Acids n Strong Acids Two types of strong






















![Calculation of [OH ] n Example 12: Calculate the p. H of a 0. Calculation of [OH ] n Example 12: Calculate the p. H of a 0.](https://slidetodoc.com/presentation_image/527bffa9e98f1eb16f99154f6966ae74/image-27.jpg)






















- Slides: 51

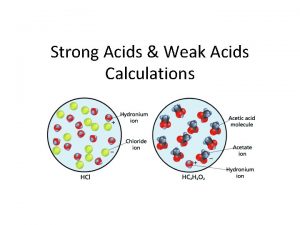
Acids n Strong Acids: Two types of strong acids, with examples that you should memorize, are n n n 1. The hydrohalic acids HCl, HBr, and HI 2. Oxoacids in which the number of O atoms exceeds the number of ionizable protons by two or more, such as HNO 3, H 2 SO 4, and HCl. O 4; for example, in H 2 SO 4, 4 O’s − 2 H’s = 2 Weak acids: There are many more weak acids than strong ones. Four types, with examples, are n n 1. The hydrohalic acid HF 2. Acids in which H is not bonded to O or to a halogen, such as HCN and H 2 S 3. Oxoacids in which the number of O atoms equals or exceeds by one the number of ionizable protons, such as HCl. O, HNO 2, and H 3 PO 4 4. Carboxylic acids (general formula RCOOH, with the ionizable proton shown in red), such as CH 3 COOH and C 6 H 5 COOH

Bases n Strong bases: Water-soluble compounds containing O 2− or OH− ions are strong bases. The cations are usually those of the most active metals: n n n 1. M 2 O or MOH, where M = Group 1 A(1) metal (Li, Na, K, Rb, Cs) 2. MO or M(OH)2, where M = Group 2 A(2) metal (Ca, Sr, Ba) [Mg. O and Mg(OH)2 are only slightly soluble in water, but the soluble portion dissociates completely. ] Weak bases: Many compounds with an electron- rich nitrogen atom are weak bases (none are Arrhenius bases). The common structural feature is an N atom with a lone electron pair: n n 1. Ammonia 2. Amines R NH 2


Bronsted-Lowry Acid-Base Model n n n This model focuses on the nature of acids and bases and the reactions between them. Definitions: Acid – proton (H+ ion) donor Base – proton (H+ ion) acceptor In an acid base reaction, a proton is transferred from an acid to a base: HB + A ⇌ HA + B Conjugate base: the species formed when a proton is removed from the acid. Conjugate acid: the species formed when a proton is added to a base.



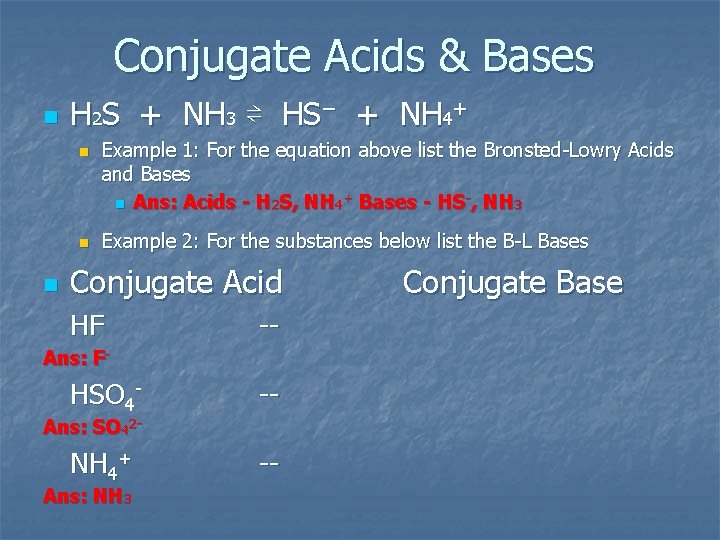
Conjugate Acids & Bases n H 2 S + NH 3 ⇌ HS− + NH 4+ n n n Example 1: For the equation above list the Bronsted Lowry Acids and Bases n Ans: Acids - H 2 S, NH 4+ Bases - HS-, NH 3 Example 2: For the substances below list the B L Bases Conjugate Acid HF Ans: F- HSO 4 NH 4+ Ans: SO 42 Ans: NH 3 Conjugate Base

Amphiprotic n n n A species that can either accept or donate a proton. For example: Water is amphiprotic: H 2 O + H 2 O ⇌ H 3 O+ + OH Example 3: What is the conjugate acid and base for n The bicarbonate ion n n Ans: H 2 CO 3, CO 32 - Hydrogen sulfate ion n Ans: H 2 S, S 2 -

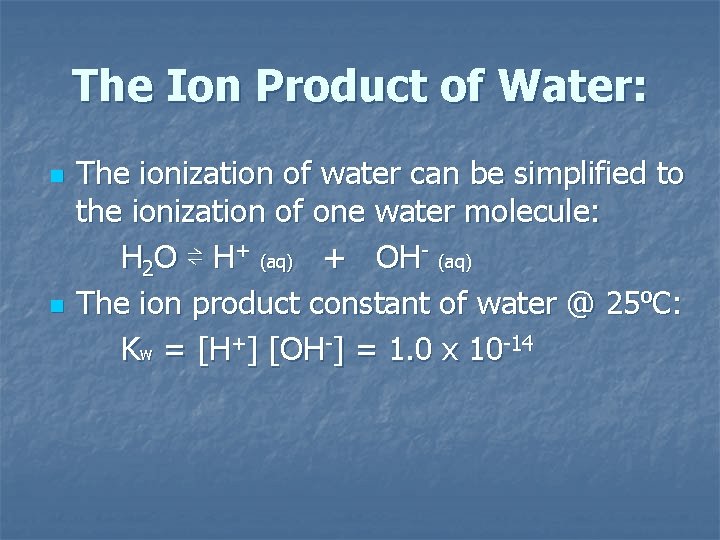
The Ion Product of Water: n n The ionization of water can be simplified to the ionization of one water molecule: H 2 O ⇌ H+ (aq) + OH (aq) The ion product constant of water @ 25⁰C: Kw = [H+] [OH ] = 1. 0 x 10 14

Ion Product Concentration of Water n n The concentrations of H+ (H+ = H 3 O+) and OH ions are can be calculated from the Kw in pure water @ 25⁰C When the H+ ions equal the OH ions the solution is said to be a neutral solution: n n n If [H+] is > 1. 0 x 10 7, [OH ] < 1. 0 x 10 7 solution is acidic If [OH ] is > 1. 0 x 10 7, [H+] < 1. 0 x 10 7 solution is basic These 2 quantities have an inversely proportional relationship

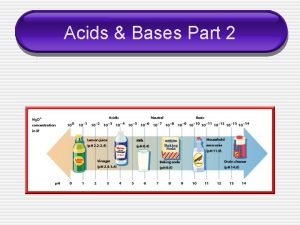
p. H n n This is an alternative method for specifying the acidity of a solution. p. H stands for the power of the hydrogen ion and can be found using the following: p. H = log[H+] = log[H 3 O+] or the same formula can be used to determine the [H+] concentration by: [H+] = 10 -p. H n Determining acidic or basic: n n n Acidic = p. H < 7, the lower the # the more acidic the solution Basic = p. H > 7, the higher the # the more basic the solution Neutral = p. H = 7


p. OH n n A similar approach is used to determine the hydroxide ion concentration: p. OH = log[OH ] Because [H+] [OH ] = 1. 0 x 10 14 then the p. H and p. OH are connected by the following: p. H + p. OH = 14. 00


Calculating p. H and p. OH n Example 4: At 25 o. C, calculate n the hydrogen ion concentration and p. H of a tap water sample in which the hydroxide ion concentration is 2. 0 x 10 7. n n The hydrogen and hydroxide ion concentrations of human blood at a p. H of 7. 40. n n Ans: 5. 0 x 10 -8 M Ans: 4. 0 x 10 -8 M, 2. 5 x 10 -7 M The p. OH of a solution in which the [H+] = 5. 0[OH ]. n Ans: 7. 35

Strong Acids & Bases n n The p. H of strong acids and bases ionize completely in water and because of this it is relatively easy to determine the p. H and p. OH. Example 5: What is the p. H of a solution prepared by dissolving 1. 00 g of barium hydroxide per liter? n Ans: 12. 07

Weak Acids & Their Equilibrium Constants Weak acids react reversibly with water to form H+ ions. n Most weak acids fall into two categories: n 1. Molecules containing an ionizable hydrogen atom: HNO 2 (aq) + H 2 O ⇌ H 3 O+ (aq) + NO 2 (aq) n 2. Cations. The ammonium ion acts as a weak acid in water. NH 4+ (aq) + H 2 O ⇌ H 3 O+ (aq) + NH 3 (aq) n Many metal cations actually act as weak acids: Al(H 2 O)6+3

Ka The equilibrium constant for a weak acid: HX ⇌ H+ + X Ka = [H+] [X ] [HX] *the smaller the Ka value the weaker the acid. n

p. Ka n n n We sometimes use a p. Ka value for weak acids: p. Ka = log. Ka The larger the p. Ka the weaker the acid. Example 6: consider acetic acid (ka=1. 8 x 10 5) and the tetraaquazinc(II) ion (ka=3. 3 x 10 10). n Write the equations to show why these species are acidic. n Which is the stronger acid? n n Ans: acetic acid What is the p. Ka of the tetraaquazinc ion? n Ans: 9. 48

Calculating Ka using an equilibrium table… n Example 7: Aspirin is a weak organic acid whose molecular formula maybe written as HC 9 H 7 O 4. A water solution of aspirin is prepared by dissolving 3. 60 g per liter. The p. H of the solution is found to be 2. 60. Calculate the Ka for aspirin. n Ans: 3. 6 x 10 -4

% Ionization n n Percent Ionization allows us to measure the percent of H+ ion that are ionized: % ionization = [H+] x 100 [HX] Example 8: What percent of H+ ions from the aspirin ionized in the previous example? n Ans: 12%

Example 9 n Nicotinic acid, HC 6 H 4 O 2 N (Ka = 1. 4 x 10 5), is another name for niacin, an important member of the vitamin B group. Determine the [H+] in a solution prepared by dissolving 0. 10 mol of nicotinic acid, HNic, in water to form one liter of solution. n [H+] = 1. 2 x 10 -3 M

Polyprotic Weak Acids n These are acids that have more than one ionizable hydrogen ion. These acids ionize in steps, with a separate equilibrium constant for each one: ⇌ HC 2 O 4 (aq) ⇌ H+ (aq) + C 2 O 4 2 H 2 C 2 O 4 (aq) H+ (aq) + HC 2 O 4 n n Ka 1 = 5. 9 x 10 2 Ka 2 = 5. 2 x 10 5 The anion formed in step one produces another H+ ion in the next step. The Ka becomes smaller with each successive step: Ka 1 > Ka 2 > Ka 3

Calculating p. H of a polyprotic acid n Example 10: The distilled water you use in the laboratory is slightly acidic because of dissolved carbon dioxide, which reacts to form carbonic acid. Calculate the p. H of a 0. 0010 M solution of carbonic acid (Ka 1 = 4. 4 x 10 7, Ka 2 = 4. 7 x 10 11). n Ans: 4. 68

Weak Bases & Their Equilibrium Constants n Most weak bases, like acids, fall into two categories: n 1. Molecules, including organic compounds known as amines: NH 3 (aq) + H 2 O ⇌ NH 4+ (aq) + OH (aq) n 2. Anion. An anion derived from a weak acid is itself a weak base: F (aq) + H 2 O ⇌ HF (aq) + OH (aq)

Weak Bases n Example 11: Write an equation to explain why each of the following produces a basic water solution. NO 2 n Na 2 CO 3 n KHCO 3 n
![Calculation of OH n Example 12 Calculate the p H of a 0 Calculation of [OH ] n Example 12: Calculate the p. H of a 0.](https://slidetodoc.com/presentation_image/527bffa9e98f1eb16f99154f6966ae74/image-27.jpg)
Calculation of [OH ] n Example 12: Calculate the p. H of a 0. 10 M Na. F (Kb = 1. 4 x 10 11). n Ans: 8. 08

Ka and Kb n The Ka of a weak acid and the Kb of a weak base will equal Kw:

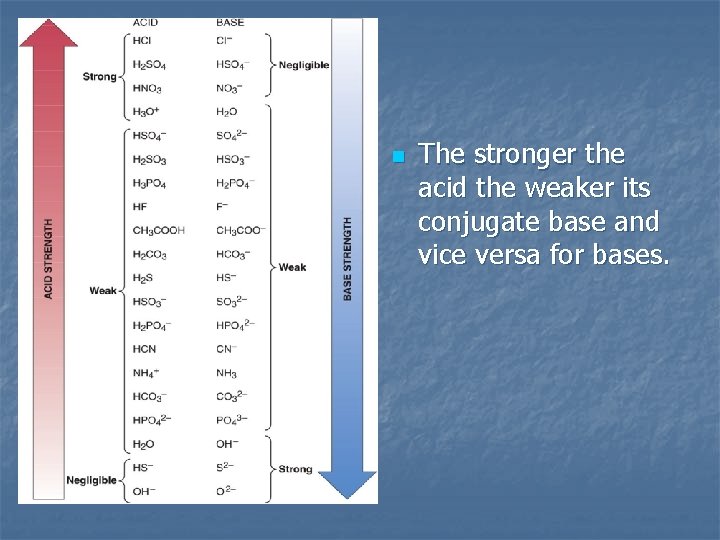
n The stronger the acid the weaker its conjugate base and vice versa for bases.

Acid Base Properties of Solutions n n A salt is an ionic solid containing a cation other than H+ and an anion other than OH. It is important to remember that anions of strong acids and cations of strong bases are neutral in solution. n n n All other cations will be acidic in nature. All other anions will be basic in nature. To predict whether a given salt solution will be acidic, basic, or neutral, consider the three factors: n n n 1. Decide what effect, if any, the cation has on the p. H of water. 2. Decide what effect, if any, the anion will have on the p. H of water. 3. Combine the 2 effects to decide the behavior of the salt. n If both cation and anion have a K value, the one with the larger value decides acidic or basic.

Salts: Acidic, Basic, Neutral? n Example 13: Consider water solutions of the following six salts and identify if the solution are acidic, basic, or neutral: n Ammonium iodide n n Zinc nitrate n n Ans: basic Ammonium fluoride n n Ans: neutral Sodium phosphate n n Ans: acidic Potassium perchlorate n n Ans: acidic Ammonium hypochlorite n Ans: basic

MC #1 n Which of the following ions is the strongest Lewis acid? (A) Na+ (B) Cl¯ (C) CH 3 COO¯ (D) Mg 2+ (E) Al 3+

MC #2 n Each of the following can act as both a Brönsted acid and a Brönsted base EXCEPT (A) HCO 3¯ (B) H 2 PO 4¯ (C) NH 4+ (D) H 2 O (E) HS¯

MC #3 n Which, if any, of the following species is in the greatest concentration in a 0. 100 molar solution of H 2 SO 4 in water? (A) H 2 SO 4 molecules (B) H 3 O+ ions (C) HSO 4¯ ions (D) SO 42¯ ions (E) All species are in equilibrium and therefore have the same concentrations.

MC #4 n Which of the following reactions does NOT proceed significantly to the right in aqueous solutions? (A) H 3 O+ + OH¯ > 2 H 2 O (B) HCN + OH¯ > H 2 O + CN¯ (C) Cu(H 2 O)42+ + 4 NH 3 > Cu(NH 3)42+ + 4 H 2 O (D) H 2 SO 4 + H 2 O > H 3 O+ + HSO 4¯ (E) H 2 O + HSO 4¯ > H 2 SO 4 + OH¯

MC #5 n If the acid dissociation constant, Ka, for an acid HA is 8 x 10¯ 4 at 25 °C, what percent of the acid is dissociated in a 0. 50 molar solution of HA at 25 °C? (A) 0. 08% (B) 0. 2% (C) 1% (D) 2% (E) 4%

MC #6 n All of the following species can function as Brönsted Lowry bases in solution EXCEPT (A) H 2 O (B) NH 3 (C) S 2¯ (D) NH 4+ (E) HCO 3¯

MC #7 n As the number of oxygen atoms increases in any series of oxygen acids, such as HXO, HXO 2, HXO 3, . . , which of the following is generally true? (A) The acid strength varies unpredictably. (B) The acid strength decreases only if X is a nonmetal. (C) The acid strength decreases only if X is a metal. (D) The acid strength decreases whether X is a nonmetal or a metal. (E) The acid strength increases.

MC #8 n A 0. 20 molar solution of a weak monoprotic acid, HA, has a p. H of 3. 00. The ionization constant of this acid is (A) 5. 0 x 10¯ 7 (B) 2. 0 x 10¯ 7 (C) 5. 0 x 10¯ 6 (D) 5. 0 x 10¯ 3 (E) 2. 0 x 10¯ 3

MC #9 n HSO 4¯ + H 2 O <===> H 3 O+ + SO 42– In the equilibrium represented above, the species that act as bases include which of the following? I. HSO 4¯ II. H 2 O III. SO 42– (A) II only (B) III only (C) I and II (D) I and III (E) II and III

MC #10 n H 2 C 2 O 4 + 2 H 2 O <===> 2 H 3 O+ + C 2 O 42¯ Oxalic acid, H 2 C 2 O 4, is a diprotic acid with K 1 = 5 x 10¯ 2 and K 2 = 5 x 10¯ 5. Which of the following is equal to the equilibrium constant for the reaction represented above? (A) 5 x 10¯ 2 (B) 5 x 10¯ 5 (C) 2. 5 x 10¯ 6 (D) 5 x 10¯ 7 (E) 2. 5 x 10¯ 8

MC #11 n A 1 molar solution of which of the following salts has the highest p. H ? (A) Na. NO 3 (B) Na 2 CO 3 (C) NH 4 Cl (D) Na. HSO 4 (E) Na 2 SO 4

MC #12 n What is the p. H of a 1. 0 x 10¯ 2 M solution of HCN? (Ka = 4. 0 x 10¯ 10. ) (A) 10 (B) Between 7 and 10 (C) 7 (D) Between 4 and 7 (E) 4

MC #13 n The net ionic equation for the reaction that occurs during the titration of nitrous aicd with sodium hydroxide is (A) HNO 2 + Na+ + OH¯ > Na. NO 2 + H 2 O (B) HNO 2 + Na. OH > Na+ + NO 2¯ + H 2 O (C) H+ + OH¯ >H 2 O (D) HNO 2 + H 2 O > NO 2¯ + H 3 O+ (E) HNO 2 + OH¯ > NO 2¯ + H 2 O

MC #14 n What is the H+ (aq) concentration in 0. 05 M HCN (aq)? (The Ka for HCN is 5. 0 x 10¯ 10) (A) 2. 5 x 10¯ 11 (B) 2. 5 x 10¯ 10 (C) 5. 0 x 10¯ 10 (D) 5. 0 x 10¯ 6 (E) 5. 0 x 10¯ 4

MC #15 A molecule or an ion is classified as a Lewis acid if it (A) accepts a proton from water (B) accepts a pair of electrons to form a bond (C) donates a pair of electrons to form a bond (D) donates a proton to water (E) has resonance Lewis electron dot structures n

FRQ #1 A pure 14. 85 g sample of the weak base ethylamine, C 2 H 5 NH 2 , is dissolved in enough distilled water to make 500. m. L of solution. n n (a) Calculate the molar concentration of the C 2 H 5 NH 2 in the solution. The aqueous ethylamine reacts with water according to the equation below. C 2 H 5 NH 2(aq) + H 2 O(l) C 2 H 5 NH 3+(aq) + OH (aq) (b) Write the equilibrium constant expression for the reaction between C 2 H 5 NH 2(aq) and water. (c) Of C 2 H 5 NH 2(aq) and C 2 H 5 NH 3+(aq), which is present in the solution at the higher concentration at equilibrium? Justify your answer. *(d) A different solution is made by mixing 500. m. L of 0. 500 M C 2 H 5 NH 2 with 500. m. L of 0. 200 M HCl. Assume that volumes are additive. The p. H of the resulting solution is found to be 10. 93. n (i) Calculate the concentration of OH (aq) in the solution. n n n (ii) Write the net ionic equation that represents the reaction that occurs when the C 2 H 5 NH 2 solution is mixed with the HCl solution. (iii) Calculate the molar concentration of the C 2 H 5 NH 3+(aq) that is formed in the reaction. (iv) Calculate the value of Kb for C 2 H 5 NH 2.

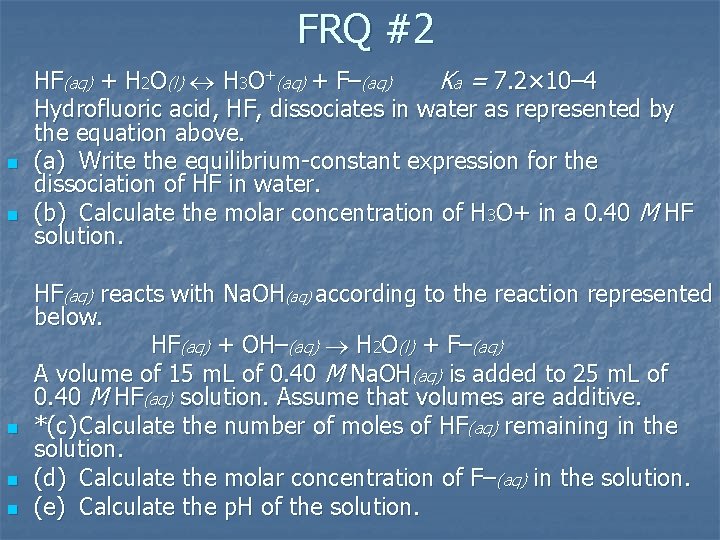
FRQ #2 n n n HF(aq) + H 2 O(l) H 3 O+(aq) + F–(aq) Ka = 7. 2× 10– 4 Hydrofluoric acid, HF, dissociates in water as represented by the equation above. (a) Write the equilibrium constant expression for the dissociation of HF in water. (b) Calculate the molar concentration of H 3 O+ in a 0. 40 M HF solution. HF(aq) reacts with Na. OH(aq) according to the reaction represented below. HF(aq) + OH–(aq) H 2 O(l) + F–(aq) A volume of 15 m. L of 0. 40 M Na. OH(aq) is added to 25 m. L of 0. 40 M HF(aq) solution. Assume that volumes are additive. *(c) Calculate the number of moles of HF(aq) remaining in the solution. (d) Calculate the molar concentration of F–(aq) in the solution. (e) Calculate the p. H of the solution.

FRQ #3 HC 3 H 5 O 2(aq) ↔ C 3 H 5 O 2– (aq) + H+(aq) Ka = 1. 34 x 10– 5 Propanoic acid, HC 3 H 5 O 2, ionizes in water according to the equation above. n n n (a) Write the equilibrium constant expression for the reaction. (b) Calculate the p. H of a 0. 265 M solution of propanoic acid. (c) A 0. 496 g sample of sodium propanoate, Na. C 3 H 5 O 2, is added to a 50. 0 m. L sample of a 0. 265 M solution of propanoic acid. Assuming that no change in the volume of the solution occurs, calculate each of the following. n n (i) The concentration of the propanoate ion, C 3 H 5 O 2–(aq) in the solution (ii) The concentration of the H+(aq) ion in the so lution. The methanoate ion, HCO 2–(aq) reacts with water to form methanoic acid and hydroxide ion, as shown in the following equation. HCO 2–(aq) + H 2 O (l) ↔ H 2 CO 2(aq) + OH–(aq) n n *(d) Given that [OH–] is 4. 18 x 10– 6 M in a 0. 309 M solution of sodium methanoate, calculate each of the following. n (i) The value of Kb for the methanoate ion, HCO 2–(aq) n (ii) The value of Ka for methanoic acid, HCO 2 H *(e) Which acid is stronger, propanoic acid or methanoic acid? Justify your answer.

FRQ #4 n n n Hypochlorous acid, HOCl, is a weak acid commonly used as a bleaching agent. The acid–dissociation constant, Ka, for the reaction represented above is 3. 2× 10– 8. (a) Calculate the [H+] of a 0. 14–molar solution of HOCl. (b) Write the correctly balanced net ionic equation for the reaction that occurs when Na. OCl is dissolved in water and calculate the numerical value of the equilibrium constant for the reaction. *(c) Calculate the p. H of a solution made by combining 40. 0 milliliters of 0. 14–molar HOCl and 10. 0 milliliters of 0. 56–molar Na. OH.

FRQ #5 n n Give a brief explanation for each of the following. (a) For the diprotic acid H 2 S, the first dissociation constant is larger than the second dissociation constant by about 105 (K 1 ~ 105 K 2). (b) In water, Na. OH is a base but HOCl is an acid. (c) HCl and HI are equally strong acids in water but, in pure acetic acid, HI is a stronger acid than HCl. (d) When each is dissolved in water, HCl is a much stronger acid than HF.
 How to remember strong acids and strong bases
How to remember strong acids and strong bases Be strong be strong be strong in the lord
Be strong be strong be strong in the lord What are the strong acids
What are the strong acids Hydronium hydroxide balance
Hydronium hydroxide balance Difference between strong and weak acids
Difference between strong and weak acids Strong acids and bases
Strong acids and bases Model 1 acid strength and conductivity
Model 1 acid strength and conductivity Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Six strong acids
Six strong acids Acids and bases
Acids and bases H2o base or acid
H2o base or acid 7 strong acids
7 strong acids What are the seven strong acids
What are the seven strong acids Seven strong acids
Seven strong acids What are the seven strong acids
What are the seven strong acids What are the 7 strong acids
What are the 7 strong acids 6 strong acids
6 strong acids Seven strong acids
Seven strong acids Strong vs weak electrolytes
Strong vs weak electrolytes Concentration and strength
Concentration and strength Is chloric acid a strong acid
Is chloric acid a strong acid Six strong acids
Six strong acids Acidity trend periodic table
Acidity trend periodic table Acids and bases
Acids and bases The 6 strong acids
The 6 strong acids Seven strong acids
Seven strong acids Strong vs weak acids
Strong vs weak acids Strong acid examples
Strong acid examples Weak acid strong base titration
Weak acid strong base titration Strong acid titration curve
Strong acid titration curve Net ionic equation titration
Net ionic equation titration Acid base song
Acid base song The chain of amino acids forms a
The chain of amino acids forms a Invisible fat examples
Invisible fat examples Happy easter symbols
Happy easter symbols Examples of strong and weak syllables
Examples of strong and weak syllables Complementary and supplementary angles formula
Complementary and supplementary angles formula Two little hands two little feet
Two little hands two little feet Osha 29cfr1910.134
Osha 29cfr1910.134 Two two four cryptarithmetic solution
Two two four cryptarithmetic solution Which two points do the two passages agree on?
Which two points do the two passages agree on? Name two pairs of supplementary angles
Name two pairs of supplementary angles When two curves coincide the two objects have the same
When two curves coincide the two objects have the same Identify a key term used in both passages.
Identify a key term used in both passages. Two nonadjacent angles formed by two intersecting lines
Two nonadjacent angles formed by two intersecting lines Two beer or not two beer shakesbeer
Two beer or not two beer shakesbeer Hamlet act two scene two
Hamlet act two scene two Two witches were watching two watches
Two witches were watching two watches Contingency table in statistics
Contingency table in statistics Shall i compare thee to a summer's day annotation
Shall i compare thee to a summer's day annotation Two witches watched two watches
Two witches watched two watches Two beer or not two beer shakesbeer
Two beer or not two beer shakesbeer