patients: 571 Page 36 Tveit KM, ESMO 2010

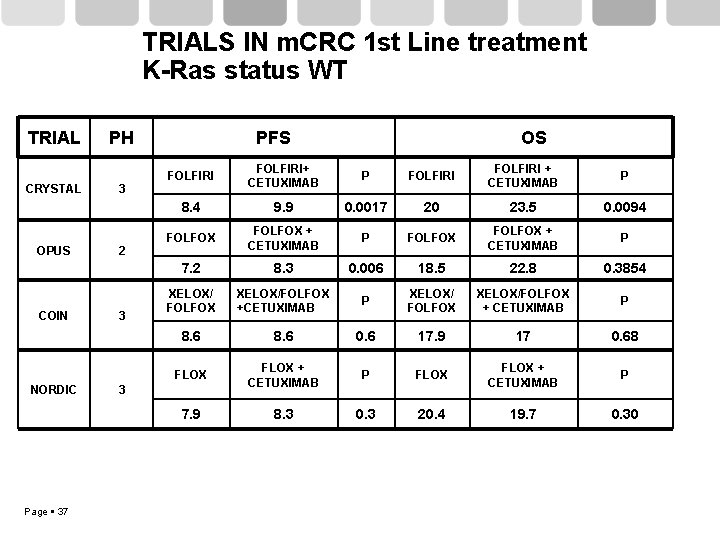
TRIALS IN m. CRC 1 st Line treatment K-Ras status WT TRIAL CRYSTAL OPUS COIN NORDIC Page 37 PH 3 2 3 PFS OS FOLFIRI+ CETUXIMAB P FOLFIRI + CETUXIMAB P 8. 4 9. 9 0. 0017 20 23. 5 0. 0094 FOLFOX + CETUXIMAB P 7. 2 8. 3 0. 006 18. 5 22. 8 0. 3854 XELOX/ FOLFOX XELOX/FOLFOX +CETUXIMAB P XELOX/ FOLFOX XELOX/FOLFOX + CETUXIMAB P 8. 6 0. 6 17. 9 17 0. 68 FLOX + CETUXIMAB P 7. 9 8. 3 0. 3 20. 4 19. 7 0. 30 3

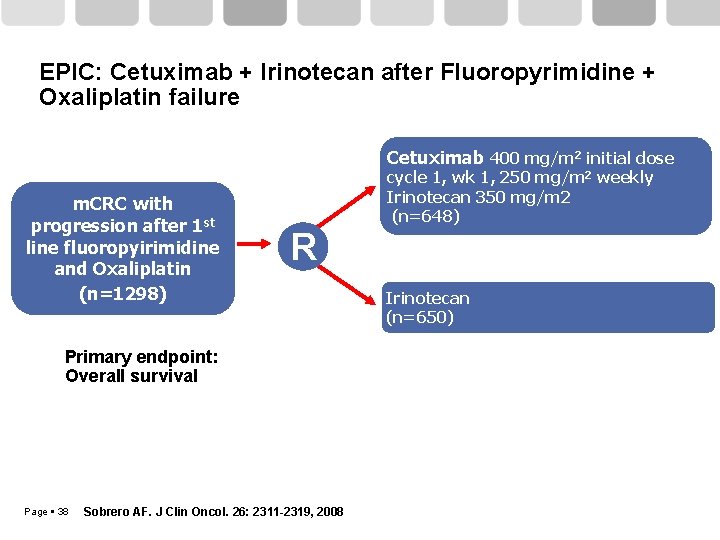
EPIC: Cetuximab + Irinotecan after Fluoropyrimidine + Oxaliplatin failure Cetuximab 400 mg/m 2 initial dose m. CRC with progression after 1 st line fluoropyirimidine and Oxaliplatin (n=1298) cycle 1, wk 1, 250 mg/m 2 weekly Irinotecan 350 mg/m 2 (n=648) R Primary endpoint: Overall survival Page 38 Sobrero AF. J Clin Oncol. 26: 2311 -2319, 2008 Irinotecan (n=650)

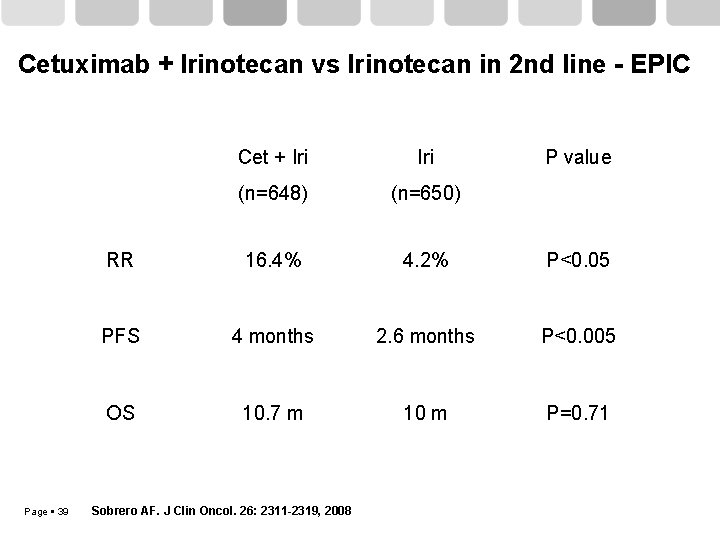
Cetuximab + Irinotecan vs Irinotecan in 2 nd line - EPIC Page 39 Cet + Iri (n=648) (n=650) RR 16. 4% 4. 2% P<0. 05 PFS 4 months 2. 6 months P<0. 005 OS 10. 7 m 10 m P=0. 71 Sobrero AF. J Clin Oncol. 26: 2311 -2319, 2008 P value

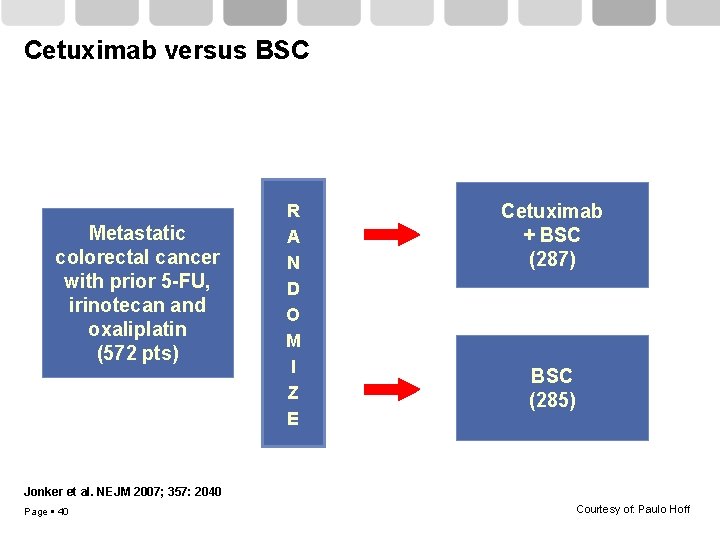
Cetuximab versus BSC Metastatic colorectal cancer with prior 5 -FU, irinotecan and oxaliplatin (572 pts) R A N D O M I Z E Cetuximab + BSC (287) BSC (285) Jonker et al. NEJM 2007; 357: 2040 Page 40 Courtesy of: Paulo Hoff

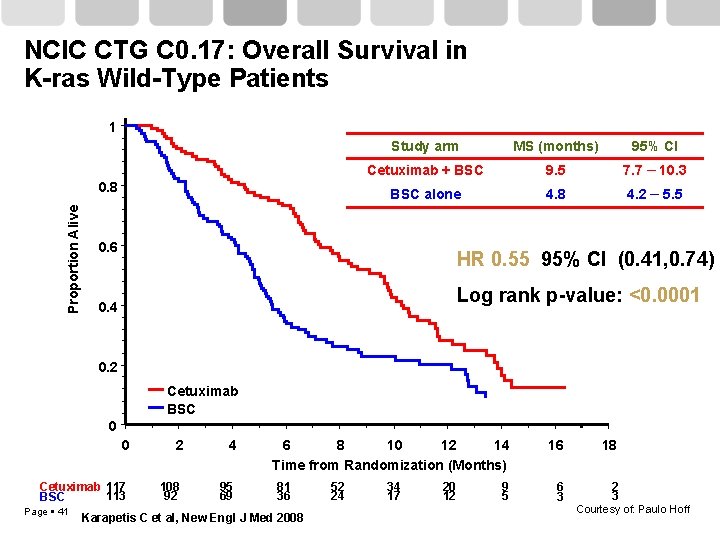
NCIC CTG C 0. 17: Overall Survival in K-ras Wild-Type Patients 1 Proportion Alive 0. 8 Study arm MS (months) 95% CI Cetuximab + BSC 9. 5 7. 7 – 10. 3 BSC alone 4. 8 4. 2 – 5. 5 0. 6 HR 0. 55 95% CI (0. 41, 0. 74) Log rank p-value: <0. 0001 0. 4 0. 2 Cetuximab BSC 0 0 Cetuximab 117 113 BSC Page 41 2 108 92 4 95 69 6 8 10 12 14 Time from Randomization (Months) 81 36 Karapetis C et al, New Engl J Med 2008 52 24 34 17 20 12 9 5 16 18 6 3 2 3 Courtesy of: Paulo Hoff

Continuum of care

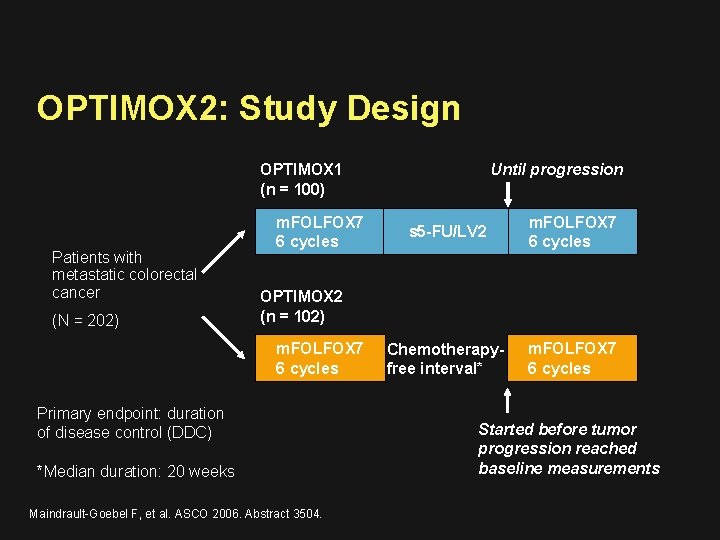
OPTIMOX 2: Study Design Until progression OPTIMOX 1 (n = 100) Patients with metastatic colorectal cancer (N = 202) m. FOLFOX 7 6 cycles s 5 -FU/LV 2 m. FOLFOX 7 6 cycles Chemotherapyfree interval* m. FOLFOX 7 6 cycles OPTIMOX 2 (n = 102) m. FOLFOX 7 6 cycles Primary endpoint: duration of disease control (DDC) *Median duration: 20 weeks Maindrault-Goebel F, et al. ASCO 2006. Abstract 3504. Started before tumor progression reached baseline measurements

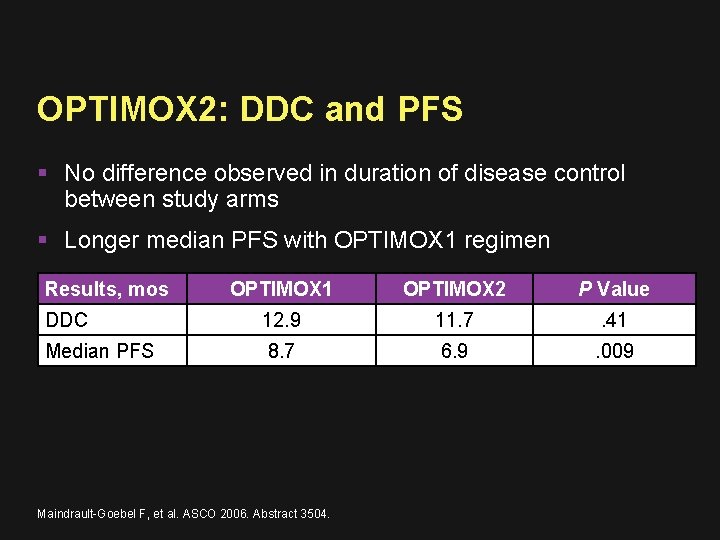
OPTIMOX 2: DDC and PFS No difference observed in duration of disease control between study arms Longer median PFS with OPTIMOX 1 regimen Results, mos OPTIMOX 1 OPTIMOX 2 P Value DDC 12. 9 11. 7 . 41 Median PFS 8. 7 6. 9 . 009 Maindrault-Goebel F, et al. ASCO 2006. Abstract 3504.

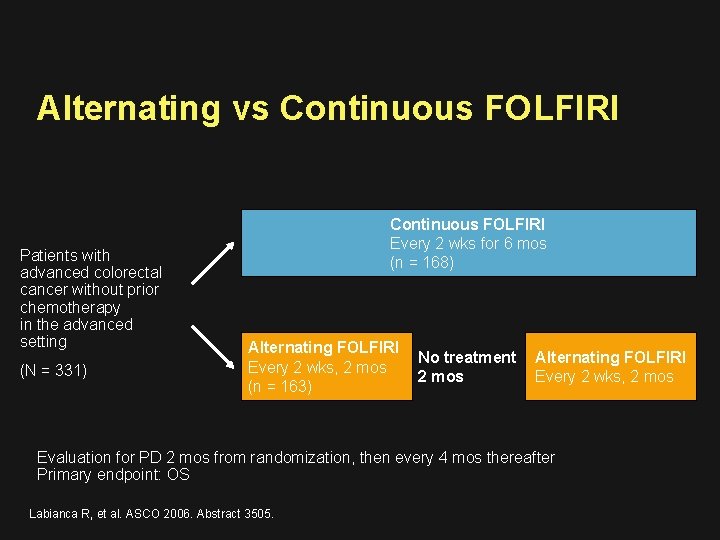
Alternating vs Continuous FOLFIRI Patients with advanced colorectal cancer without prior chemotherapy in the advanced setting (N = 331) Continuous FOLFIRI Every 2 wks for 6 mos (n = 168) Alternating FOLFIRI Every 2 wks, 2 mos (n = 163) No treatment 2 mos Alternating FOLFIRI Every 2 wks, 2 mos Evaluation for PD 2 mos from randomization, then every 4 mos thereafter Primary endpoint: OS Labianca R, et al. ASCO 2006. Abstract 3505.

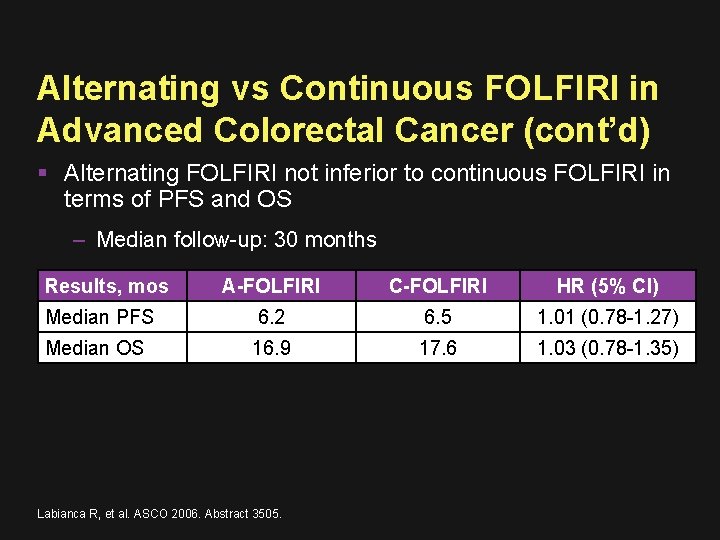
Alternating vs Continuous FOLFIRI in Advanced Colorectal Cancer (cont’d) Alternating FOLFIRI not inferior to continuous FOLFIRI in terms of PFS and OS – Median follow-up: 30 months Results, mos A-FOLFIRI C-FOLFIRI HR (5% CI) Median PFS 6. 2 6. 5 1. 01 (0. 78 -1. 27) Median OS 16. 9 17. 6 1. 03 (0. 78 -1. 35) Labianca R, et al. ASCO 2006. Abstract 3505.

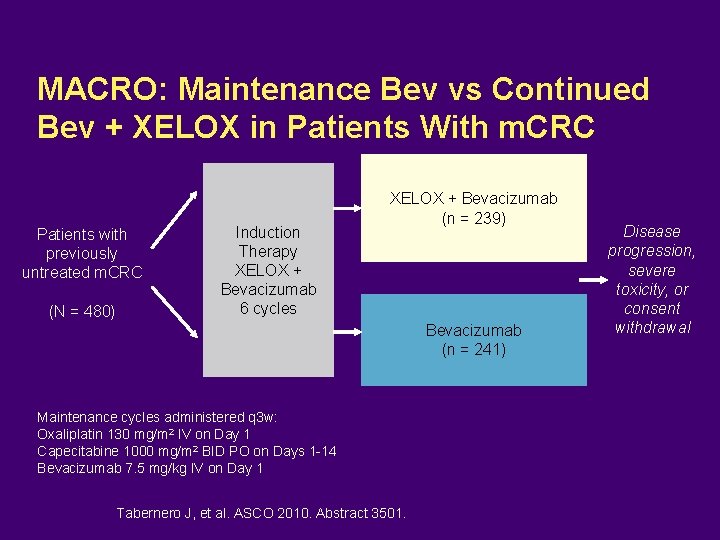
MACRO: Maintenance Bev vs Continued Bev + XELOX in Patients With m. CRC Patients with previously untreated m. CRC (N = 480) Induction Therapy XELOX + Bevacizumab 6 cycles XELOX + Bevacizumab (n = 239) Bevacizumab (n = 241) Maintenance cycles administered q 3 w: Oxaliplatin 130 mg/m 2 IV on Day 1 Capecitabine 1000 mg/m 2 BID PO on Days 1 -14 Bevacizumab 7. 5 mg/kg IV on Day 1 Tabernero J, et al. ASCO 2010. Abstract 3501. Disease progression, severe toxicity, or consent withdrawal

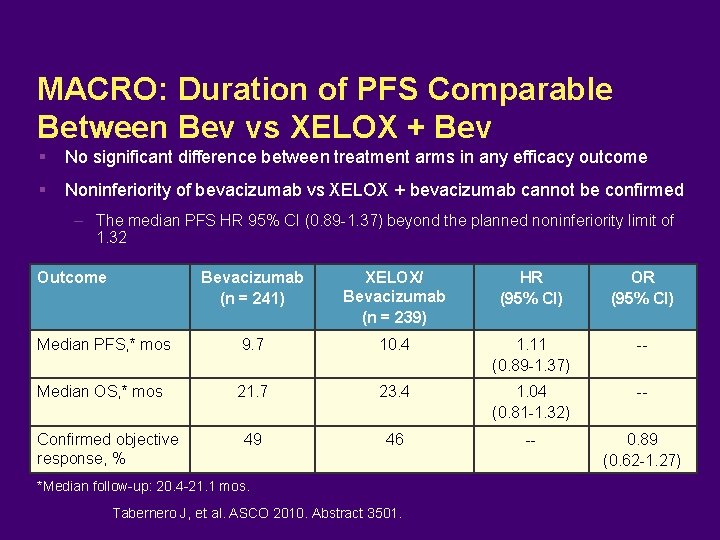
MACRO: Duration of PFS Comparable Between Bev vs XELOX + Bev No significant difference between treatment arms in any efficacy outcome Noninferiority of bevacizumab vs XELOX + bevacizumab cannot be confirmed – The median PFS HR 95% CI (0. 89 -1. 37) beyond the planned noninferiority limit of 1. 32 Outcome Bevacizumab (n = 241) XELOX/ Bevacizumab (n = 239) HR (95% CI) OR (95% CI) Median PFS, * mos 9. 7 10. 4 1. 11 (0. 89 -1. 37) -- Median OS, * mos 21. 7 23. 4 1. 04 (0. 81 -1. 32) -- 49 46 -- 0. 89 (0. 62 -1. 27) Confirmed objective response, % *Median follow-up: 20. 4 -21. 1 mos. Tabernero J, et al. ASCO 2010. Abstract 3501.

BRi. TE: * continuation of bevacizumab post-first progression significantly increases OS (time from initiation of first-line treatment to death) Post-progression therapy Estimated probability 1. 0 Bevacizumab post-PD (n=642) No bevacizumab post-PD (n=531) No treatment (n=253) 0. 8 0. 6 0. 4 Post-progression bevacizumab HR=0. 48 (95% CI: 0. 41– 0. 57) p<0. 001 0. 2 0 Page 49 12. 6 0 5 10 *Non-randomised, observational trial 19. 9 15 20 OS (months) 31. 8 25 30 35 Grothey, et al. ASCO 2007 (poster) Grothey, et al. JCO 2008

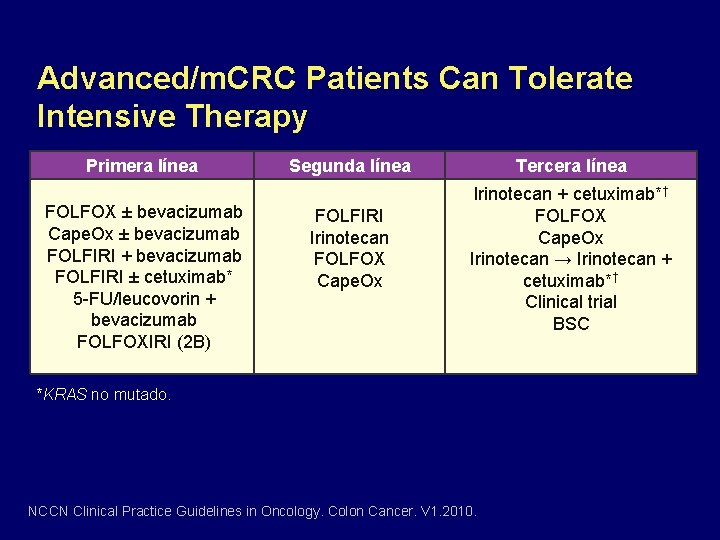
Advanced/m. CRC Patients Can Tolerate Intensive Therapy Primera línea FOLFOX ± bevacizumab Cape. Ox ± bevacizumab FOLFIRI + bevacizumab FOLFIRI ± cetuximab* 5 -FU/leucovorin + bevacizumab FOLFOXIRI (2 B) Segunda línea FOLFIRI Irinotecan FOLFOX Cape. Ox Tercera línea Irinotecan + cetuximab*† FOLFOX Cape. Ox Irinotecan → Irinotecan + cetuximab*† Clinical trial BSC *KRAS no mutado. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. V 1. 2010.

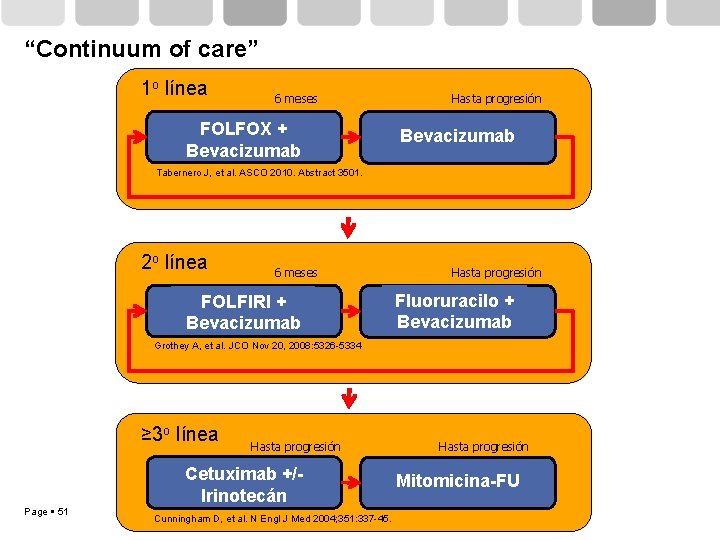
“Continuum of care” 1 o línea 6 meses FOLFOX + Bevacizumab Hasta progresión Bevacizumab Tabernero J, et al. ASCO 2010. Abstract 3501. 2 o línea 6 meses FOLFIRI + Bevacizumab Hasta progresión Fluoruracilo + Bevacizumab Grothey A, et al. JCO Nov 20, 2008: 5326 -5334 ≥ 3 o línea Page 51 Hasta progresión Cetuximab +/Irinotecán Cunningham D, et al. N Engl J Med 2004; 351: 337 -45. Hasta progresión Mitomicina-FU

Conclusiones Debe recibir Irinotecán, Oxaliplatino y Fluoruracilo… Bevacizumab + QT en primera línea metastásica Cetuximab +/- Irinotecán en última línea Disminución de intensidad (y toxicidad) es válida (sábados) Suspender el tratamiento: disminuye la supervivencia (domingos)
 Gog 240
Gog 240 Investigacionales
Investigacionales When is colon used
When is colon used Colons
Colons Semicolon example of use
Semicolon example of use Colon and semicolon
Colon and semicolon Semi colon vs colon
Semi colon vs colon A colon in a sentence
A colon in a sentence When to use a semi colon and colon
When to use a semi colon and colon Primary secondary tertiary medical care
Primary secondary tertiary medical care Big bend continuum of care
Big bend continuum of care Continuum care greensboro nc
Continuum care greensboro nc Summit county continuum of care
Summit county continuum of care Continuum of care
Continuum of care Managed care continuum
Managed care continuum Continuum of care
Continuum of care Pikes peak continuum of care
Pikes peak continuum of care Continuum of care reform
Continuum of care reform Asam continuum of care
Asam continuum of care Health and social care values unit 2
Health and social care values unit 2 Duty of care care certificate
Duty of care care certificate Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Acul magnetic al unei busole se orienteaza
Acul magnetic al unei busole se orienteaza Care certificate standard 8 answers
Care certificate standard 8 answers Palliative care vs hospice care
Palliative care vs hospice care Hip fracture care clinical care standard
Hip fracture care clinical care standard Cum se înmulțesc mamiferele
Cum se înmulțesc mamiferele Fusus coli
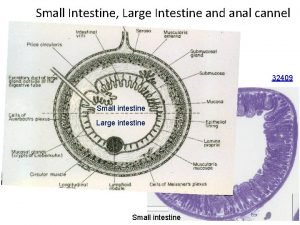
Fusus coli Anal canal
Anal canal Genes supresores de tumores
Genes supresores de tumores Funciones del colon
Funciones del colon When is a colon used in a sentence examples
When is a colon used in a sentence examples Clasificacion de dukes cancer colon
Clasificacion de dukes cancer colon Colon in matlab
Colon in matlab Dave barry a journey into my colon
Dave barry a journey into my colon Programa sag
Programa sag Minor salivary glands
Minor salivary glands Edith colon
Edith colon Sacculations
Sacculations Rectum and anal canal
Rectum and anal canal Thank you name with comma
Thank you name with comma Analogy colon
Analogy colon Blood supply of colon
Blood supply of colon Brandon levan
Brandon levan Colon looping
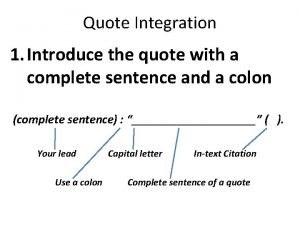
Colon looping Colon quote integration
Colon quote integration Polibutin colon irritable
Polibutin colon irritable Laxative effect
Laxative effect Midgut
Midgut Transverse colon
Transverse colon Clasificacion molecular cancer de endometrio
Clasificacion molecular cancer de endometrio Schema intestin
Schema intestin Cayo tortuga colon
Cayo tortuga colon