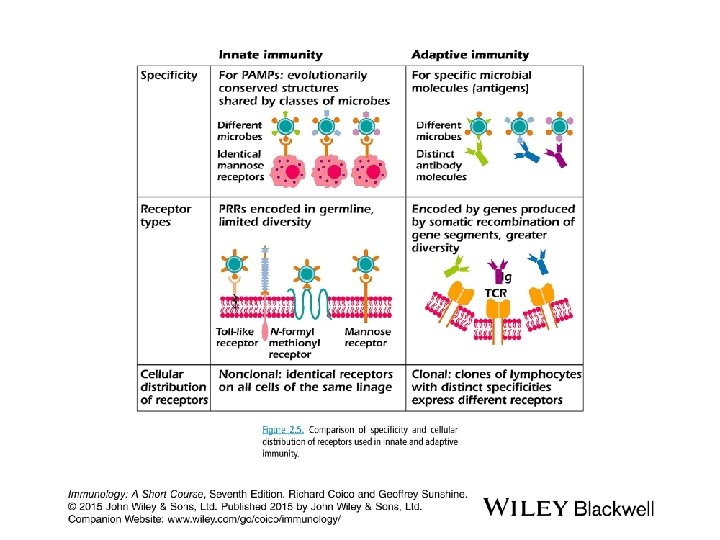
Elements of Innate and Acquired Immunity Innate Immunity




















- Slides: 48

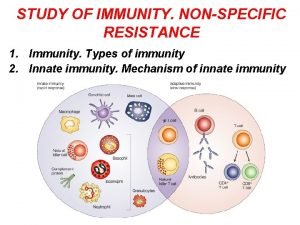
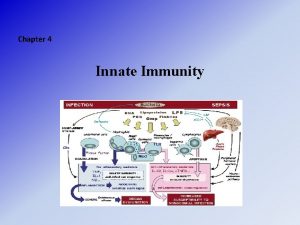
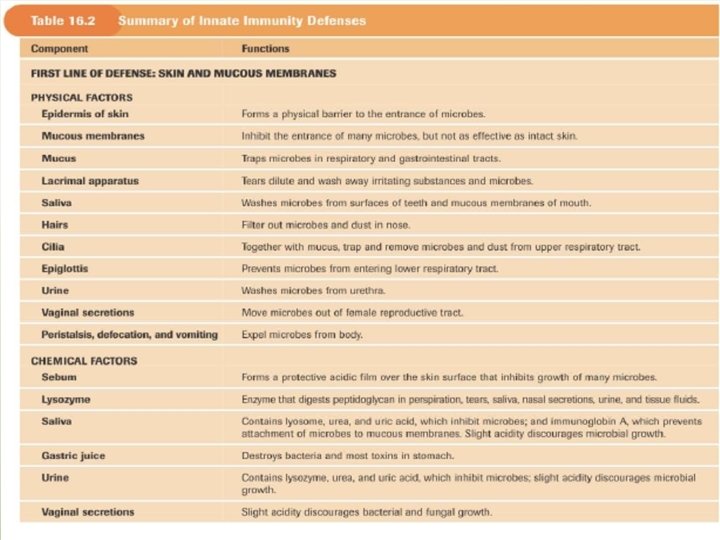
Elements of Innate and Acquired Immunity Innate Immunity It represents a nonspecific first line of defense against pathogens • Intact skin • Acid p. H of sweat • Lysozymes • Interferons • Complement • Ciliated cells of the respiratory tract

Mucous membranes trap microorganisms Hairs in nostrils Cough reflex Pulmonary or alveolar macrophages Hydrolytic enzymes in saliva Low p. H of the stomach Proteolytic enzymes and bile in small intestine • Low p. H of the vagina • •



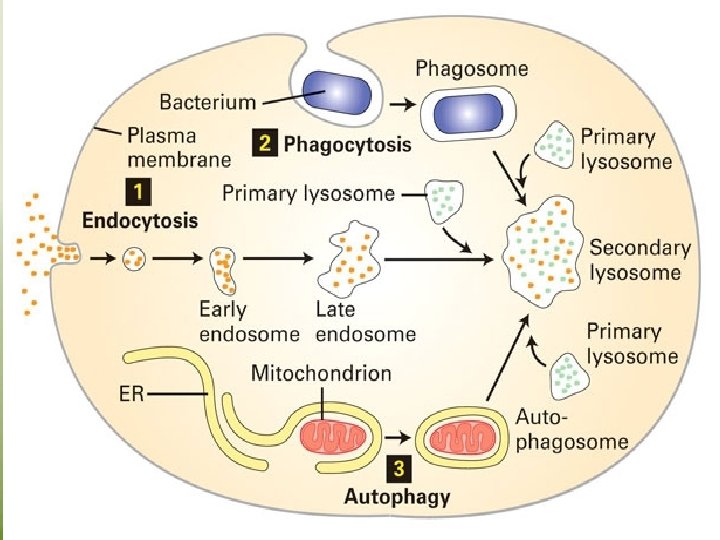
Endocytosis It is the ingestion of macromolecules present in extra cellular tissue fluid. This occurs either by nonspecific membrane invagination or by receptor mediated endocytosis with endocytic vesicle – endosomelysosomes yield breakdown products. Phagocytosis The ingestion of invading particles e. g. bacteria – by phagocytic cells. Phagocytosis is aided by opsonins “antibodies, complement components”. The foreign particle is entrapped in a phagocytic vacuole


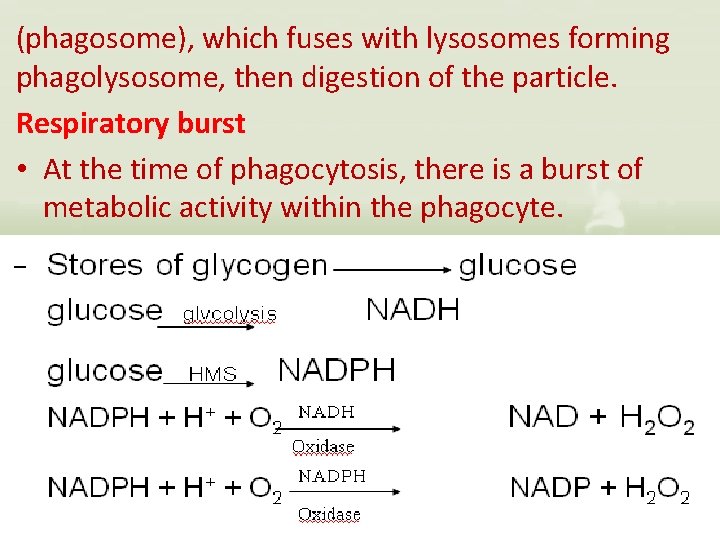
(phagosome), which fuses with lysosomes forming phagolysosome, then digestion of the particle. Respiratory burst • At the time of phagocytosis, there is a burst of metabolic activity within the phagocyte.



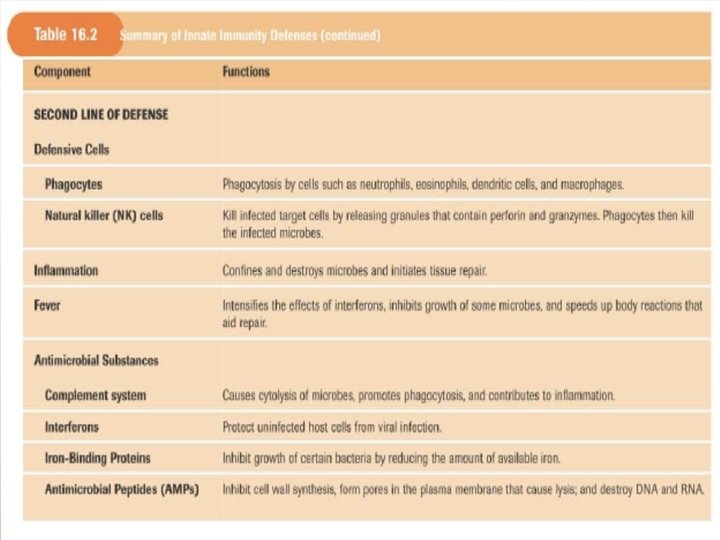
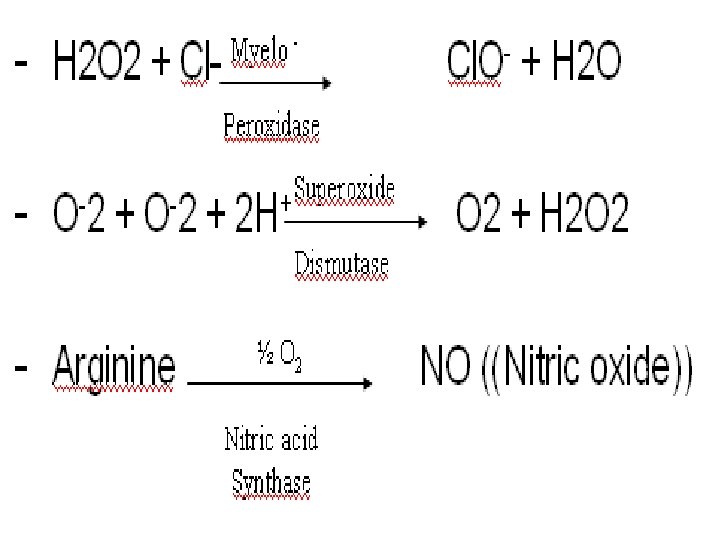
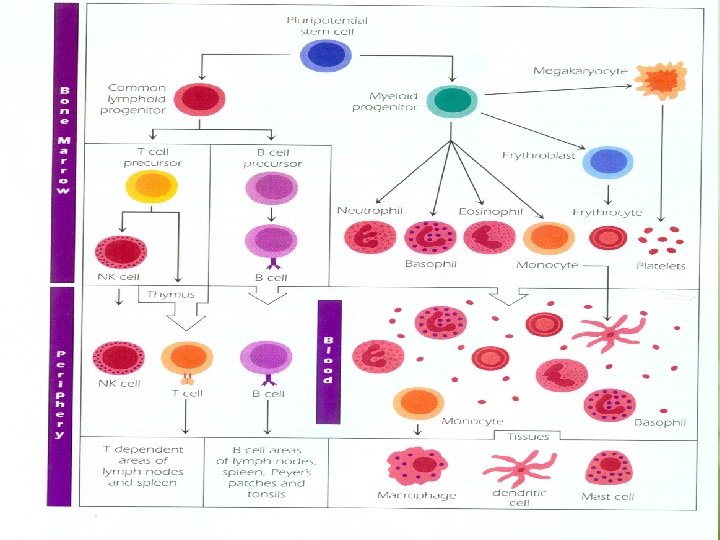
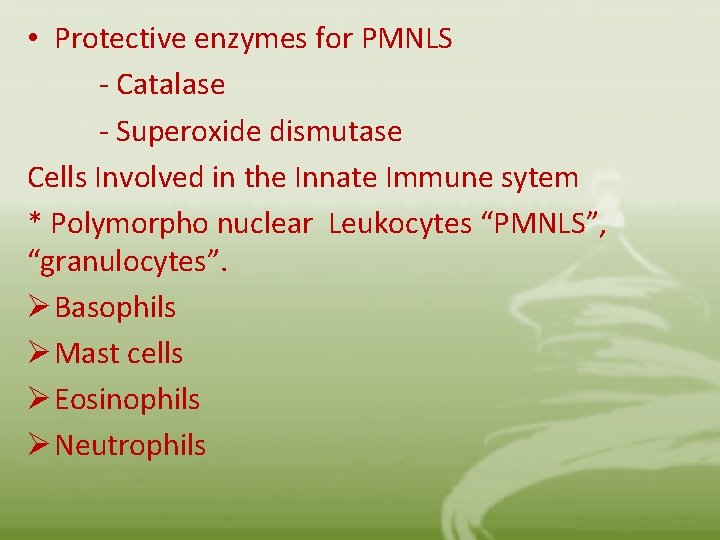
• Protective enzymes for PMNLS - Catalase - Superoxide dismutase Cells Involved in the Innate Immune sytem * Polymorpho nuclear Leukocytes “PMNLS”, “granulocytes”. Ø Basophils Ø Mast cells Ø Eosinophils Ø Neutrophils

• They contain the enzyme – rich lysosomes. • They produce peroxide and superoxide radicals which are toxic to many microorganisms • Some lysosomes contain bactericidal proteins, such as lactoferrin. • Defects in PMN cell function are accompanied by chronic or recurrent infection. • Macrophages: • They are phagocytes derived from blood monocytes. • Kupffer cells, in the liver • Alveolar macrophages, in the lung • Splenic macrophages, in the white plup • Peritoneal macrophages, free floating in peritoneal fluid. • Microglial cells, in the central nervous system.

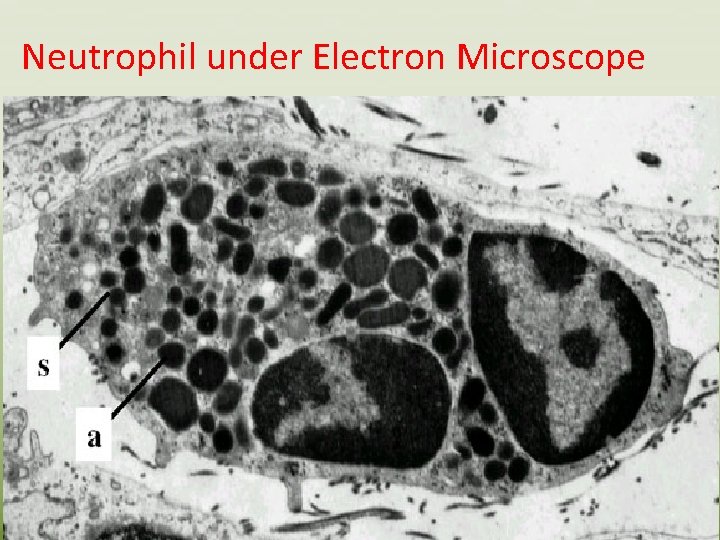
Neutrophil under Electron Microscope

• Neutrophils are produced at a rate of 7 million per minute and released from bone marrow. • They are short –lived “ 2 -3 days” • They represent 95% of the circulating granulocytes. • They contain antibiotic proteins which are stored in two main types of granules: • 1 - The primary “azurophilic “ granules, they are lysosomes containing acid hydrolases , myeloperoxidase, lysozyme, defensins and bacterial permeability inducing protein”BPI”

• 2 - The secondary granules “ specific to neutrophils” • contain lactoferrin and lysozyme • N. B: • Other small granules contain gelatinase : • Neutrophils can also release granules and cytotoxic substances extracellularly when they are activated by immune complexes through their Fc receptors. • N. B Monocytes contain azurophilic granules

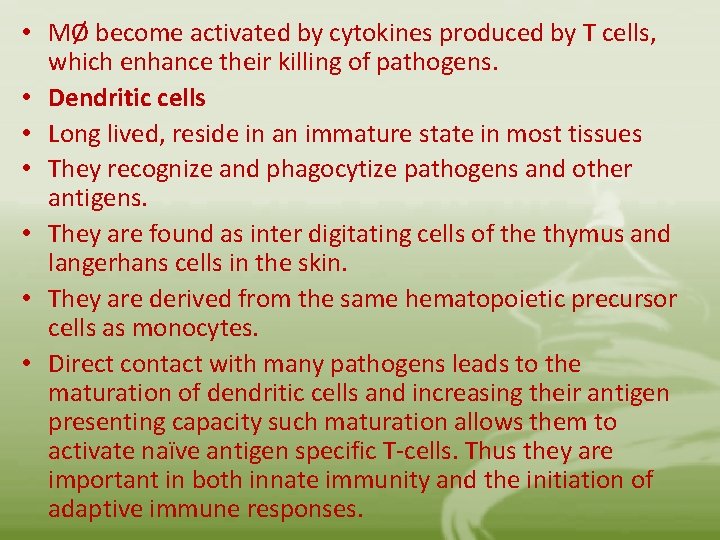
• Each of these MØ Populations is included in the reticuloendothelial system “RES”, which is widely distributed throughout the body. • The major function of RES is to phagocytize microorganisms and foreign substances. • The RES also functions in the destruction of aged and imperfect cells, such as erythrocytes. • The major functions of MØ are: - Engulf, break down trapped materials into simple substances - Act as antigen presenting cells APCs

• MØ become activated by cytokines produced by T cells, which enhance their killing of pathogens. • Dendritic cells • Long lived, reside in an immature state in most tissues • They recognize and phagocytize pathogens and other antigens. • They are found as inter digitating cells of the thymus and langerhans cells in the skin. • They are derived from the same hematopoietic precursor cells as monocytes. • Direct contact with many pathogens leads to the maturation of dendritic cells and increasing their antigen presenting capacity such maturation allows them to activate naïve antigen specific T-cells. Thus they are important in both innate immunity and the initiation of adaptive immune responses.

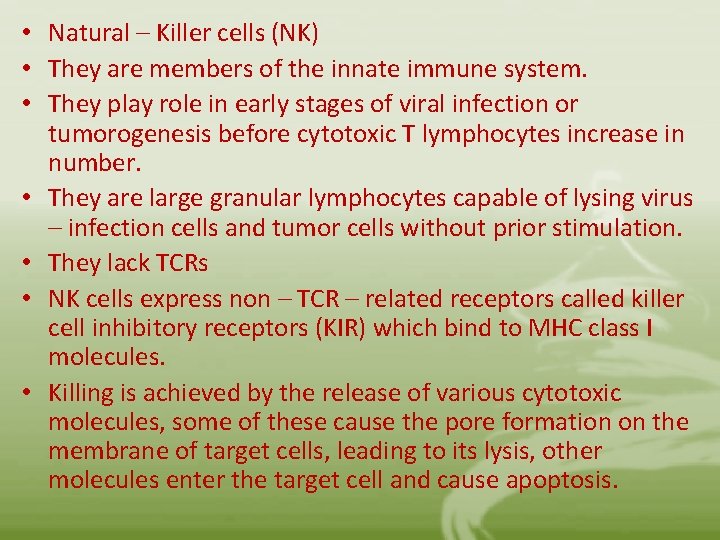
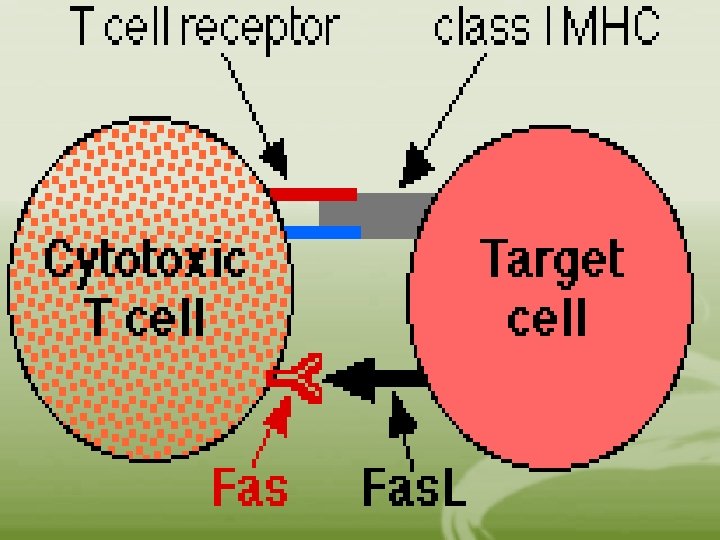
• Natural – Killer cells (NK) • They are members of the innate immune system. • They play role in early stages of viral infection or tumorogenesis before cytotoxic T lymphocytes increase in number. • They are large granular lymphocytes capable of lysing virus – infection cells and tumor cells without prior stimulation. • They lack TCRs • NK cells express non – TCR – related receptors called killer cell inhibitory receptors (KIR) which bind to MHC class I molecules. • Killing is achieved by the release of various cytotoxic molecules, some of these cause the pore formation on the membrane of target cells, leading to its lysis, other molecules enter the target cell and cause apoptosis.

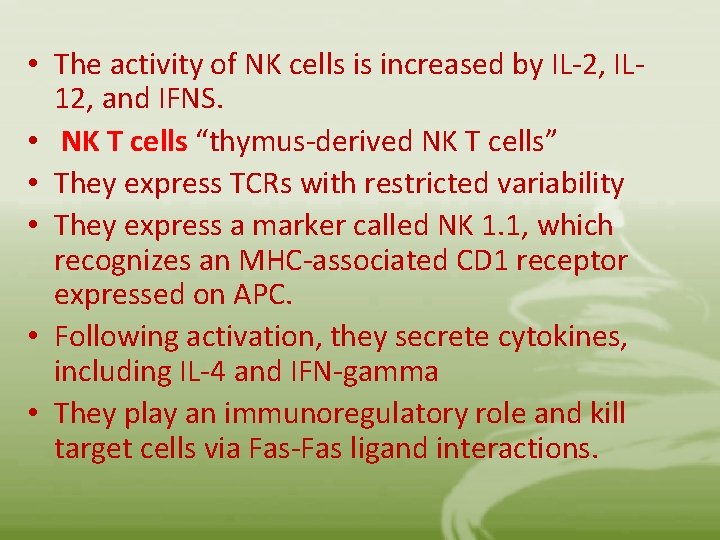
• The activity of NK cells is increased by IL-2, IL 12, and IFNS. • NK T cells “thymus-derived NK T cells” • They express TCRs with restricted variability • They express a marker called NK 1. 1, which recognizes an MHC-associated CD 1 receptor expressed on APC. • Following activation, they secrete cytokines, including IL-4 and IFN-gamma • They play an immunoregulatory role and kill target cells via Fas-Fas ligand interactions.


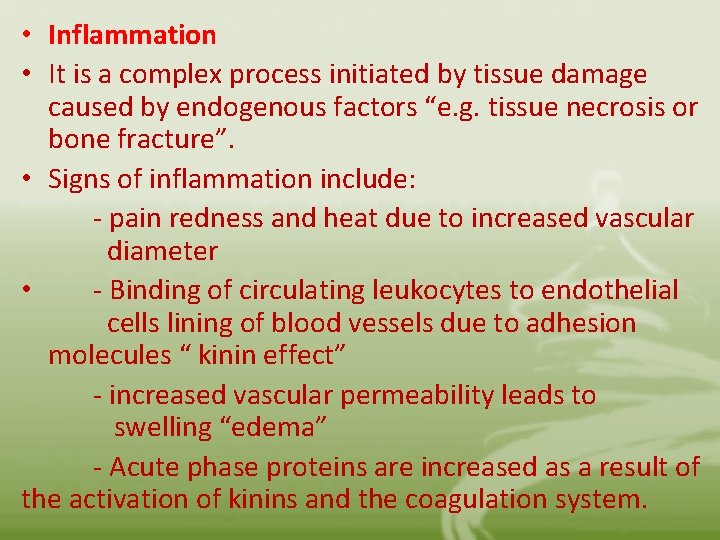
• Inflammation • It is a complex process initiated by tissue damage caused by endogenous factors “e. g. tissue necrosis or bone fracture”. • Signs of inflammation include: - pain redness and heat due to increased vascular diameter • - Binding of circulating leukocytes to endothelial cells lining of blood vessels due to adhesion molecules “ kinin effect” - increased vascular permeability leads to swelling “edema” - Acute phase proteins are increased as a result of the activation of kinins and the coagulation system.

Kinins effects “e. g. bradykinin” • Cause muscle contraction • Stimulate nerves causing pain • Increases vascular permeability which leads to expression of endothelial cell adhesion molecules. The coagulation pathway • Formation of physical barrier “clot” that prevents microorganisms from entering the blood stream. The systemic inflammatory response • Induction of fever • Leukocytosis • Increased synthesis of hydrocortisone and ACTH

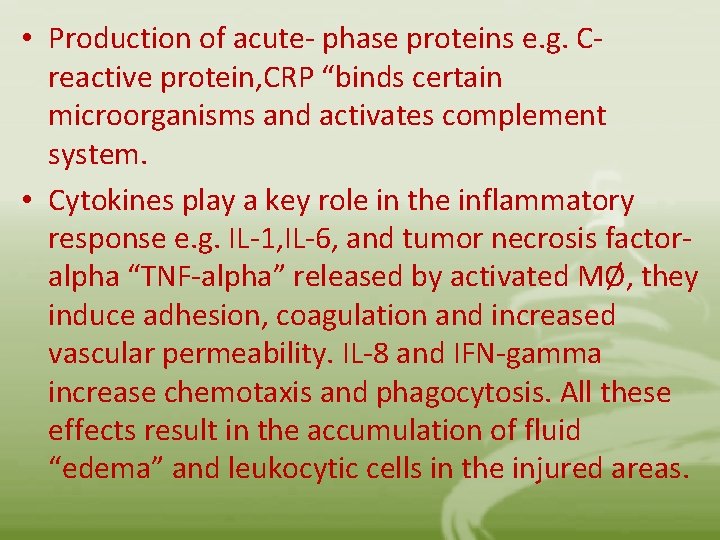
• Production of acute- phase proteins e. g. Creactive protein, CRP “binds certain microorganisms and activates complement system. • Cytokines play a key role in the inflammatory response e. g. IL-1, IL-6, and tumor necrosis factoralpha “TNF-alpha” released by activated MØ, they induce adhesion, coagulation and increased vascular permeability. IL-8 and IFN-gamma increase chemotaxis and phagocytosis. All these effects result in the accumulation of fluid “edema” and leukocytic cells in the injured areas.

• Cells involved in the inflammatory response are phagocytic cells. – PMNLS accumulate within 30 -60 min phagocytize and release lysosomal enzymes. – Within 4 -6 h the area will be infiltrated by mononuclear cells e. g. mØ and lymphocytes. mØ are phagocytic and act as APCs. • If injury or invasion of microorganisms continues, the inflammatory response will be augmented by elements of acquired immunity and CMI. • If inflammation is chronic e. g. chronic activation of immune response “ rheumatoid arthritis, glomerulonephritis”, anti – inflammatory agents e. g. aspirin and cortisone, are used , they do not affect the root cause of the inflammation.

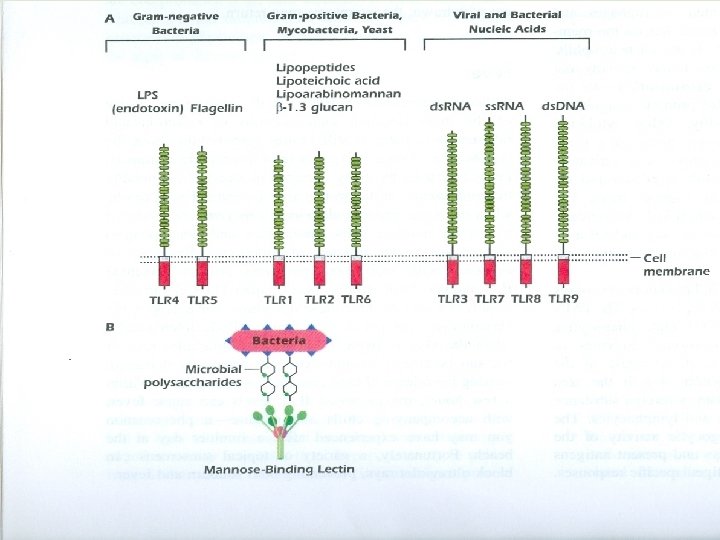
Receptors involved in the Innate Immune system Pattern Recognition Receptors • F-met-Leu-phe receptor on neutrophils, a chemotactic receptor that binds the N-formylated peptides of certain bacteria and guides neutrophils to the site of infection • Mannan – binding “MB” lectin § These enable phagocytes to recognize pathogenic microbe – expressed polysaccharides. § Ligation of the phagocyte MB lectin “receptor” with these sugars initiated and activated the MB lectin pathway of complement.

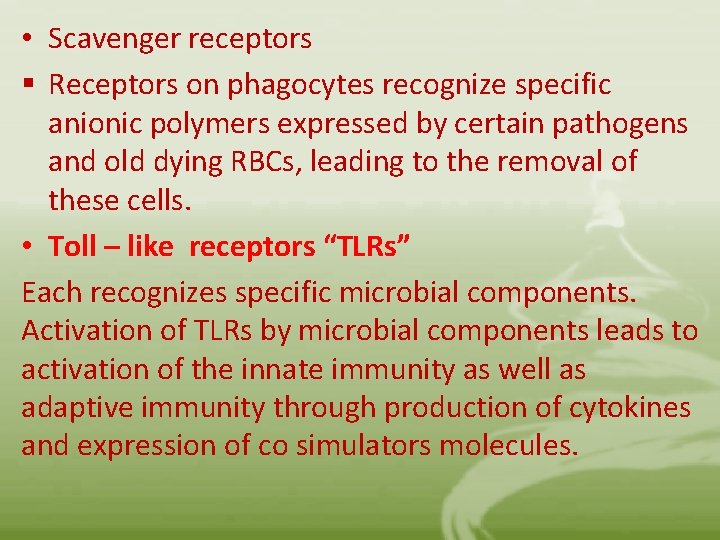
• Scavenger receptors § Receptors on phagocytes recognize specific anionic polymers expressed by certain pathogens and old dying RBCs, leading to the removal of these cells. • Toll – like receptors “TLRs” Each recognizes specific microbial components. Activation of TLRs by microbial components leads to activation of the innate immunity as well as adaptive immunity through production of cytokines and expression of co simulators molecules.



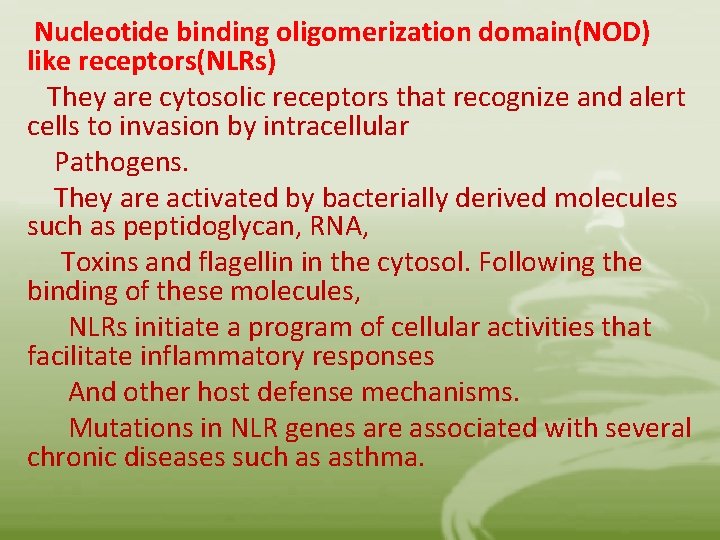
Nucleotide binding oligomerization domain(NOD) like receptors(NLRs) They are cytosolic receptors that recognize and alert cells to invasion by intracellular Pathogens. They are activated by bacterially derived molecules such as peptidoglycan, RNA, Toxins and flagellin in the cytosol. Following the binding of these molecules, NLRs initiate a program of cellular activities that facilitate inflammatory responses And other host defense mechanisms. Mutations in NLR genes are associated with several chronic diseases such as asthma.

Adaptive (Acquired) Immunity characteristics: • Generation of antigen – specific lymphocytes “effector cells” • Generation of Memory cells which prevent reinfection with the same organism. • It takes more time to develop “>96 h” • It exhibits specificity against foreign substances.

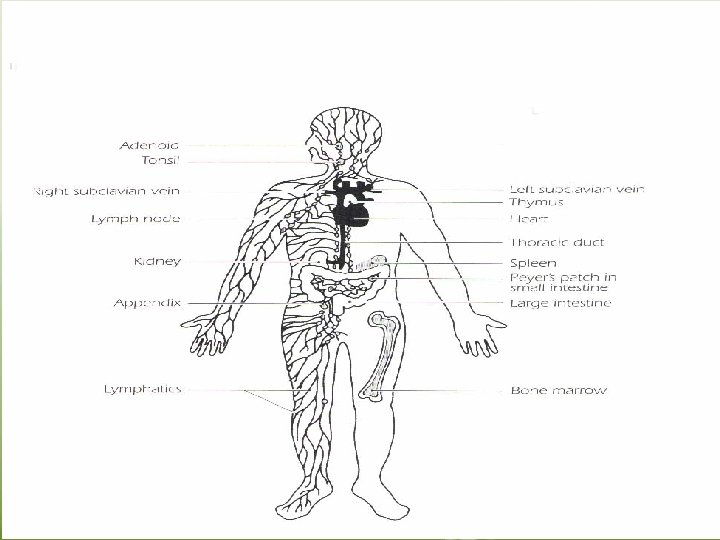
Lymphatic organs • These organs are the sites in which lymphocyte maturation, differentiation and proliferation take place. A. The primary “central” lymphoid organs. . . Thymus gland bone marrow. Maturation of T and B lymphocytes into antigen – recognizing lymphocytes and acquiring their antigen – specific receptors take place in primary lymphoid organs. B. Secondary “peripheral”lymphoid organs They are those organs in which antigen – driven proliferation and differentiation take place. These include lymph nodes, spleen, gut-associated lymphoid tissues such as tonsils.


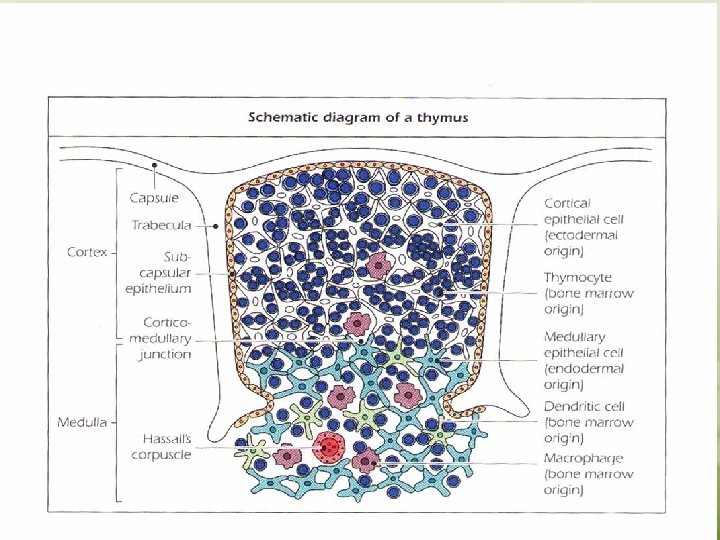
q Thymus Gland. . . See the figure • Progenitor cells from the bone marrow migrate to the thymus, where they differentiate into T lymphocytes • The thymus gland is a lymphoepitheloid bilobed structure • It is derived from the endoderm of the third and fourth pharyngeal pouches. • During fetal development, the size of the thymus increases, the growth continues until puberty, thereafter, the thymus undergoes atrophy with aging. • The cortex and medulla are infiltrated with thymocytes. T lymphocytes mature in cortex and migrate to the medulla. Macrophages and dendritic cells are found in medulla.


Macrophages are involved in clearing apoptotic thymocytes. Mature T lymphocytes migrate to the secondary lymphoid organs in which they encounter and respond to foreign antigens. • T lymphocytes recognize and respond to foreign antigen via their specific receptors “TCR” which is acquired during differentiation in the thymus. • Only 5 -10% of maturing lymphocytes survive and eventually leave thymus, 90 -95% of all thymocytes die in the thymus. • Lymphocytes which die have developed specificity to self structures or have failed to make functional receptors and, therefore, are eliminated.

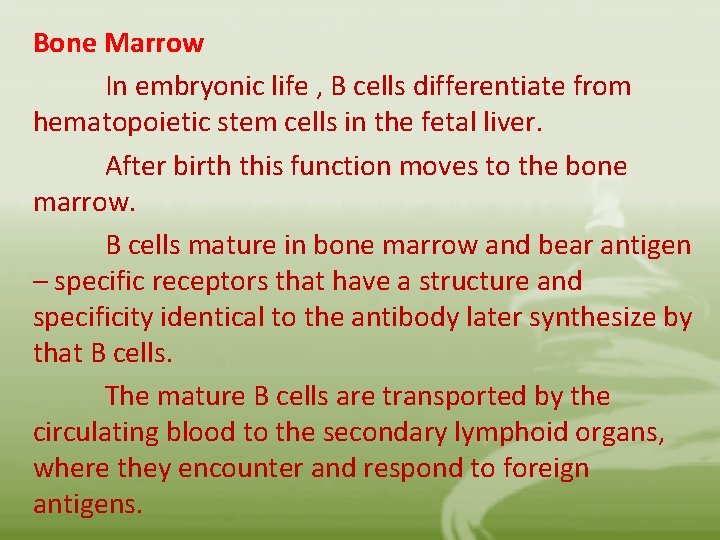
Bone Marrow In embryonic life , B cells differentiate from hematopoietic stem cells in the fetal liver. After birth this function moves to the bone marrow. B cells mature in bone marrow and bear antigen – specific receptors that have a structure and specificity identical to the antibody later synthesize by that B cells. The mature B cells are transported by the circulating blood to the secondary lymphoid organs, where they encounter and respond to foreign antigens.

Secondary Lymphoid Organs • The major secondary lymphoid organs are the spleen and lymph nodes. In addition, tonsils, appendix, peyer’s patches, and lymphoid aggregates spread throughout mucosal tissue “mucosal – associated lymphoid tissue – MALT”. Those associated with the gut “gut – associated lymphoid tissue – GALT, bronchus – associated lymphoid tissue BALT” • The secondary lymphoid organs have two major functions: 1. Trapping and concentrating foreign substances 2. The main sites of production of antibodies and the induction of antigen – specific T Lymphocytes.

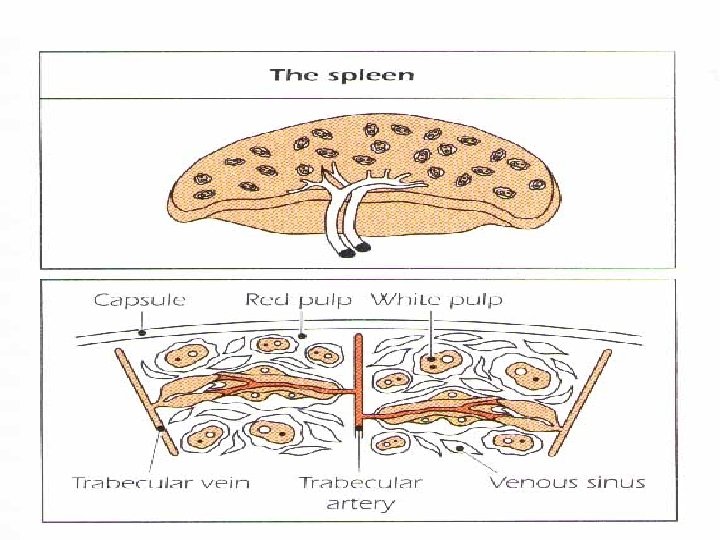
The spleen • The largest organ of the secondary lymphoid organs. • Traps and concentrates foreign substances carried in the blood. • The major organ of antibody synthesis • White pulp is rich in lymphoid cells • Red pulp contains many sinuses, large quantities of RBCs and MØ , some lymphocytes. • B – cells are present mainly in germinal centers, T cells in the peripheral region. • Approximately 50% of spleen cells are B lymphocytes, 30 -40% are T lymphocytes. • After antigenic stimulation, the germinal centers contain large numbers of B cells and plasma cells, these cells synthesize and release antibodies.


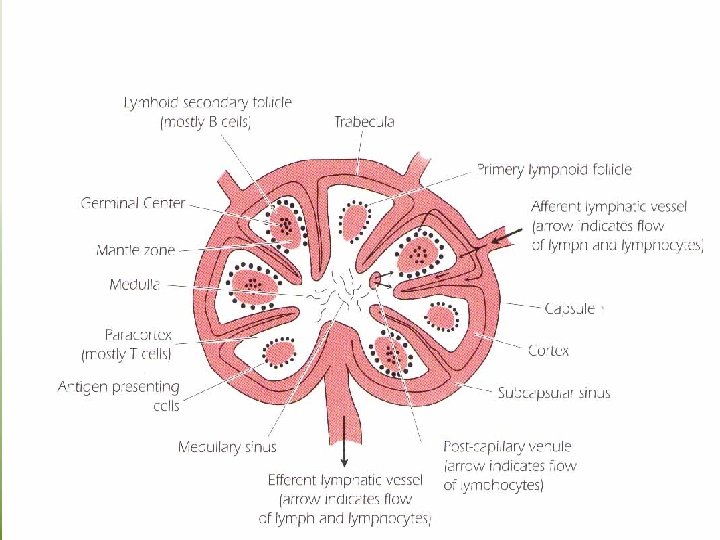
Lymph nodes • lymph nodes are small, ovoid structures • They are close to major junction of the lymphatic channels, which are connected to the thoracic duct. • The thoracic duct transports lymph and lymphocytes to the vena cava, the vessel that carries blood to the right side of the heart, from where it is redistributed throughout the body. • The cortical region contains primary lymphoid follicles. • After antigenic stimulation, they enlarge to form secondary lymphoid follicles with germinal centers containing B lymphocytes. They generate clones of cells with higher affinity receptors “antibody” for the antigenic epitope that triggered the initial response.


• The deep cortical area or Para cortical region contains T cells and dendritic cells which present antigen fragments to T cells • The medullary area of the lymph node contains antibody – secreting plasma cells that have travelled from the cortex to the medulla via lymphatic vessels. Lymphocyte circulation


• The migration of lymphocytes between various lymphoid and nonlymphoid tissue and their homing to a particular site is highly regulated by means of various cell – surface adhesion molecules “CAMs” and receptors to these molecules. • Blood lymphocytes cross the endothelial vascular lining of post capillary vascular sites termed high endothelial venules “HEVs” this process is called extravasation. • Recirculating lymphocytes selectively bind to specific receptors on the HEV of lymphoid tissue. • Recirculating monocytes and granulocytes also express adhesion molecule receptors and migrate to tissue sites using a similar mechanism.

• The traffic of lymphocytes between lymphoid and non lymphoid tissue ensures that on exposure to an antigen, the antigen and the lymphocytes specific to that antigen are sequestered in the lymphoid tissue, where the lymphocytes undergo proliferation, and differentiation. The differentiated cells (memory cells) leave the lymphoid organ and are dessiminated in the body to reconcentrate at the site where antigen persists and, at that place, exert their protective function.

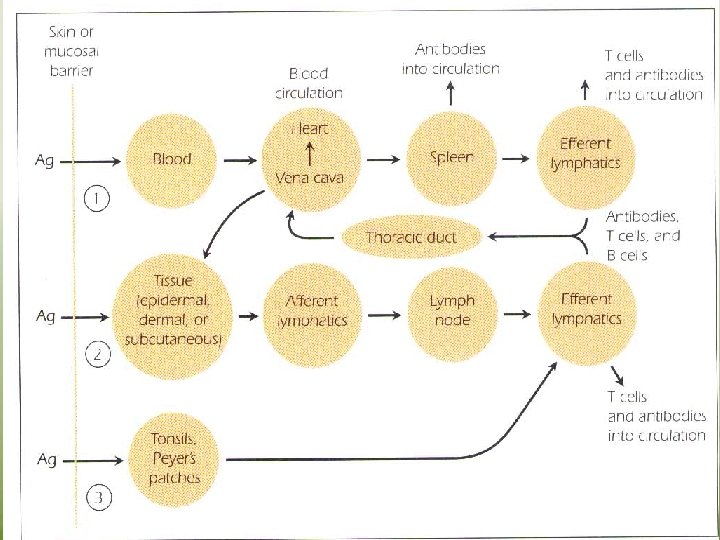
The Fate of Antigen After Penetration • The RES traps foreign antigens and subject them to ingestion and degradation by the phagocytic cells of the system • There is constant movement of lymphocytes which direct them to be deposited in strategic places along the lymphatic vessels. • The system also provides Loci “ the secondary lymphoid organs” where antigen, mØ, T cells and B cells can interact within a very small area to initiate an immune response. • Three major route may be followed by an antigen after it has penetrated the interior of the body

1. If the antigen enters the body through the blood stream, it is carried to spleen, where it interacts with APCs “dendritic cells, MØ , B cells”. APCs activate – antigen – specific T cells. The spleen releases antibodies into the circulation. Lymphocytes leave the spleen through the efferent lymphatics to reenter the circulation via the thoracic duct. 2. - The antigen may lodge in epidermal , dermal or subcutaneons tissue, where it may cause an inflammatory response. - When antigen is trapped by APCs, it is transported through the afferent lymphatic channels into the regional lymph nodes. In the lymph node, the antigen, MØ, dendritic cells, T cells and B cells interact to generate an immune response.

Antigen –specific T cells and antibodies which have been synthesised in the lymph node , enter the circulation via the thoracic duct. Antitgen may enter GIT or respiratory tract, where it lodges in the MALT. Where it interact with MØ and lymphocytes. Antibodies are deposited in local tissue, in addition, lymphocytes entering the efferent lymphatics are carried through the thoracic duct to the circulation and redistributed to various tissues.

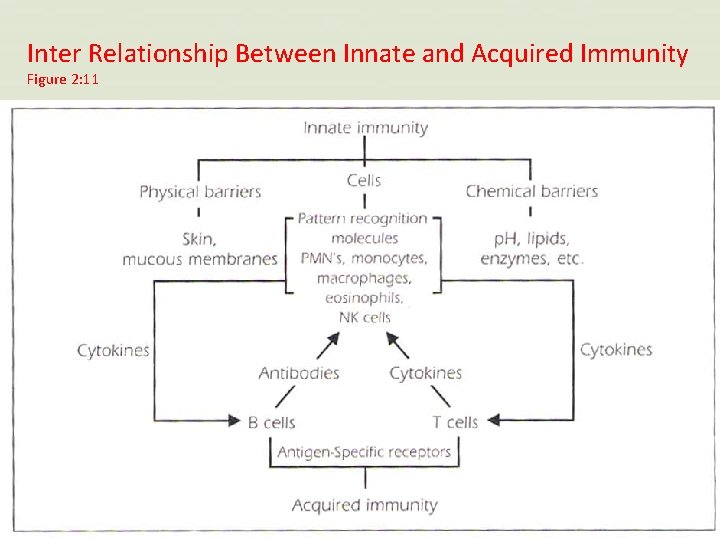
Inter Relationship Between Innate and Acquired Immunity Figure 2: 11
 Difference between acquired immunity and innate immunity
Difference between acquired immunity and innate immunity Acquired immunity
Acquired immunity Acquired immunity definition
Acquired immunity definition Acquired immunity
Acquired immunity Naturally acquired passive immunity definition
Naturally acquired passive immunity definition Is conscience innate or acquired
Is conscience innate or acquired Innate immunity examples
Innate immunity examples Innate immunity examples
Innate immunity examples Neutrophil extracellular traps
Neutrophil extracellular traps Innate immunity first line of defense
Innate immunity first line of defense Innate immunity
Innate immunity Innate immunity first line of defense
Innate immunity first line of defense Innate immunity first line of defense
Innate immunity first line of defense Innate immunity first line of defense
Innate immunity first line of defense Salmonella life cycle
Salmonella life cycle Innate immunity
Innate immunity Inherited and acquired traits
Inherited and acquired traits Training is the act of increasing the
Training is the act of increasing the Father of principles of management
Father of principles of management How knowledge is acquired represented and organized
How knowledge is acquired represented and organized The difference between humoral and cell mediated immunity
The difference between humoral and cell mediated immunity Chapter 13 lymphatic system and immunity
Chapter 13 lymphatic system and immunity What is immunity
What is immunity Chapter 16 lymphatic system and immunity
Chapter 16 lymphatic system and immunity Chapter 22 lymphatic system and immunity
Chapter 22 lymphatic system and immunity Acquired thrombophilia
Acquired thrombophilia Acquired thrombophilia
Acquired thrombophilia Milstrip
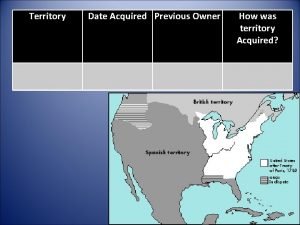
Milstrip British cession date
British cession date Systemic acquired resistance in plants
Systemic acquired resistance in plants Inherited vs acquired traits
Inherited vs acquired traits Inherited traits
Inherited traits What are some acquired traits
What are some acquired traits Inherited traits and learned behaviors 5th grade
Inherited traits and learned behaviors 5th grade Acquired physical traits
Acquired physical traits Whats a acquired trait
Whats a acquired trait Systemic acquired resistance in plants
Systemic acquired resistance in plants Causes of hemolysis
Causes of hemolysis Signs of hemolysis
Signs of hemolysis Acquired physical traits
Acquired physical traits Examples of biotic components
Examples of biotic components Acquired needs theory
Acquired needs theory Anmeia
Anmeia Infer how the pigs acquired another case of whiskey.
Infer how the pigs acquired another case of whiskey. Acquired traits examples
Acquired traits examples Acquired trait
Acquired trait Lance brothers enterprises acquired
Lance brothers enterprises acquired Mexican cession date acquired
Mexican cession date acquired Acquired progressive kinking of the hair
Acquired progressive kinking of the hair