Acids Concentration vs Strength WEAK STRONG CONCENTRATED H
- Slides: 7

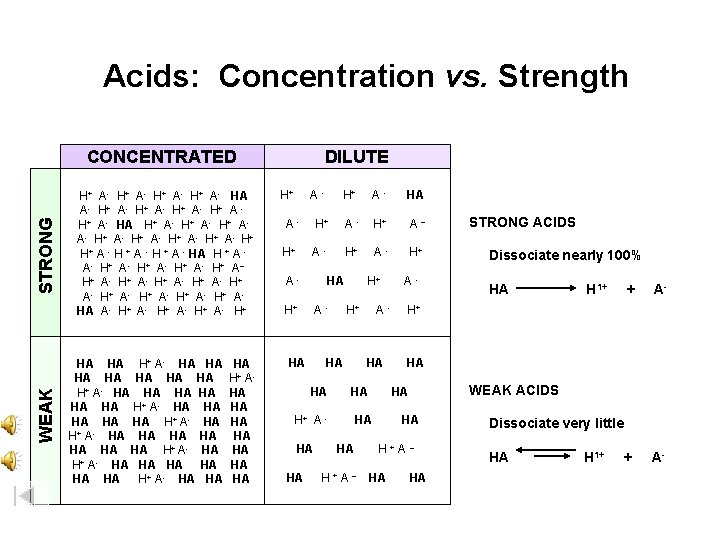
Acids: Concentration vs. Strength WEAK STRONG CONCENTRATED H+ A- HA A- H + A H+ A- HA H+ A- H+ AA- H+ A- H+ H+ A - HA H + A A - H+ A - H + A – H + A - H+ A - H + A HA A- H+ HA HA H+ A- HA HA HA HA HA H+ A- HA HA H+ A- HA HA HA H+ AHA HA DILUTE H+ A - H+ A H+ - A- - HA H+ A HA HA H+ A A H+ - A HA HA H+ A HA HA - H+ H+ - HA HA A H+A– - - Dissociate nearly 100% HA H 1+ + A- HA WEAK ACIDS HA H+A– HA STRONG ACIDS H+ HA HA HA A – HA Dissociate very little HA H 1+

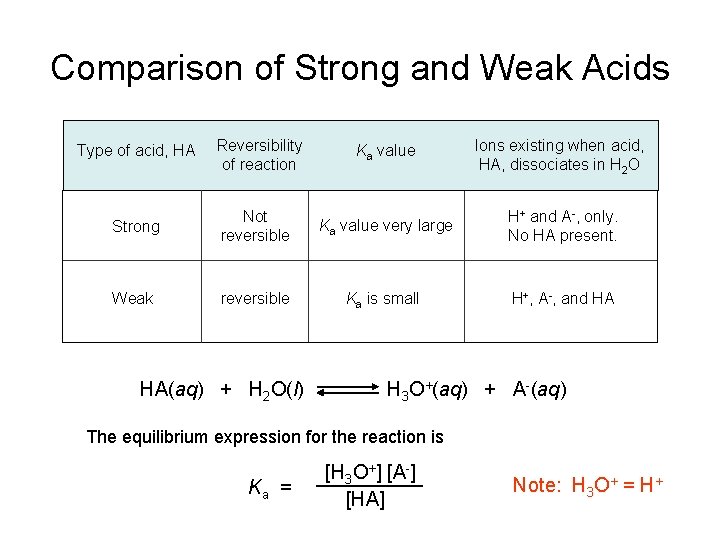
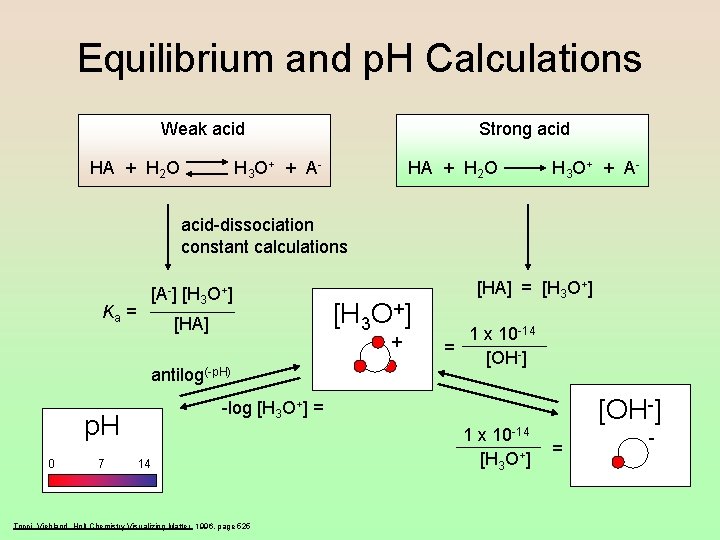
Comparison of Strong and Weak Acids Type of acid, HA Reversibility of reaction Ka value Ions existing when acid, HA, dissociates in H 2 O Strong Not reversible Ka value very large H+ and A-, only. No HA present. Weak reversible Ka is small H+, A-, and HA HA(aq) + H 2 O(l) H 3 O+(aq) + A-(aq) The equilibrium expression for the reaction is Ka = [H 3 O+] [A-] [HA] Note: H 3 O+ = H+

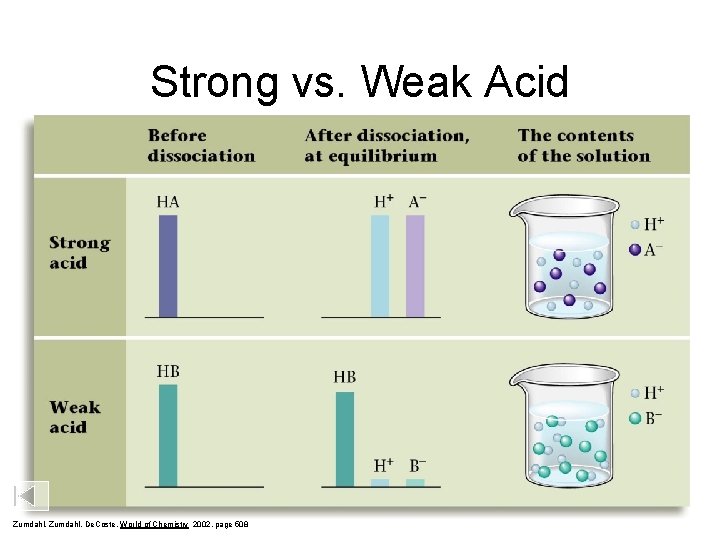
Strong vs. Weak Acid Zumdahl, De. Coste, World of Chemistry 2002, page 508

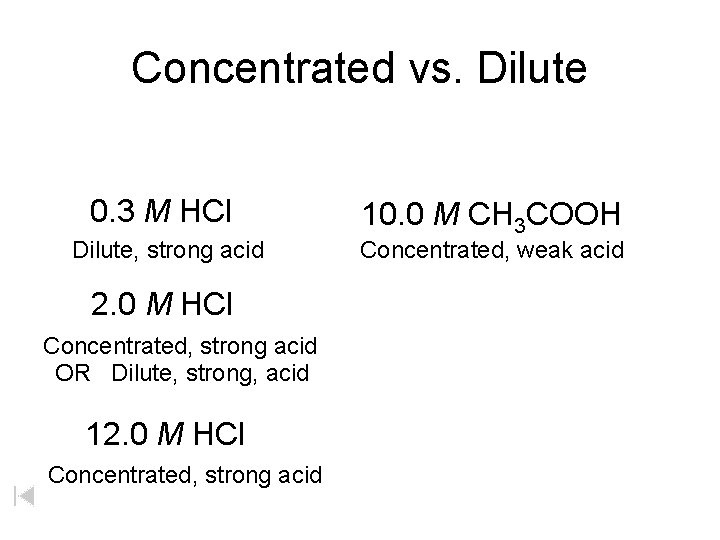
Concentrated vs. Dilute 0. 3 M HCl Dilute, strong acid 2. 0 M HCl Concentrated, strong acid OR Dilute, strong, acid 12. 0 M HCl Concentrated, strong acid 10. 0 M CH 3 COOH Concentrated, weak acid

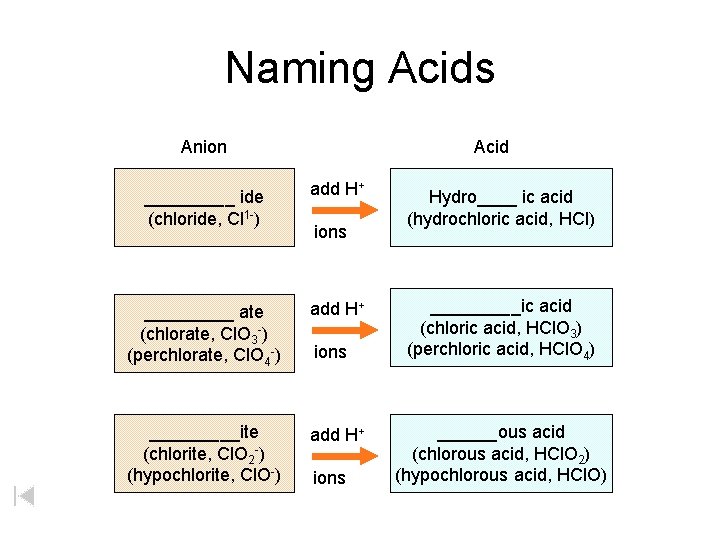
Naming Acids Anion Acid _____ ide (chloride, Cl 1 -) add H+ _____ ate (chlorate, Cl. O 3 -) (perchlorate, Cl. O 4 -) add H+ _____ite (chlorite, Cl. O 2 -) (hypochlorite, Cl. O-) add H+ ions Hydro____ ic acid (hydrochloric acid, HCl) _____ic acid (chloric acid, HCl. O 3) (perchloric acid, HCl. O 4) ______ous acid (chlorous acid, HCl. O 2) (hypochlorous acid, HCl. O)

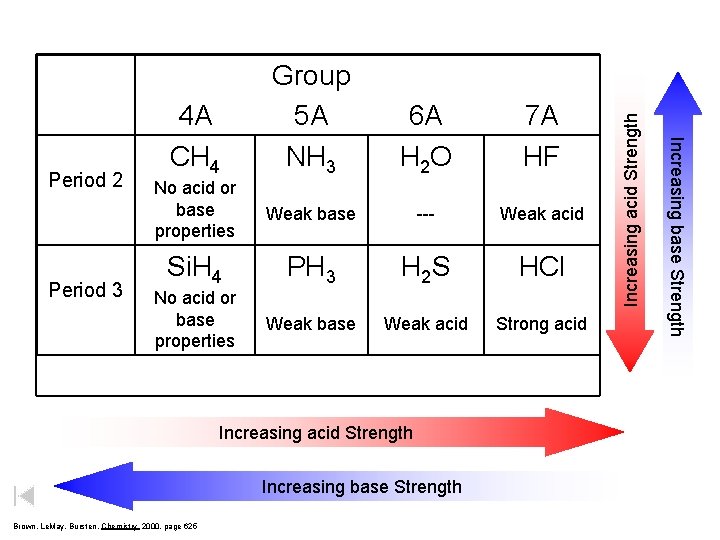
7 A HF No acid or base properties Weak base --- Weak acid Si. H 4 PH 3 H 2 S HCl No acid or base properties Weak base Weak acid Strong acid Increasing acid Strength Increasing base Strength Brown, Le. May, Bursten, Chemistry 2000, page 625 Increasing acid Strength Period 3 6 A H 2 O 4 A CH 4 Increasing base Strength Period 2 Group 5 A NH 3

Equilibrium and p. H Calculations Weak acid HA + H 2 O Strong acid H 3 O+ + A - HA + H 2 O H 3 O+ + A - acid-dissociation constant calculations Ka = [A-] [H 3 O+] [HA] + antilog(-p. H) 7 1 x 10 -14 = [OH-] -log [H 3 O+] = p. H 0 [H 3 O+] [HA] = [H 3 O+] 14 Tocci, Viehland, Holt Chemistry Visualizing Matter 1996, page 525 1 x 10 -14 [H 3 O+] = -