Chemistry The Central Science 10 th edition Theodore



![p. H = −log [H+] or p. H = −log [H 3 O+] H p. H = −log [H+] or p. H = −log [H 3 O+] H](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-14.jpg)
![[H 3 O+] & [OH–] = Acidic Neutral Basic [H 3 O+] & [OH–] = Acidic Neutral Basic](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-15.jpg)
![“base 10” scale Neutral [H+] p. H p. OH [OH–] 1 x 10– 0 “base 10” scale Neutral [H+] p. H p. OH [OH–] 1 x 10– 0](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-17.jpg)
![[H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14. [H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14.](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-18.jpg)
![[H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14. [H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14.](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-19.jpg)
![p. H p. OH [H+] [OH–] Calculations on equation sheet p. H = –log[H+] p. H p. OH [H+] [OH–] Calculations on equation sheet p. H = –log[H+]](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-20.jpg)
![p. H & p. OH Calculations p. H = –log[H+] = 1 x 10– p. H & p. OH Calculations p. H = –log[H+] = 1 x 10–](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-21.jpg)


![I Do Know This Stuff! 2) The [H 3 O+] for four solutions is I Do Know This Stuff! 2) The [H 3 O+] for four solutions is](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-25.jpg)

![Calculating Percent Ionization NOT on [H 3 O+]eq % ionization = [HA]in 100% equation Calculating Percent Ionization NOT on [H 3 O+]eq % ionization = [HA]in 100% equation](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-33.jpg)
![Calculating p. H from Ka [H 3 O+] [C 2 H 3 O 2−] Calculating p. H from Ka [H 3 O+] [C 2 H 3 O 2−]](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-35.jpg)
![Kb can be used to find [OH−], then p. OH, & p. H. Weak Kb can be used to find [OH−], then p. OH, & p. H. Weak](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-38.jpg)
![Calculating p. H of Basic Solutions [NH 4+] [OH−] Kb = [NH 3] Kb Calculating p. H of Basic Solutions [NH 4+] [OH−] Kb = [NH 3] Kb](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-42.jpg)
![Calculating p. H of Basic Solutions [OH−] = 1. 6 10− 3 M Therefore, Calculating p. H of Basic Solutions [OH−] = 1. 6 10− 3 M Therefore,](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-43.jpg)
- Slides: 48

Chemistry, The Central Science, 10 th edition Theodore L. Brown; H. Eugene Le. May, Jr. ; and Bruce E. Bursten Unit 9 (Chp 16): Acid-Base Equilibria (Ka, Kb, Kw, p. H, p. OH) John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall, Inc.

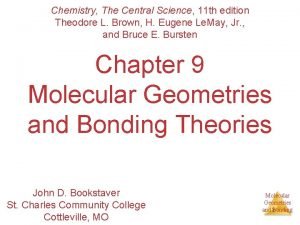
H H Cl H O + – O H Cl H H HCl + H 2 O H 3 O+ + Cl– Ø Acid: proton (H+) donor Ø Base: proton (H+) acceptor NH 3 + H 2 O NH 4 H H H N H O H H H N H + + OH– + H – O H

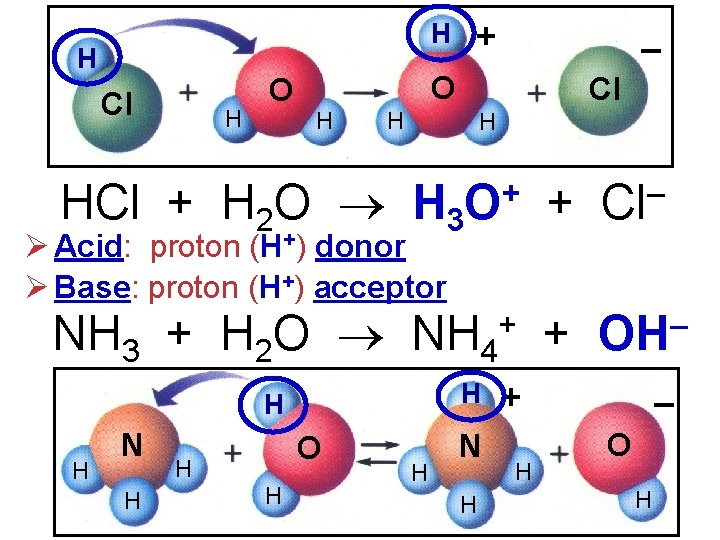
Acid: have a removable (acidic) proton. HCl NH 4+ CH 3 COOH H Cl– : NH 3 CH 3 COO– Base: have a pair of nonbonding electrons.

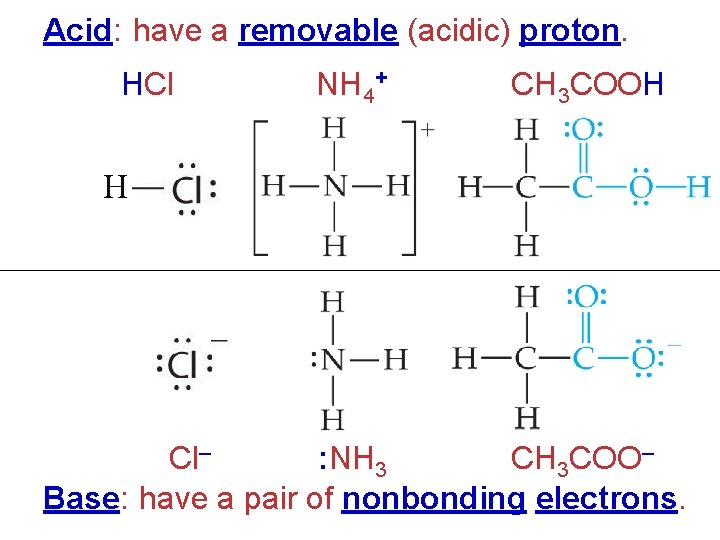
amphoteric: can be both acid & base Amino Acids: Tryptophan HCO 3− − HSO 4 H 2 O

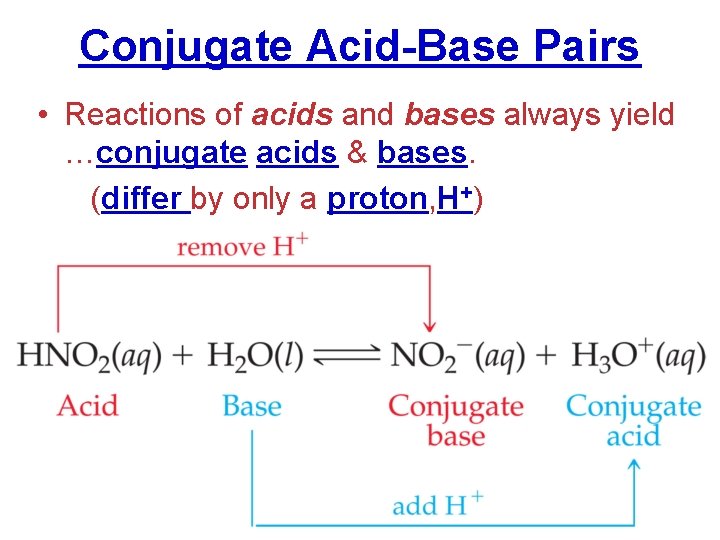
Conjugate Acid-Base Pairs • Reactions of acids and bases always yield …conjugate acids & bases. (differ by only a proton, H+)

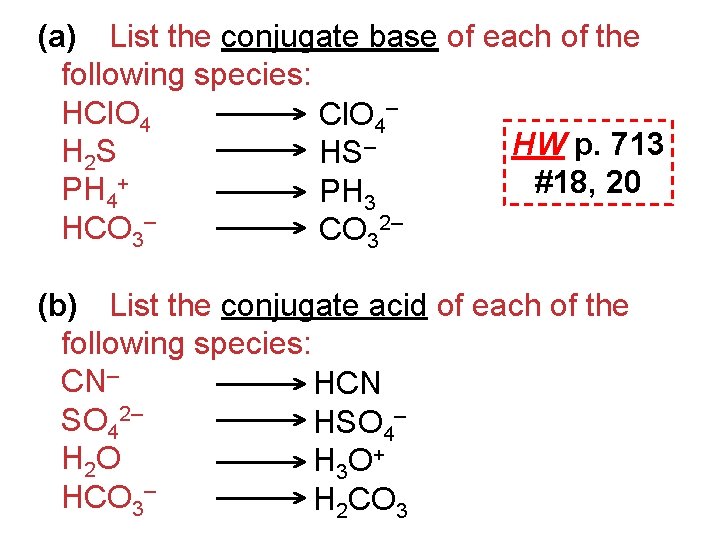
(a) List the conjugate base of each of the following species: HCl. O 4– HW p. 713 H 2 S HS– #18, 20 PH 4+ PH 3 HCO 3– CO 32– (b) List the conjugate acid of each of the following species: CN– HCN SO 42– HSO 4– H 2 O H 3 O + HCO 3– H 2 CO 3

Strong Acids • Recall the six Strong Acids are… HNO 3 , H 2 SO 4 , HCl, HBr, HI, and HCl. O 4. • strong electrolytes (completely ionized) (exist totally as ions in aqueous solution) HNO 3 + H 2 O H 3 O+ + NO 3– • For the monoprotic strong acids, [acid] = [H 3 O+] 0. 50 M HCl means… [H 3 O+] = 0. 50 M

Strong Bases • Strong Bases are the soluble hydroxides (OH–) of… Group 1 (Li, Na, K, Rb, Cs) and Ca 2+, Ba 2+, Sr 2+. • strong electrolytes (completely dissociated into ions in aqueous solution). Na. OH Na+ + OH– • and accept H’s easily in aqueous solution. OH– + HA H 2 O + A–

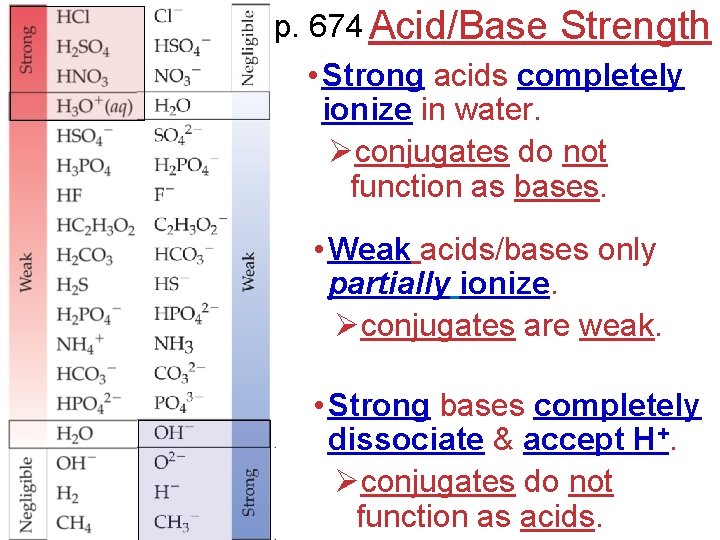
p. 674 Acid/Base Strength • Strong acids completely ionize in water. Øconjugates do not function as bases. • Weak acids/bases only partially ionize. Øconjugates are weak. • Strong bases completely dissociate & accept H+. Øconjugates do not function as acids.

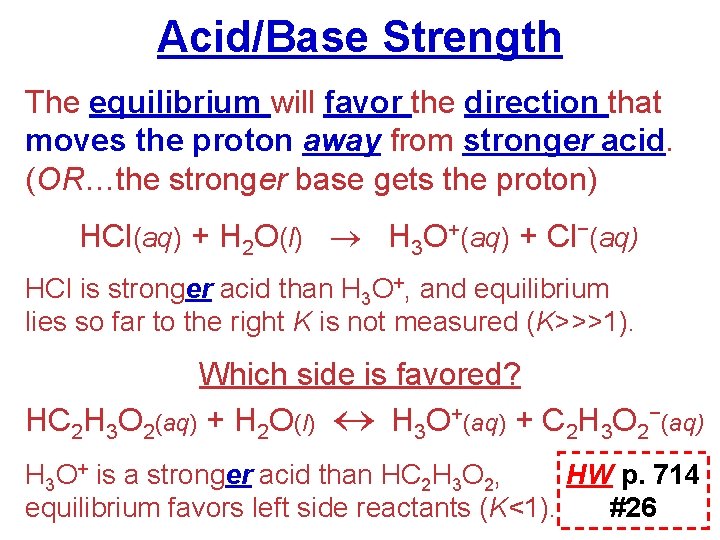
Acid/Base Strength The equilibrium will favor the direction that moves the proton away from stronger acid. (OR…the stronger base gets the proton) HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl−(aq) HCl is stronger acid than H 3 O+, and equilibrium lies so far to the right K is not measured (K>>>1). Which side is favored? HC 2 H 3 O 2(aq) + H 2 O(l) H 3 O+(aq) + C 2 H 3 O 2−(aq) H 3 O+ is a stronger acid than HC 2 H 3 O 2, HW p. 714 equilibrium favors left side reactants (K<1). #26

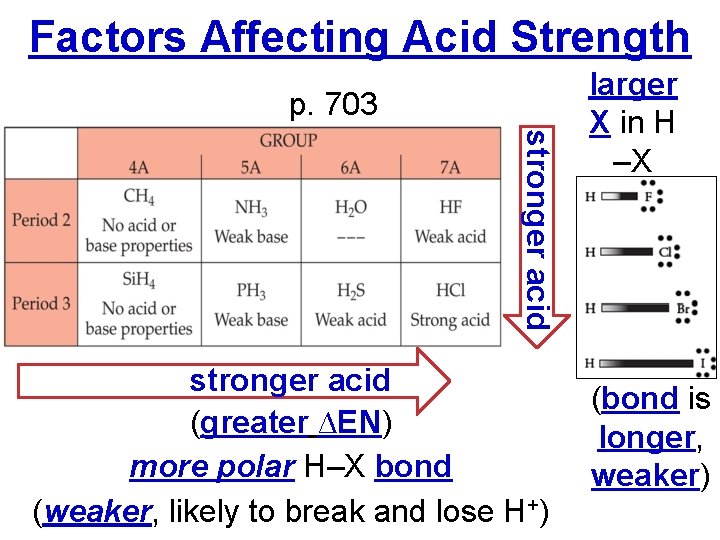
Factors Affecting Acid Strength p. 703 stronger acid (greater ∆EN) more polar H–X bond (weaker, likely to break and lose H+) larger X in H –X (bond is longer, weaker)

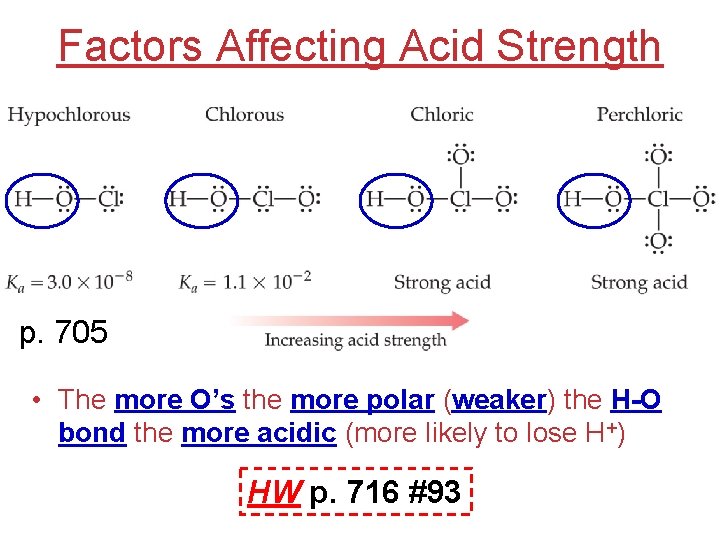
Factors Affecting Acid Strength p. 705 • The more O’s the more polar (weaker) the H-O bond the more acidic (more likely to lose H+) HW p. 716 #93

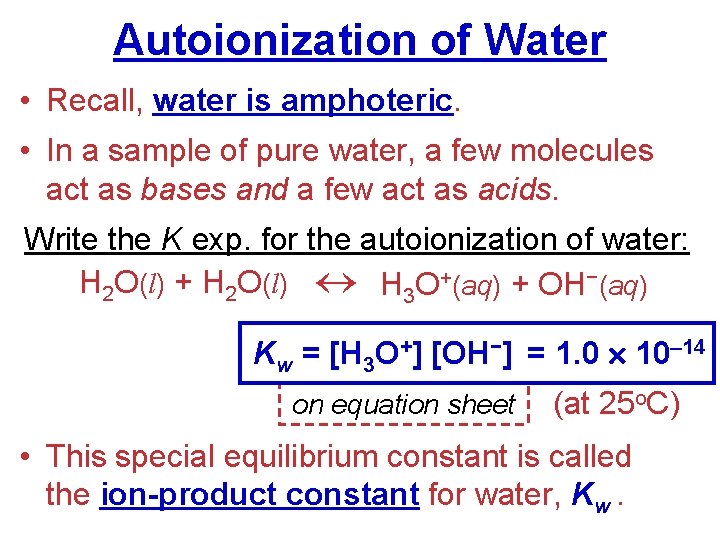
Autoionization of Water • Recall, water is amphoteric. • In a sample of pure water, a few molecules act as bases and a few act as acids. Write the K exp. for the autoionization of water: H 2 O(l) + H 2 O(l) H 3 O+(aq) + OH−(aq) Kw = [H 3 O+] [OH−] = 1. 0 10– 14 on equation sheet (at 25 o. C) • This special equilibrium constant is called the ion-product constant for water, Kw.
![p H log H or p H log H 3 O H p. H = −log [H+] or p. H = −log [H 3 O+] H](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-14.jpg)
p. H = −log [H+] or p. H = −log [H 3 O+] H 2 O(l) + H 2 O(l) H 3 O+(aq) + OH−(aq) In pure water, [H 3 O+] = [OH−] Kw = [H 3 O+] [OH−] = 1. 0 10− 14 x 2 = 1. 0 10− 14 [H 3 O+] = 1. 0 10− 7 M (in pure water) p. H = −log(1. 0 10− 7) = 7. 00 • Acids: higher [H 3 O+], 1 x 10–(<7) , so p. H <7 • Bases: lower [H 3 O+], 1 x 10–(>7) , so p. H >7
![H 3 O OH Acidic Neutral Basic [H 3 O+] & [OH–] = Acidic Neutral Basic](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-15.jpg)
[H 3 O+] & [OH–] = Acidic Neutral Basic

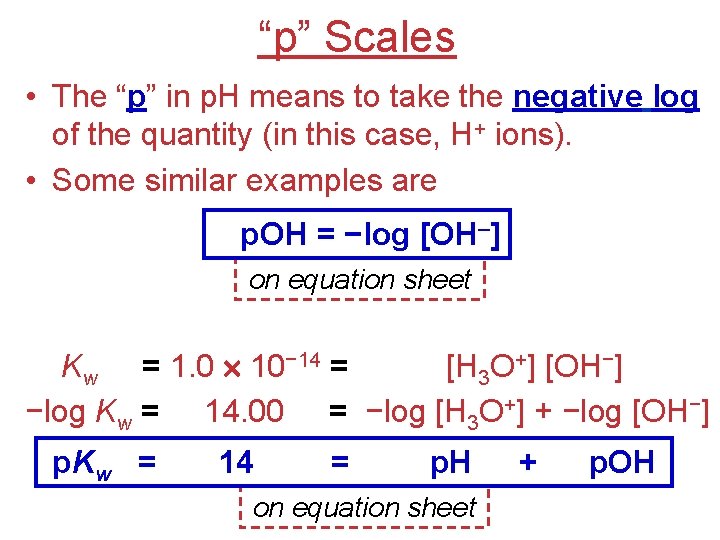
“p” Scales • The “p” in p. H means to take the negative log of the quantity (in this case, H+ ions). • Some similar examples are p. OH = −log [OH–] on equation sheet Kw = 1. 0 10− 14 = [H 3 O+] [OH−] −log Kw = 14. 00 = −log [H 3 O+] + −log [OH−] p. Kw = 14 = p. H on equation sheet + p. OH
![base 10 scale Neutral H p H p OH OH 1 x 10 0 “base 10” scale Neutral [H+] p. H p. OH [OH–] 1 x 10– 0](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-17.jpg)
“base 10” scale Neutral [H+] p. H p. OH [OH–] 1 x 10– 0 0. 0 14. 0 1 x 10– 14 1 x 10– 1 1. 0 13. 0 1 x 10– 13 1 x 10– 2 2. 0 1 x 10– 12 1 x 10– 3 3. 0 11. 0 1 x 10– 11 1 x 10– 4 4. 0 10. 0 1 x 10– 10 1 x 10– 5 5. 0 9. 0 1 x 10– 9 1 x 10– 6 6. 0 8. 0 1 x 10– 8 1 x 10– 7 7. 0 1 x 10– 7 1 x 10– 8 8. 0 6. 0 1 x 10– 6 1 x 10– 9 9. 0 5. 0 1 x 10– 5 1 x 10– 10 10. 0 4. 0 1 x 10– 4 1 x 10– 11 11. 0 3. 0 1 x 10– 3 1 x 10– 12 12. 0 1 x 10– 2 1 x 10– 13 13. 0 1 x 10– 14 14. 0 0. 0 1 x 10– 0 Kw = [H+]x[OH–] 1 x 10– 14 1 x 10– 14 1 x 10– 14 1 x 10– 14
![H Neutral p H p OH OH 1 x 10 0 0 0 14 [H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14.](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-18.jpg)
[H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14. 0 1 x 10– 14 1 x 10– 1 1. 0 13. 0 1 x 10– 13 1 x 10– 2 2. 0 1 x 10– 12 1 x 10– 3 3. 0 11. 0 1 x 10– 11 1 x 10– 4 4. 0 10. 0 1 x 10– 10 1 x 10– 5 5. 0 9. 0 1 x 10– 9 1 x 10– 6 6. 0 8. 0 1 x 10– 8 1 x 10– 7 7. 0 1 x 10– 7 1 x 10– 8 8. 0 6. 0 1 x 10– 6 1 x 10– 9 9. 0? 5. 0? 1 x 10 ? – 5 1 x 10– 10 10. 0 4. 0 1 x 10– 4 1 x 10– 11 11. 0 3. 0 1 x 10– 3 1 x 10– 12 12. 0 1 x 10– 2 1 x 10– 13 13. 0 1 x 10– 14 14. 0 0. 0 1 x 10– 0 Kw = [H+]x[OH–] 1 x 10– 14 1 x 10– 14 1 x 10– 14 1 x 10– 14
![H Neutral p H p OH OH 1 x 10 0 0 0 14 [H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14.](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-19.jpg)
[H+] Neutral p. H p. OH [OH–] 1 x 10– 0 0. 0 14. 0 1 x 10– 14 1 x 10– 1 1. 0 13. 0 1 x 10– 13 1 x 10– 2 2. 0 1 x 10– 12 1 x 10 ? – 3 3. 0? 11. 0 ? 1 x 10– 11 1 x 10– 4 4. 0 10. 0 1 x 10– 10 1 x 10– 5 5. 0 9. 0 1 x 10– 9 1 x 10– 6 6. 0 8. 0 1 x 10– 8 1 x 10– 7 7. 0 1 x 10– 7 1 x 10– 8 8. 0 6. 0 1 x 10– 6 1 x 10– 9 9. 0 5. 0 1 x 10– 5 1 x 10– 10 10. 0 4. 0 1 x 10– 4 1 x 10– 11 11. 0 3. 0 1 x 10– 3 1 x 10– 12 12. 0 1 x 10– 2 1 x 10– 13 13. 0 1 x 10– 14 14. 0 0. 0 1 x 10– 0 Kw = [H+]x[OH–] 1 x 10– 14 1 x 10– 14 1 x 10– 14 1 x 10– 14
![p H p OH H OH Calculations on equation sheet p H logH p. H p. OH [H+] [OH–] Calculations on equation sheet p. H = –log[H+]](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-20.jpg)
p. H p. OH [H+] [OH–] Calculations on equation sheet p. H = –log[H+] p. OH = –log[OH–] 10 –p. H = [H+] NOT 10 –p. OH = [OH–] on equation sheet Kw – 14 + – = [H ] [OH ] = 1. 0 x 10 14 = p. H + p. OH on equation sheet NOT
![p H p OH Calculations p H logH 1 x 10 p. H & p. OH Calculations p. H = –log[H+] = 1 x 10–](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-21.jpg)
p. H & p. OH Calculations p. H = –log[H+] = 1 x 10– 14 [OH–] ALL on the Equation Sheet! p. OH = 14 – p. H = 14 – p. OH – OH p. OH = –log[OH–] [H+] = 10–p. H + H [OH–] = 10–p. OH [OH–] = 1 x 10– 14 [H+] p. OH

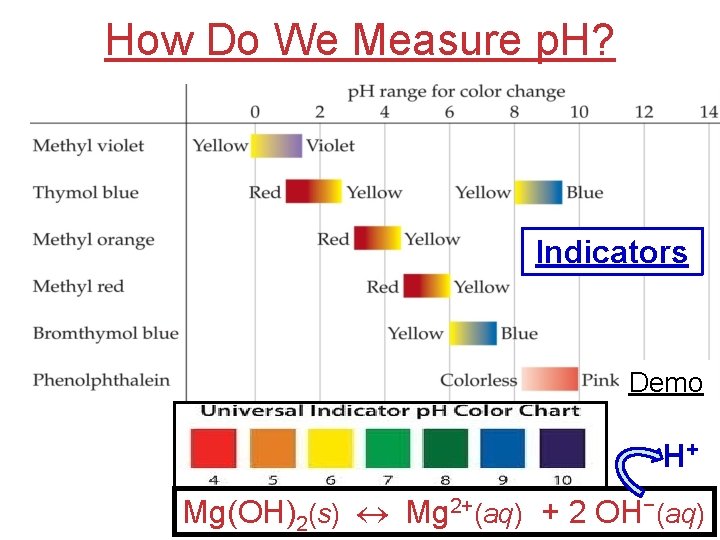
How Do We Measure p. H? Indicators Demo H+ Mg(OH)2(s) Mg 2+(aq) + 2 OH−(aq)

How Do We Measure p. H? For accurate measurements, use a p. H meter, which measures voltage in solution by tracking the number of H+ particles that contact the device.

I Think I Know This Stuff. 1) Which one of the following solutions is the most basic? A) soap p. H ≈ 10 B) milk p. H ≈ 6. 5 C) vinegar p. H ≈ 2. 5 D) ammonia p. H ≈ 11 E) tomato juice F) blood p. H ≈ 4. 0 p. H ≈ 7. 4
![I Do Know This Stuff 2 The H 3 O for four solutions is I Do Know This Stuff! 2) The [H 3 O+] for four solutions is](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-25.jpg)
I Do Know This Stuff! 2) The [H 3 O+] for four solutions is given below. Which one of the solutions is the most acidic? A) 1 x 10– 3 M B) 1 x 10– 7 M C) 1 x 10– 9 M D) 1 x 10– 14 M

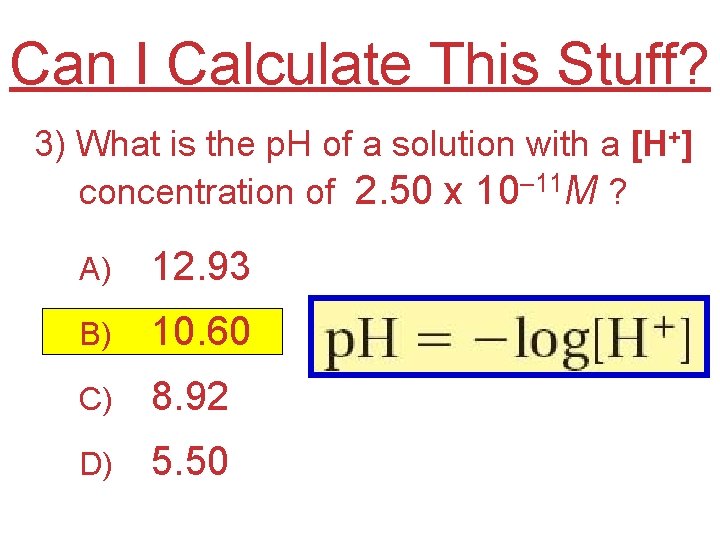
Can I Calculate This Stuff? 3) What is the p. H of a solution with a [H+] concentration of 2. 50 x 10– 11 M ? A) 12. 93 B) 10. 60 C) 8. 92 D) 5. 50

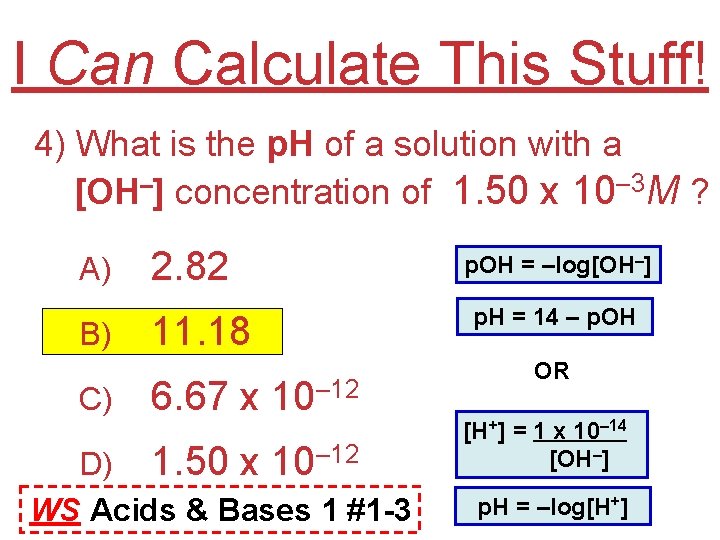
I Can Calculate This Stuff! 4) What is the p. H of a solution with a [OH–] concentration of 1. 50 x 10– 3 M ? A) B) C) 2. 82 11. 18 6. 67 x 10– 12 D) 1. 50 x 10– 12 WS Acids & Bases 1 #1 -3 p. OH = –log[OH–] p. H = 14 – p. OH OR [H+] = 1 x 10– 14 [OH–] p. H = –log[H+]

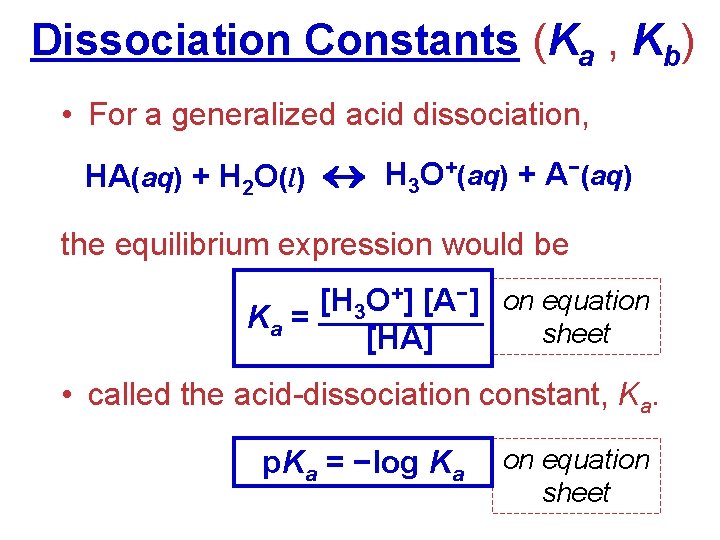
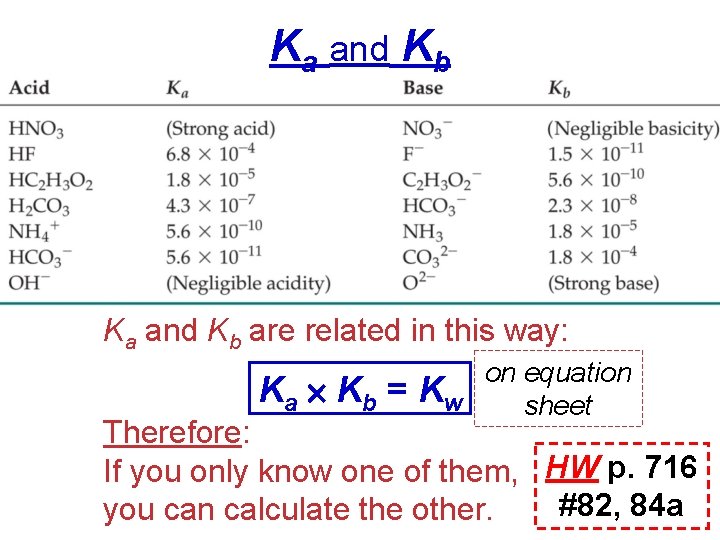
Dissociation Constants (Ka , Kb) • For a generalized acid dissociation, HA(aq) + H 2 O(l) H 3 O+(aq) + A−(aq) the equilibrium expression would be [H 3 O+] [A−] on equation Ka = sheet [HA] • called the acid-dissociation constant, Ka. p. Ka = −log Ka on equation sheet

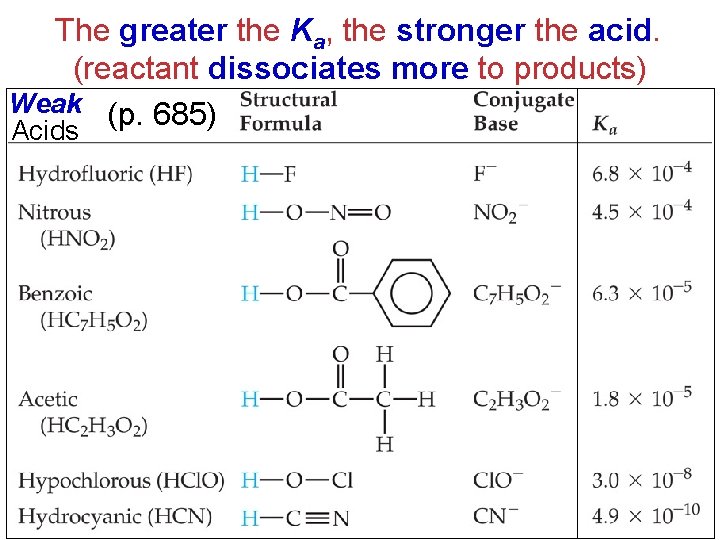
The greater the Ka, the stronger the acid. (reactant dissociates more to products) Weak (p. 685) Acids

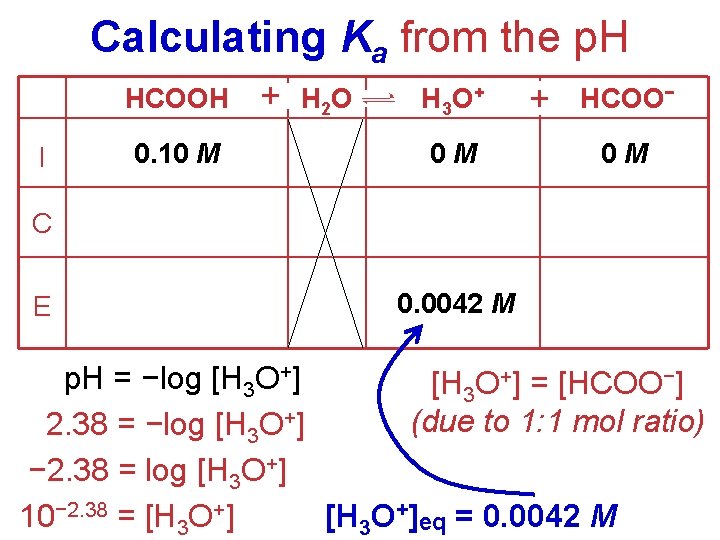
Calculating Ka from the p. H • The p. H of a 0. 10 M solution of formic acid, HCOOH, at 25 o. C is 2. 38. Calculate Ka formic acid at 25 o. C. HCOOH(aq) + H 2 O(l) H 3 O+(aq) + HCOO–(aq) [H 3 O+] [HCOO−] • We know: Ka = [HCOOH] • We need: all 3 concentrations at equilibrium. • We are given: • [HCOOH]initial = 0. 10 M • get [H 3 O+]eq from p. H.

Calculating Ka from the p. H HCOOH I 0. 10 M + H 2 O H 3 O + 0 M + HCOO− 0 M C E 0. 0042 M p. H = −log [H 3 O+] = [HCOO−] (due to 1: 1 mol ratio) 2. 38 = −log [H 3 O+] − 2. 38 = log [H 3 O+]eq = 0. 0042 M 10− 2. 38 = [H 3 O+]

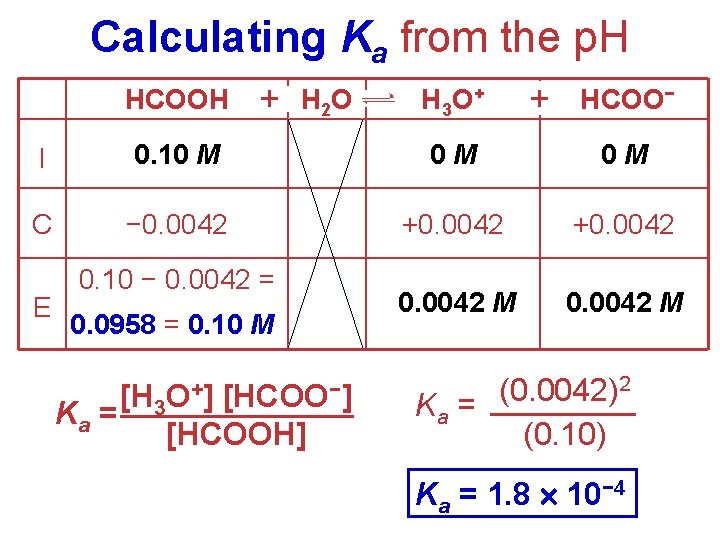
Calculating Ka from the p. H HCOOH + H 2 O H 3 O + + HCOO− I 0. 10 M 0 M 0 M C − 0. 0042 +0. 0042 M E 0. 10 − 0. 0042 = 0. 0958 = 0. 10 M Ka = [H 3 O+] [HCOO−] [HCOOH] 2 (0. 0042) Ka = (0. 10) Ka = 1. 8 10− 4
![Calculating Percent Ionization NOT on H 3 Oeq ionization HAin 100 equation Calculating Percent Ionization NOT on [H 3 O+]eq % ionization = [HA]in 100% equation](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-33.jpg)
Calculating Percent Ionization NOT on [H 3 O+]eq % ionization = [HA]in 100% equation sheet HCOOH(aq) + H 2 O(l) H 3 O+(aq) + HCOO–(aq) HCOOH + H 2 O I 0. 10 M C − 0. 0042 E 0. 10 − 0. 0042 = 0. 0958 = 0. 10 M H 3 O + 0 M +0. 0042 M + HCOO− 0 M +0. 0042 M 0. 0042 % ionization = 100% = 4. 2% 0. 10

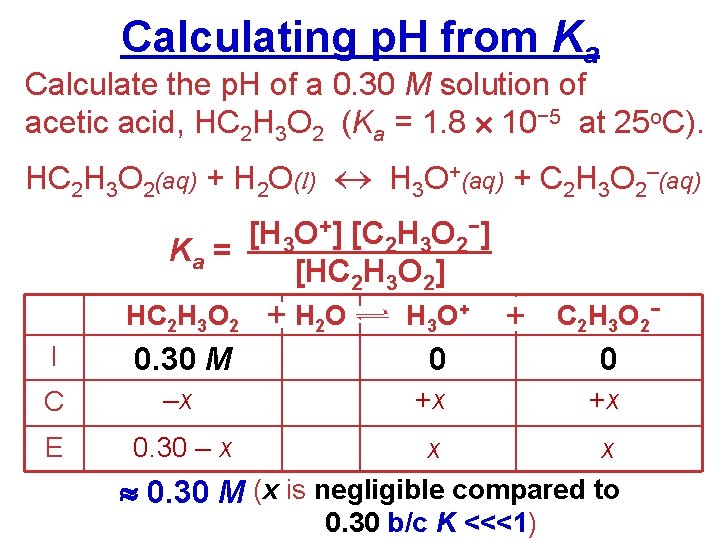
Calculating p. H from Ka Calculate the p. H of a 0. 30 M solution of acetic acid, HC 2 H 3 O 2 (Ka = 1. 8 10− 5 at 25 o. C). HC 2 H 3 O 2(aq) + H 2 O(l) H 3 O+(aq) + C 2 H 3 O 2–(aq) [H 3 O+] [C 2 H 3 O 2−] Ka = [HC 2 H 3 O 2] HC 2 H 3 O 2 + H 2 O H 3 O + + C 2 H 3 O 2− I 0. 30 M 0 0 C –x +x +x E 0. 30 – x x x 0. 30 M (x is negligible compared to 0. 30 b/c K <<<1)
![Calculating p H from Ka H 3 O C 2 H 3 O 2 Calculating p. H from Ka [H 3 O+] [C 2 H 3 O 2−]](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-35.jpg)
Calculating p. H from Ka [H 3 O+] [C 2 H 3 O 2−] Ka = [HC 2 H 3 O 2] 2 (x) 1. 8 10− 5 = (0. 30) (1. 8 10− 5) (0. 30) = x 2 5. 4 10− 6 = x 2 − 3 = x 2. 3 10 HW p. 715 #56, 62 ab p. H = −log [H 3 O+] − 3) p. H = −log (2. 3 10 WS Acids & Bases 1 #7 -9 WS Acids & Bases 2 #1 -2 p. H = 2. 64

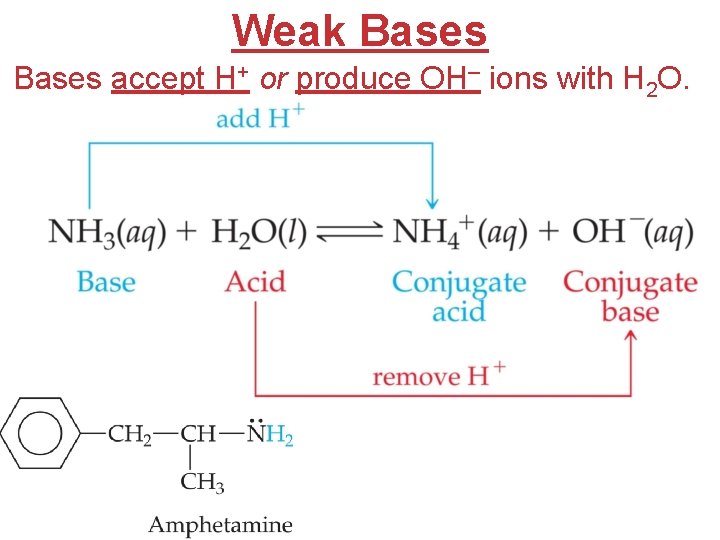
Weak Bases accept H+ or produce OH– ions with H 2 O.

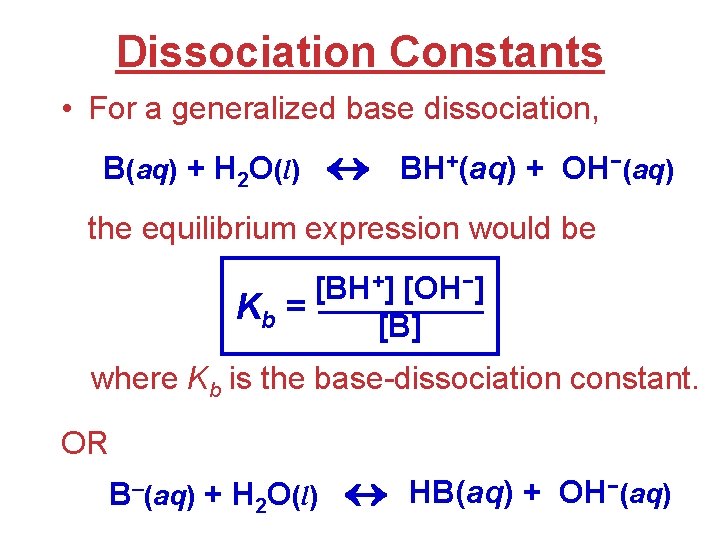
Dissociation Constants • For a generalized base dissociation, B(aq) + H 2 O(l) BH+(aq) + OH−(aq) the equilibrium expression would be [BH+] [OH−] Kb = [B] where Kb is the base-dissociation constant. OR B–(aq) + H 2 O(l) HB(aq) + OH−(aq)
![Kb can be used to find OH then p OH p H Weak Kb can be used to find [OH−], then p. OH, & p. H. Weak](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-38.jpg)
Kb can be used to find [OH−], then p. OH, & p. H. Weak (p. 694) Bases

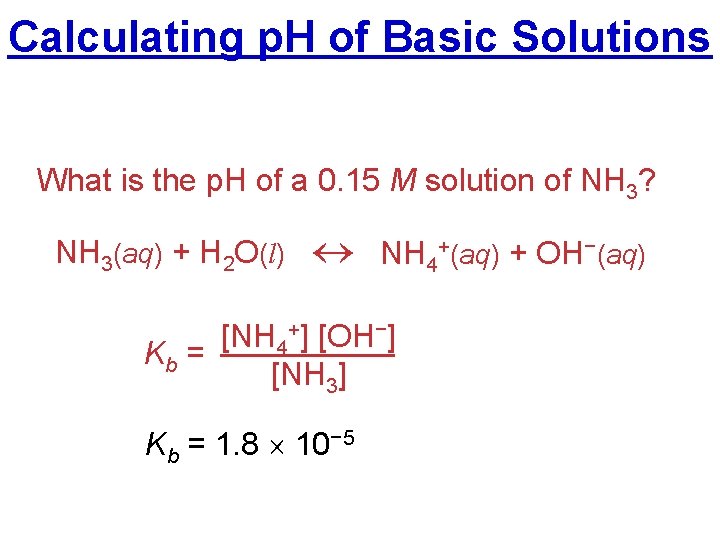
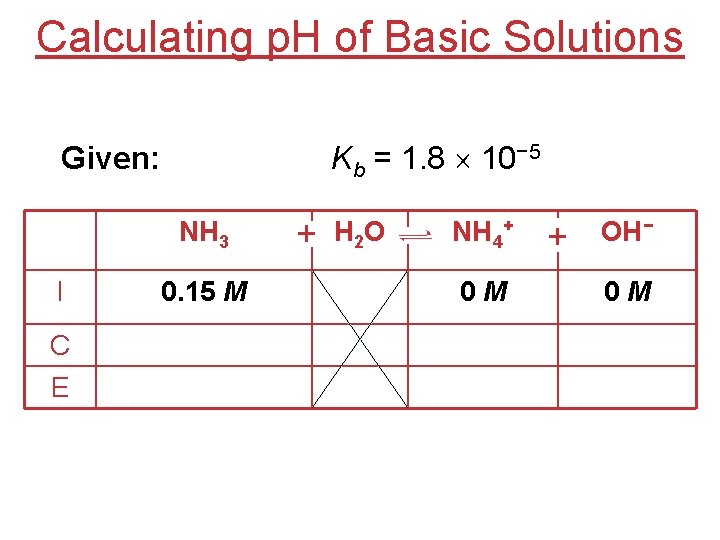
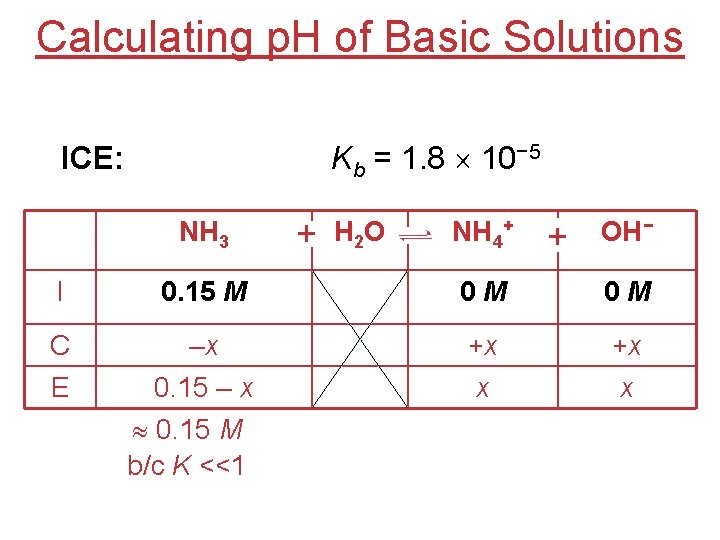
Calculating p. H of Basic Solutions What is the p. H of a 0. 15 M solution of NH 3? NH 3(aq) + H 2 O(l) NH 4+(aq) + OH−(aq) [NH 4+] [OH−] Kb = [NH 3] Kb = 1. 8 10− 5

Calculating p. H of Basic Solutions Given: Kb = 1. 8 10− 5 NH 3 I C E 0. 15 M + H 2 O NH 4+ 0 M + OH− 0 M

Calculating p. H of Basic Solutions ICE: Kb = 1. 8 10− 5 NH 3 + H 2 O NH 4+ + OH− I 0. 15 M 0 M 0 M C –x +x +x x x E 0. 15 – x 0. 15 M b/c K <<1
![Calculating p H of Basic Solutions NH 4 OH Kb NH 3 Kb Calculating p. H of Basic Solutions [NH 4+] [OH−] Kb = [NH 3] Kb](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-42.jpg)
Calculating p. H of Basic Solutions [NH 4+] [OH−] Kb = [NH 3] Kb = 1. 8 10− 5 2 (x) 1. 8 10− 5 = (0. 15) (1. 8 10− 5) (0. 15) = x 2 2. 7 10− 6 = x 2 1. 6 10− 3 = x 2 [OH−] = 1. 6 10− 3 M
![Calculating p H of Basic Solutions OH 1 6 10 3 M Therefore Calculating p. H of Basic Solutions [OH−] = 1. 6 10− 3 M Therefore,](https://slidetodoc.com/presentation_image_h/1c56b6f41cdb46efdcd0a91a78f0a2c5/image-43.jpg)
Calculating p. H of Basic Solutions [OH−] = 1. 6 10− 3 M Therefore, p. OH = −log [OH−] p. OH = −log (1. 6 10− 3) p. OH = 2. 80 p. H + p. OH = 14 p. H + 2. 80 = 14. 00 p. H = 11. 20 HW p. 716 #76, 78

Ka and Kb are related in this way: Ka Kb = on equation Kw sheet Therefore: If you only know one of them, HW p. 716 #82, 84 a you can calculate the other.

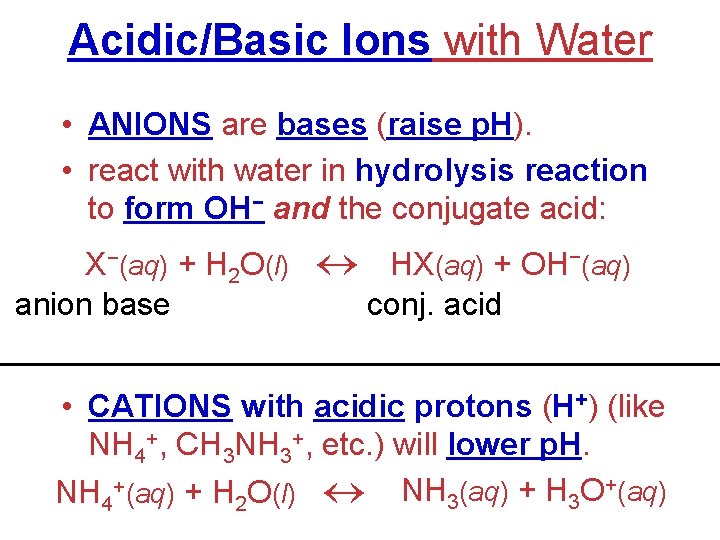
Acidic/Basic Ions with Water • ANIONS are bases (raise p. H). • react with water in hydrolysis reaction to form OH− and the conjugate acid: X−(aq) + H 2 O(l) HX(aq) + OH−(aq) anion base conj. acid • CATIONS with acidic protons (H+) (like NH 4+, CH 3 NH 3+, etc. ) will lower p. H. NH 4+(aq) + H 2 O(l) NH 3(aq) + H 3 O+(aq)

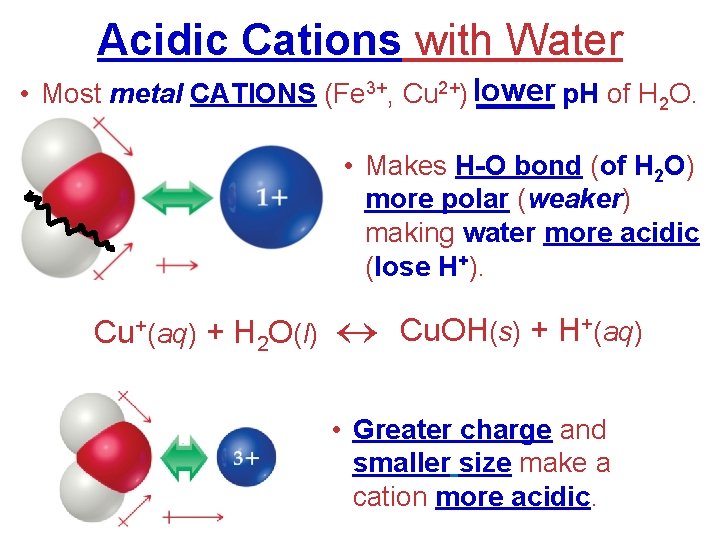
Acidic Cations with Water lower • Most metal CATIONS (Fe 3+, Cu 2+) _____ p. H of H 2 O. • Makes H-O bond (of H 2 O) more polar (weaker) making water more acidic (lose H+). Cu+(aq) + H 2 O(l) Cu. OH(s) + H+(aq) • Greater charge and smaller size make a cation more acidic.

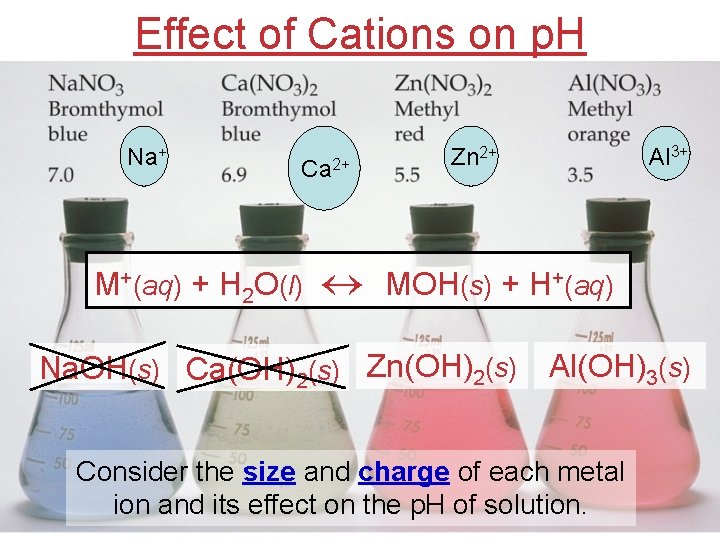
Effect of Cations on p. H Na+ Ca 2+ Zn 2+ Al 3+ M+(aq) + H 2 O(l) MOH(s) + H+(aq) Na. OH(s) Ca(OH)2(s) Zn(OH)2(s) Al(OH)3(s) Consider the size and charge of each metal ion and its effect on the p. H of solution.

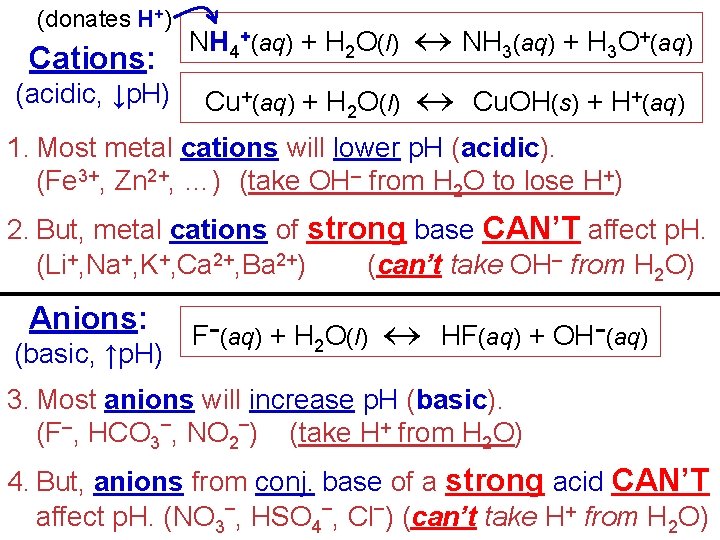
(donates H+) Cations: NH 4+(aq) + H 2 O(l) NH 3(aq) + H 3 O+(aq) (acidic, ↓p. H) Cu+(aq) + H O(l) Cu. OH(s) + H+(aq) 2 1. Most metal cations will lower p. H (acidic). (Fe 3+, Zn 2+, …) (take OH– from H 2 O to lose H+) 2. But, metal cations of strong base CAN’T affect p. H. (Li+, Na+, K+, Ca 2+, Ba 2+) (can’t take OH– from H 2 O) Anions: (basic, ↑p. H) F−(aq) + H 2 O(l) HF(aq) + OH−(aq) 3. Most anions will increase p. H (basic). (F–, HCO 3–, NO 2–) (take H+ from H 2 O) 4. But, anions from conj. base of a strong acid CAN’T affect p. H. (NO 3–, HSO 4–, Cl–) (can’t take H+ from H 2 O)
 Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Theodore roosevelt contribution to forensic science
Theodore roosevelt contribution to forensic science What's your favourite lesson
What's your favourite lesson Mis chapter 6
Mis chapter 6 Mis
Mis Transition state energy diagram
Transition state energy diagram Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Is alkane an organic compound
Is alkane an organic compound Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Nomenclature of ethers
Nomenclature of ethers Ap chemistry notes zumdahl
Ap chemistry notes zumdahl Organic chemistry third edition david klein
Organic chemistry third edition david klein General chemistry
General chemistry Lesson 81 drop in molecular views answer key
Lesson 81 drop in molecular views answer key Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Halohydrin
Halohydrin Thermodynamic control
Thermodynamic control Organic chemistry second edition david klein
Organic chemistry second edition david klein Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Aldo leopold and theodore roosevelt
Aldo leopold and theodore roosevelt Teddy stoddard story
Teddy stoddard story Théodore bouchonneau
Théodore bouchonneau Theodore friedman md
Theodore friedman md Théodore de samos
Théodore de samos Theodore newcomb bennington college study
Theodore newcomb bennington college study Dr theodore stern
Dr theodore stern Theodore bedell
Theodore bedell Theodore streleski
Theodore streleski What are some quotes from open house by theodore roethke
What are some quotes from open house by theodore roethke Theodore boone kid lawyer characters
Theodore boone kid lawyer characters Host 1
Host 1 Theodore s rappaport wireless communications
Theodore s rappaport wireless communications Theodore roosevelt imperialist policies
Theodore roosevelt imperialist policies Theodore roosevelt
Theodore roosevelt From my books surcease of sorrow figurative language
From my books surcease of sorrow figurative language Theodore james ryken
Theodore james ryken Theodore schwann
Theodore schwann Theodore schwann
Theodore schwann Theodore mirvis
Theodore mirvis Achillea
Achillea Kelompok sosial menurut theodore caplow
Kelompok sosial menurut theodore caplow Théodor géricault
Théodor géricault Theodore finch quotes
Theodore finch quotes The cay movie
The cay movie