ACQUIRED COAGULATION FACTOR DISORDERS Majid vafaie MD Acquired




![• Therapeutic anticoagulants (heparin, lowmolecularweight-heparin [LMWH], warfarin). • Platelet receptor antagonists (l. Ib/Il. • Therapeutic anticoagulants (heparin, lowmolecularweight-heparin [LMWH], warfarin). • Platelet receptor antagonists (l. Ib/Il.](https://slidetodoc.com/presentation_image_h/d5c5fb8c84a8c9b606e63038f0c8c1dc/image-8.jpg)




























- Slides: 50


ACQUIRED COAGULATION FACTOR DISORDERS Majid vafaie MD

Acquired Hemorrhagic Disorders • • • Drug-Induced Bleeding Disseminated Intravascular Coagulation Liver Disease Vitamin K Deficiency Massive Transfusion Coagulopathy Acquired Inhibitors of Coagulation Proteins

• Many drugs have been associated with abnormal platelet function, although not all result in bleeding manifestations. • The major classes include anti-inflammatory agents, antibiotics, cardiovascular drugs, psychotropic drugs, anticonvulsants, and anticoagulants.

• Aspirin is the most common example; it acts by irreversibly acetylating a serine residue at the active site of cyclooxygenase • whereas other nonsteroidal antiinflammatories (e. g. , ibuprofen, naproxen) are reversible inhibitors

• B-Lactam antibiotics can interfere with platelet function through binding to the platelet membrane • Calcium channel blockers, such as nifedipineand tricyclic antidepressants, • such as amytriptyline can give rise to decreased platelet aggregation responses but are unlikely to result in clinical bleeding.

Valproic acid induced coagulopathy • • • thrombocytopenia platelet dysfunction acquired von. Willebrand'sdisease decreased VK-dependent clotting factors hypofibrinogenemia decreased factor XIII levels
![Therapeutic anticoagulants heparin lowmolecularweightheparin LMWH warfarin Platelet receptor antagonists l IbIl • Therapeutic anticoagulants (heparin, lowmolecularweight-heparin [LMWH], warfarin). • Platelet receptor antagonists (l. Ib/Il.](https://slidetodoc.com/presentation_image_h/d5c5fb8c84a8c9b606e63038f0c8c1dc/image-8.jpg)
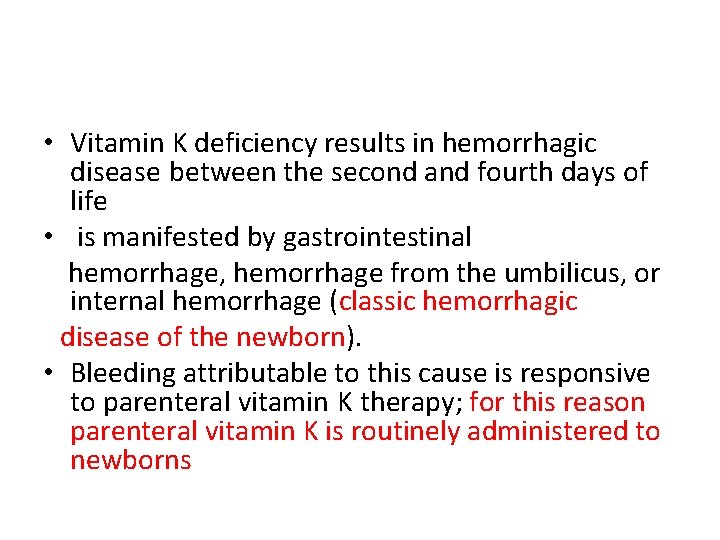
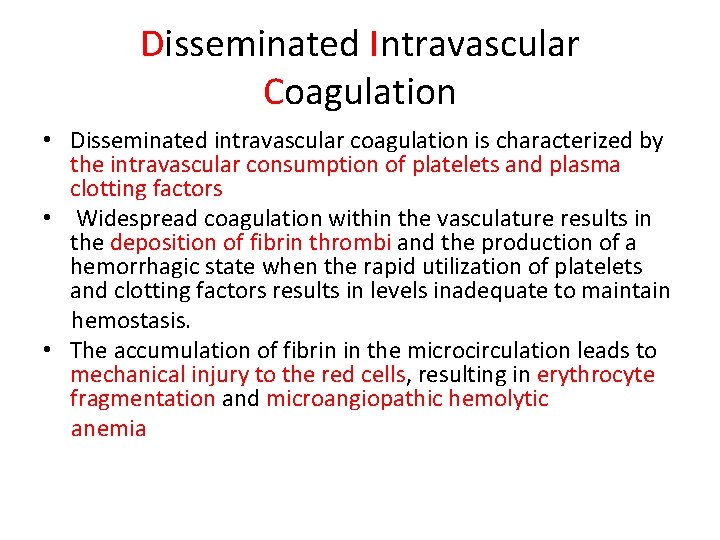
• Therapeutic anticoagulants (heparin, lowmolecularweight-heparin [LMWH], warfarin). • Platelet receptor antagonists (l. Ib/Il. Ia inhibitors, e. g. , abciximab, eptifibatide, tirofiban; adenosine diphosphate receptor antagonists, e. g. , clopidogrel) are increasingly being used in patients with congenital heart disease

• discontinuation of the offending drug should resolve the hemorrhagic manifestations • Administration of VK will in most cases rapidly reverse warfarin-induced bleeding • Alternatively, (FFP) can be used to replace Vk dependent clotting factors rapidly • Recombinant factor VIla (r. Vl. Ia) has proved effective at rapidly reversing warfarin-induced coagulopathy

• Transfusions of fresh platelets are indicated in patients with life-threatening or unremitting bleeding as a result of drug-induced platelet dysfunction. • In cases of minor bleeding in which the drug cannot readily be discontinued (e. g. , anticonvulsants), desmopressin has been used with some success

Vitamin K Deficiency • The normal full-term infant is born with levels of factors II, VII, IX and X that are low by • adult standards (Table 13 -3). • The coagulation factors fall even lower over the first few days of life, reaching their nadir on about the third day

• This is due to the low body stores of vitamin K at birth. • As little as 25 μg vitamin K can prevent this fall in activity of the vitamin Kdependent clotting factors. • The vitamin K content of cow’s milk is about 6 μg/dl and that of breast milk 1. 5 μg/dl.

• It is a combination of low initial stores and subsequent poor intake of vitamin K that occasionally produces an aggravation of the coagulation defect causing primary hemorrhagic disease of the newborn

• Vitamin K deficiency results in hemorrhagic disease between the second and fourth days of life • is manifested by gastrointestinal hemorrhage, hemorrhage from the umbilicus, or internal hemorrhage (classic hemorrhagic disease of the newborn). • Bleeding attributable to this cause is responsive to parenteral vitamin K therapy; for this reason parenteral vitamin K is routinely administered to newborns

• In premature infants of low birth weight, both the vitamin K stores and the level of coagulation factors are even lower than in term infants. • The response to vitamin K is slow and inconsistent, suggesting that the immature liver has reduced synthetic capability

• Maternal ingestion of certain drugs may cause early hemorrhagic disease of the newborn as a result of neonatal hypoprothrombinemia due to a reduction in factors VII, IX and X. • These drugs include oral anticoagulants and anticonvulsants (phenytoin, primidone and phenobarbital

• Late hemorrhagic disease of the newborn occurs from one week after birth until 6 months of life due to the absence of adequate vitamin K prophylaxis at birth, malabsorption, liver disease, or idiopathically • Consider investigating for biliary atresia, alpha 1 antitrypsin deficiency if hemorrhagic disease of the newborn occurs after adequate vitamin K prophylaxis at birth.





Hepatic Dysfunction • Any transient inability of the newborn’s liver to synthesize necessary coagulation factors, even in the presence of vitamin K, can result in hemorrhagic disease that is nonrespon to vitamin K therapy • Hepatic dysfunction as a result of immaturity, infection, hypoxia, or underperfusion of the liver can all result in transient inability of the liver to synthesize coagulation factors

• This is more prominent in small premature infants. • The sites of bleeding in these cases are usually pulmonary and intracerebral with a high mortality • In liver disease vitamin K-dependent factors and factor V and fibrinogen are

• In liver disease vitamin K-dependent factors and factor V and fibrinogen are usually decreased, fibrin split products may be elevated due to impaired clearance • In contrast, factor VIII levels are usually normal.


• There is no response to vitamin K. • There is usually a clinical response to clotting factor replacement therapy, using fresh frozen plasma and cryoprecipitate (replacement guidelines are the same as those • outlined in Table 13 -7).

Disseminated Intravascular Coagulation • Disseminated intravascular coagulation is characterized by the intravascular consumption of platelets and plasma clotting factors • Widespread coagulation within the vasculature results in the deposition of fibrin thrombi and the production of a hemorrhagic state when the rapid utilization of platelets and clotting factors results in levels inadequate to maintain hemostasis. • The accumulation of fibrin in the microcirculation leads to mechanical injury to the red cells, resulting in erythrocyte fragmentation and microangiopathic hemolytic anemia

• Widespread activation of the coagulation cascade rapidly results in the depletion of many clotting factors as fibrinogen is converted to fibrin throughout the body

Two important mechanisms • The generation of thrombin results in intravascular coagulation and rapidly falling • platelet count, fibrinogen and FV, FVIII and FXIII levels • Paradoxically, in vitro bioassays for these factors may be elevated owing to generalized activation of the coagulation system

• Concurrently, plasminogen is converted to its enzymatic form (plasmin) by t-PA. • Plasmin digests fibrinogen and fibrin (secondary fibrinolysis) into fibrin split products (FSPs), resulting in clot lysis.

• Diagnosis of DIC relies on presence of a welldefined clinical situation associated with a thrombo-hemorrhagic disorder.


Diagnostic test • • Increased PT and (a. PTT) Decreased fibrinogen Decreased platelet count Fibrin degradation (elevated fibrin degradation products, elevated D dimmers) Presence of fragmented red blood cells (e. g. , schistocytes, triangle cells, helmet cells, burr cells) Increased PF 4 (platelet factor 4) Increased FPA (fibrinopeptide A) Decreased FV, FVIII, FXIII


low-grade DIC • • • Kasabach–Merritt syndrome Chronic inflammatory disorders Arteriovenous fistulae Vascular prosthesis Glomerulonephritis Low-grade DIC has the potential to accelerate into fulminant DIC

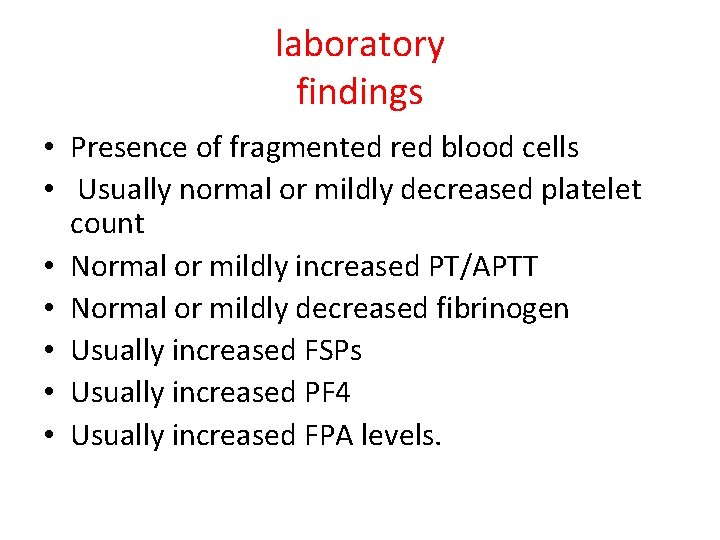
laboratory findings • Presence of fragmented red blood cells • Usually normal or mildly decreased platelet count • Normal or mildly increased PT/APTT • Normal or mildly decreased fibrinogen • Usually increased FSPs • Usually increased PF 4 • Usually increased FPA levels.

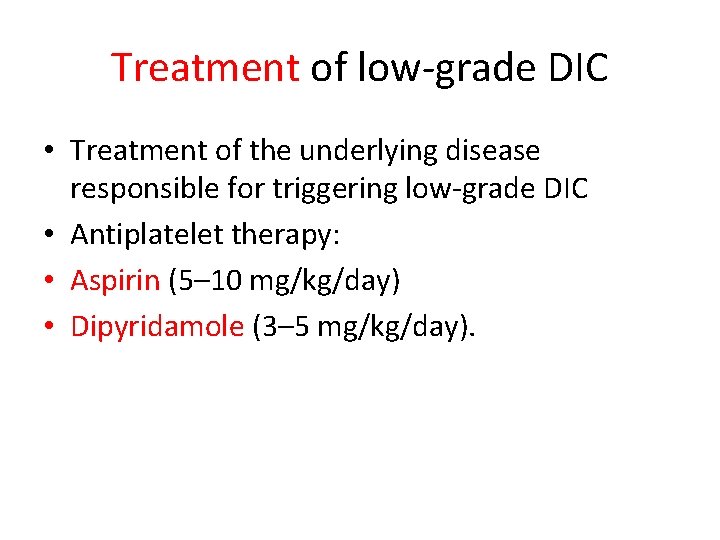
Treatment of low-grade DIC • Treatment of the underlying disease responsible for triggering low-grade DIC • Antiplatelet therapy: • Aspirin (5– 10 mg/kg/day) • Dipyridamole (3– 5 mg/kg/day).

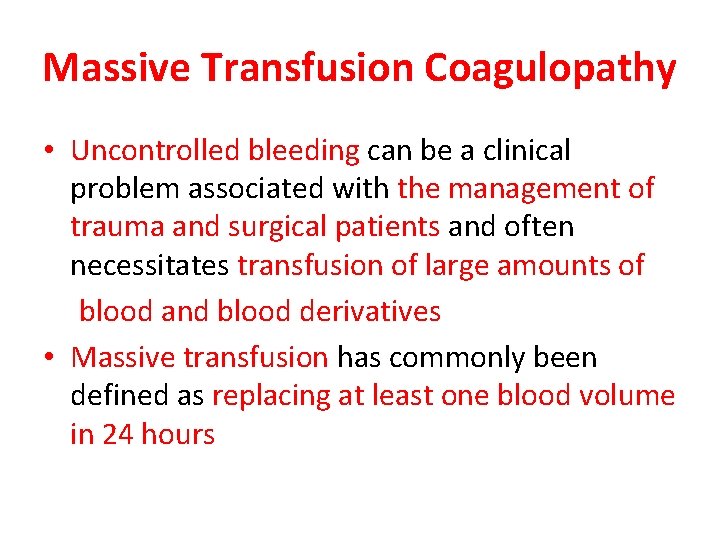
Massive Transfusion Coagulopathy • Uncontrolled bleeding can be a clinical problem associated with the management of trauma and surgical patients and often necessitates transfusion of large amounts of blood and blood derivatives • Massive transfusion has commonly been defined as replacing at least one blood volume in 24 hours

• Patients with this degree of bleeding are at risk for defective hemostasis related to the transfusions, in addition to the underlying precipitating trauma or surgical insult

• The resulting bleeding diathesis may be complex and include evidence of DIC, depletion of hemostatic factors through blood loss, tissue injury, and consumption of factors, dilutional coagulopathy secondary to aggressive blood component resuscitation, hypothermia, platelet dysfunction, and excessive fibrinolysis.

• r. VIIa has demonstrated effectiveness in patients with bleeding associated with massive transfusion who were refractory to conventional treatments

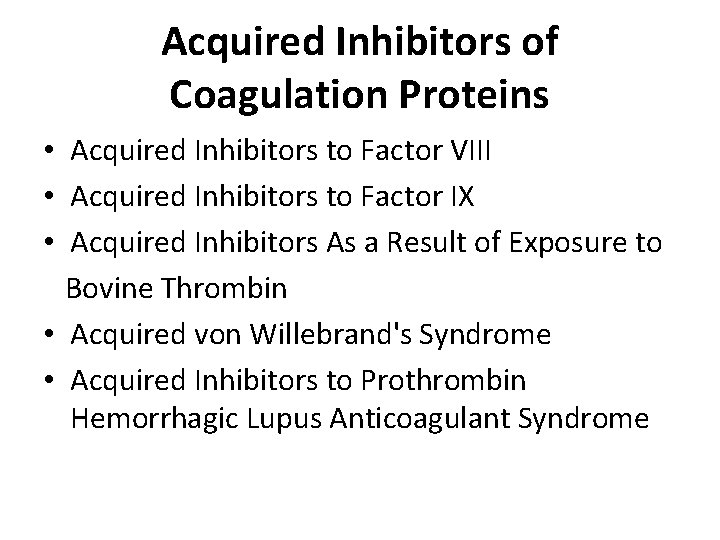
Acquired Inhibitors of Coagulation Proteins • Acquired Inhibitors to Factor VIII • Acquired Inhibitors to Factor IX • Acquired Inhibitors As a Result of Exposure to Bovine Thrombin • Acquired von Willebrand's Syndrome • Acquired Inhibitors to Prothrombin Hemorrhagic Lupus Anticoagulant Syndrome

• Anti-factor VIII autoantibodies are rare in children but can result in severe bleeding and significant morbidity • These antibodies are associated with underlying medical conditions in half of those affected, including malignancy, autoimmune disease, Iymphoproliferative disorders, or drugs (e. g. , penicillin)

• Patients can exhibit bleeding symptoms ranging from easy bruising to intracranial hemorrhage • Laboratory screening studies will show a prolonged APTT that does not fully correct upon mixing with normal plasma in a 1 : 1 mix ("inhibitor screen")

• Hemostasis can be achieved with high doses of factor VIII concentrates, activated prothrombin complex concentrates, and r. VIIa

Acquired Inhibitors to Factor IX • Few cases of spontaneous factor IX inhibitors have been reported in nonhemophiliacs, and most adult cases have been related to underlying systemic disorders such as systemic lupus erythematosus (SLE), hepatitis, multiple sclerosis, rheumatic fever, collagen vascular disease, postpartum status, and prostatectomy.

• Treatment has included any combination of corticosteroids, gamma globulin, or cyclophosphamide, with rapid resolution of the inhibitor • Recombinant factor IX and r. VIIa can be considered for the acute management of bleeding

Acquired von Willebrand's Syndrome • Acquired von Willebrand's syndrome (AVWS) is a rare bleeding disorder with clinical and laboratory findings similar to those of inherited von willebrand's disease • AVWS usually occurs in individuals with no personal or family history of von Willebrand's disease and is accompanied by bleeding symptoms in about three quarters of patients

• Iymphoproliferative and myeloproliferative disorders, solid tumors, immunologic and cardiovascular disorders, and other miscellaneous conditions, including drug associations

• AVWS has also been reported in children, primarily associated with congenital heart disease, collagen vascular diseases, Wilms' tumor, hypothyroidism, and certain drugs • Drug-associated AVWS has been described in up to 20% of pediatric patients taking valproic acid