Chemistry The Central Science 10 th edition Theodore




- Slides: 29

Chemistry, The Central Science, 10 th edition Theodore L. Brown; H. Eugene Le. May, Jr. ; and Bruce E. Bursten Unit 3 (Chp 1, 2, 3): Matter, Measurement, & Stoichiometry John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall Inc.

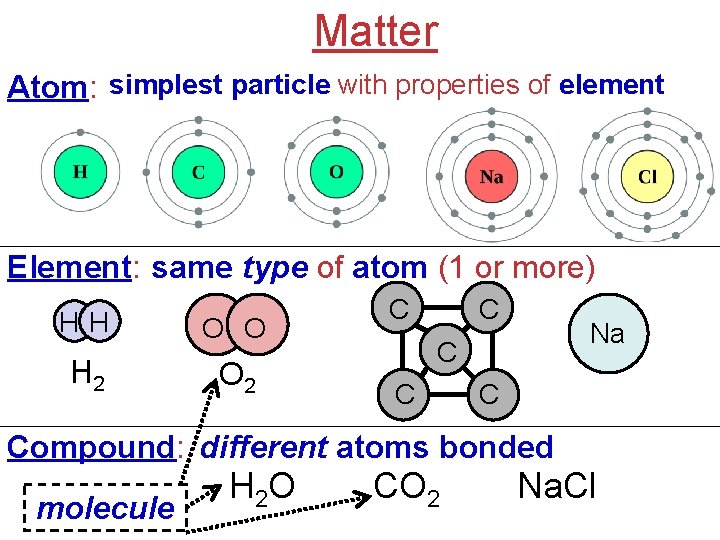
Matter Atom: simplest particle with properties of element Element: same type of atom (1 or more) HH O O H 2 O 2 C C Na C Compound: different atoms bonded molecule H 2 O CO 2 Na. Cl

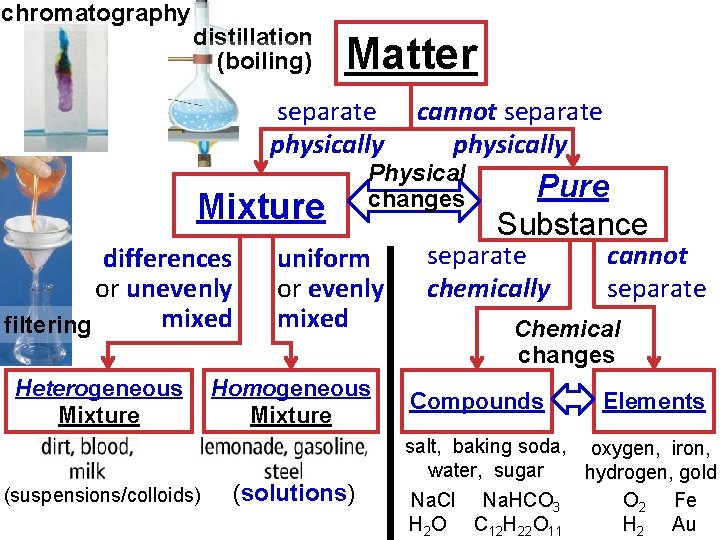
chromatography distillation (boiling) Matter separate physically Mixture differences or unevenly mixed filtering Heterogeneous Mixture (suspensions/colloids) Physical changes uniform or evenly mixed Homogeneous Mixture (solutions) cannot separate physically Pure Substance separate chemically cannot separate Chemical changes Compounds Elements salt, baking soda, oxygen, iron, water, sugar hydrogen, gold Na. Cl Na. HCO 3 O 2 Fe H 2 O C 12 H 22 O 11 H 2 Au

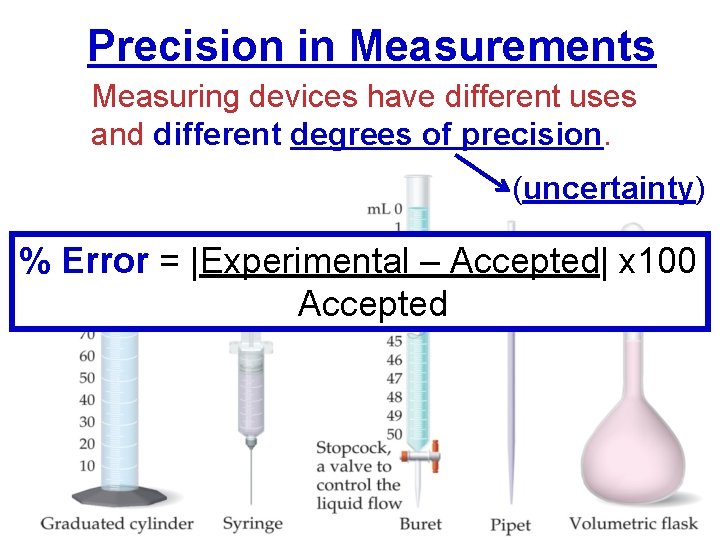
Precision in Measurements Measuring devices have different uses and different degrees of precision. (uncertainty) % Error = |Experimental – Accepted| x 100 Accepted

Box & Dot 0’s Significant Digits nonzero trailing 0’s if decimal 0. 0003700400 grams 500. 0. 0500 1. Nonzero digits 2. Captive Zeroes (between two sig digs) 3. Leading Zeroes (at the beginning of a number) are NEVER significant 4. Trailing Zeroes: Significant ONLY if there’s a decimal What if 500 is significant to the 10’s digit? 5. 0 x 102

Sigs Digs in Operations + or – round answers to keep the fewest decimal places 3. 48 + 2. 2 = 5. 68 x or ÷ 5. 7 round answers to keep the fewest significant digits 6. 40 x 2. 0 = 12. 8 13

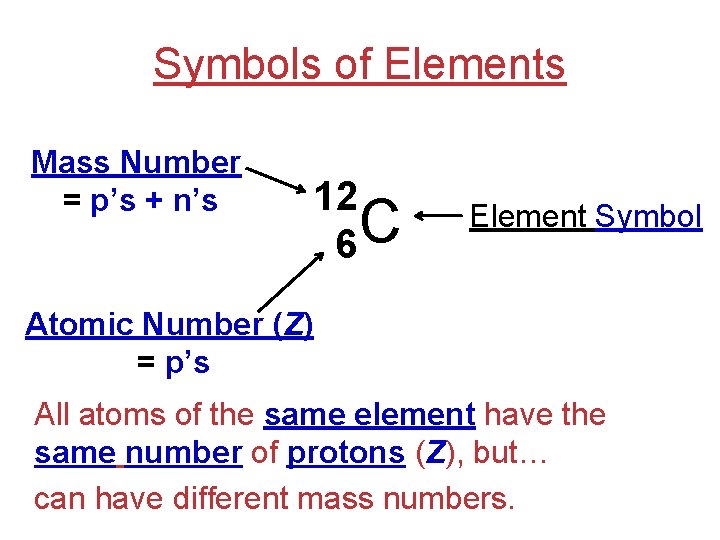
Symbols of Elements Mass Number = p’s + n’s 12 C 6 Element Symbol Atomic Number (Z) = p’s All atoms of the same element have the same number of protons (Z), but… can have different mass numbers.

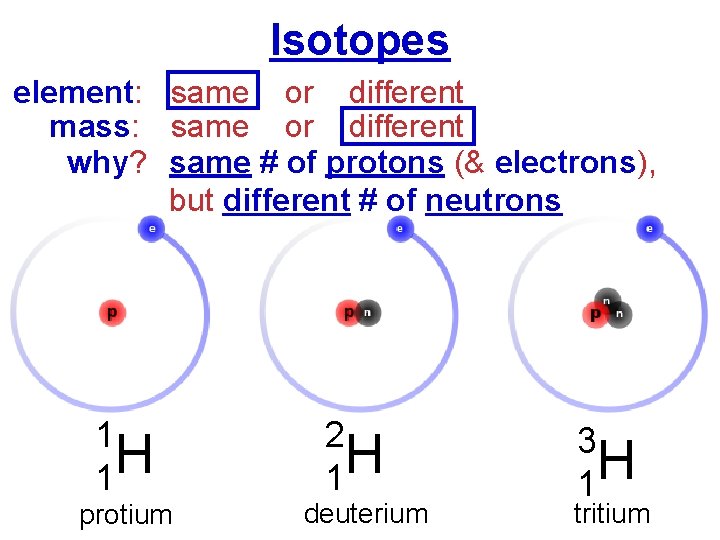
Isotopes element: same or different mass: same or different why? same # of protons (& electrons), but different # of neutrons 1 H 1 protium 2 H 1 deuterium 3 H 1 tritium

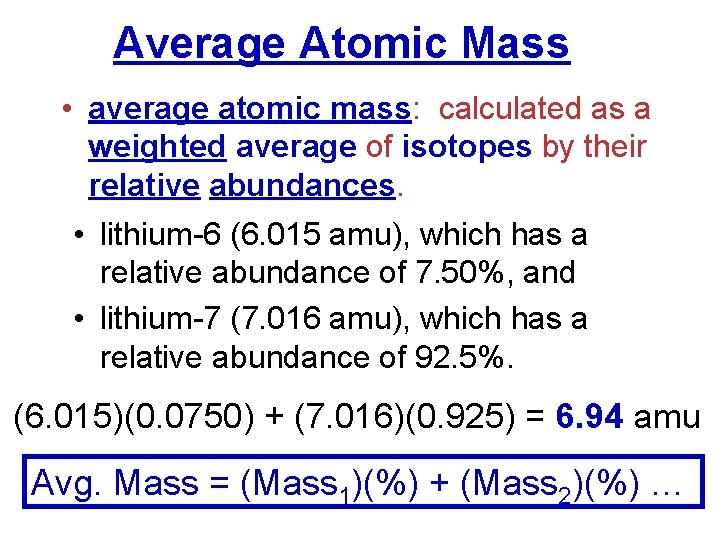
Average Atomic Mass • average atomic mass: calculated as a weighted average of isotopes by their relative abundances. • lithium-6 (6. 015 amu), which has a relative abundance of 7. 50%, and • lithium-7 (7. 016 amu), which has a relative abundance of 92. 5%. (6. 015)(0. 0750) + (7. 016)(0. 925) = 6. 94 amu Avg. Mass = (Mass 1)(%) + (Mass 2)(%) …

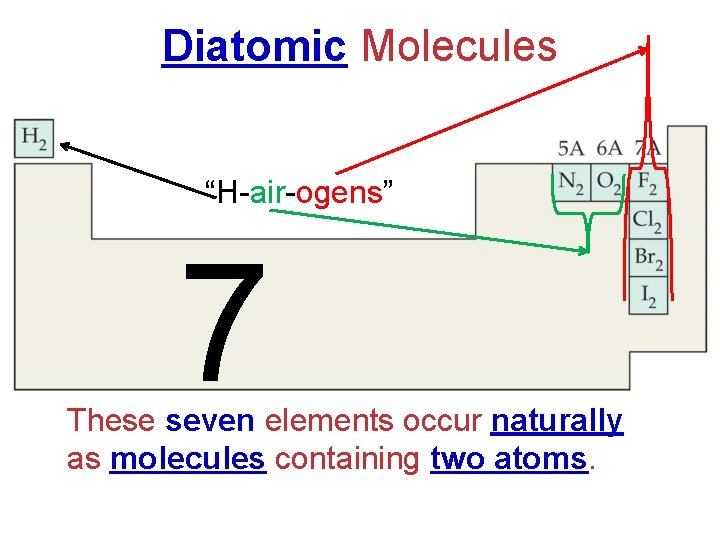
Diatomic Molecules “H-air-ogens” 7 These seven elements occur naturally as molecules containing two atoms.

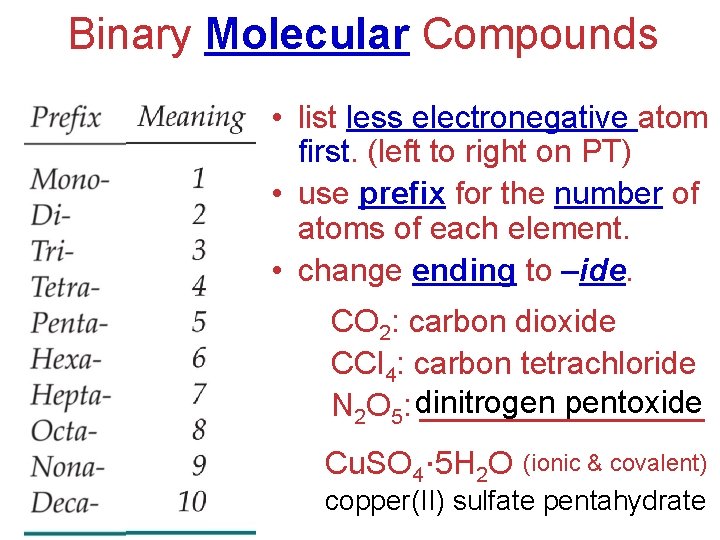
Binary Molecular Compounds • list less electronegative atom first. (left to right on PT) • use prefix for the number of atoms of each element. • change ending to –ide. CO 2: carbon dioxide CCl 4: carbon tetrachloride pentoxide N 2 O 5: dinitrogen ________ Cu. SO 4∙ 5 H 2 O (ionic & covalent) copper(II) sulfate pentahydrate

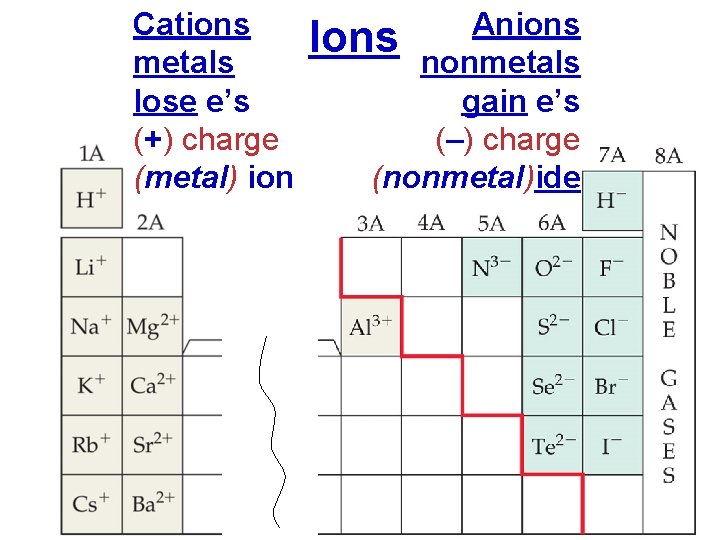
Cations metals lose e’s (+) charge (metal) ion Anions Ions nonmetals gain e’s (–) charge (nonmetal)ide

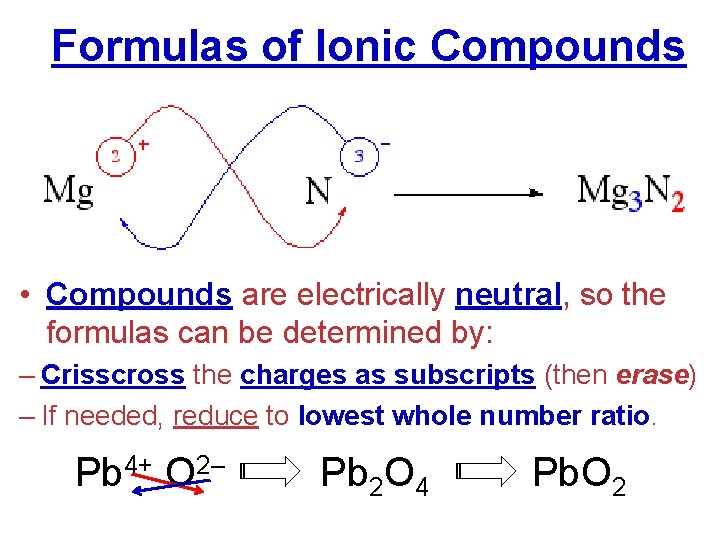
Formulas of Ionic Compounds • Compounds are electrically neutral, so the formulas can be determined by: – Crisscross the charges as subscripts (then erase) – If needed, reduce to lowest whole number ratio. Pb 4+ O 2– Pb 2 O 4 Pb. O 2

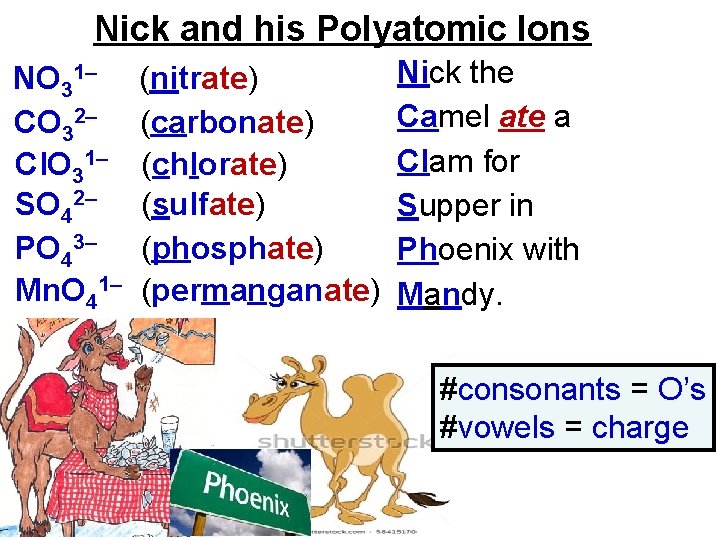
Nick and his Polyatomic Ions NO 31– CO 32– Cl. O 31– SO 42– PO 43– Mn. O 41– (nitrate) (carbonate) (chlorate) (sulfate) (phosphate) (permanganate) Nick the Camel ate a Clam for Supper in Phoenix with Mandy. #consonants = O’s #vowels = charge

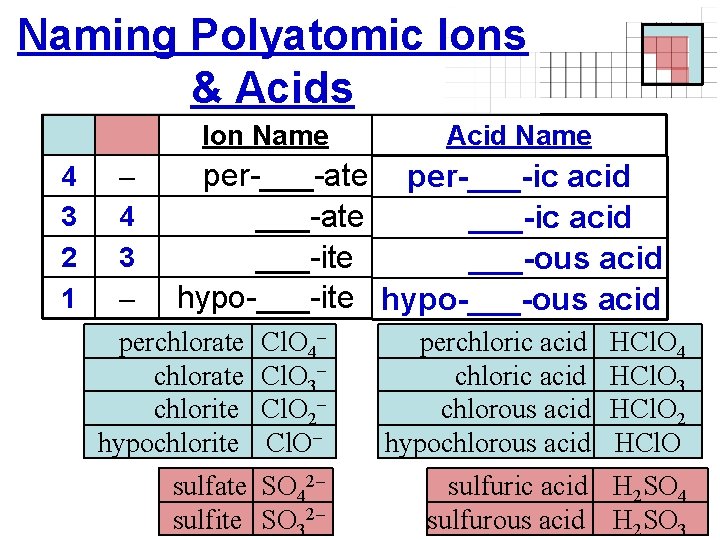
Naming Polyatomic Ions & Acids Ion Name 4 3 2 1 – 4 3 – Acid Name per-___-ate per-___-ic acid ___-ate ___-ic acid ___-ite ___-ous acid hypo-___-ite hypo-___-ous acid perchlorate chlorite hypochlorite Cl. O 4– Cl. O 3– Cl. O 2– Cl. O– sulfate SO 42– sulfite SO 32– perchloric acid chlorous acid hypochlorous acid HCl. O 4 HCl. O 3 HCl. O 2 HCl. O sulfuric acid H 2 SO 4 sulfurous acid H 2 SO 3

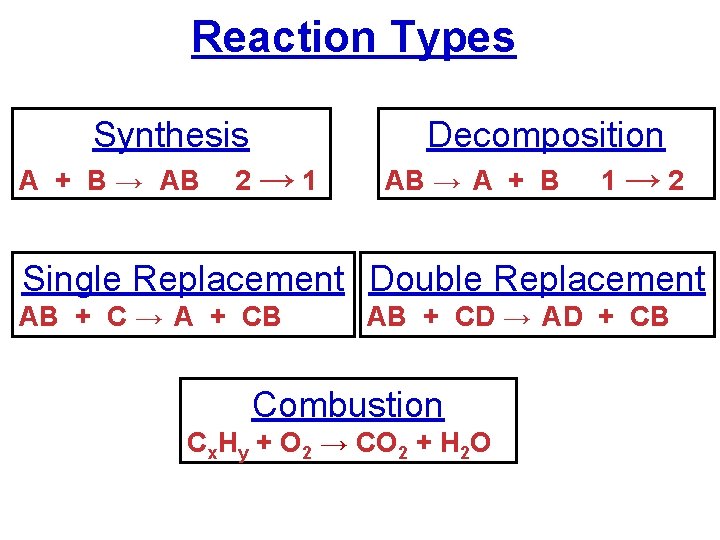
Reaction Types Synthesis A + B → AB 2→ 1 Decomposition AB → A + B 1 → 2 Single Replacement Double Replacement AB + C → A + CB AB + CD → AD + CB Combustion Cx. Hy + O 2 → CO 2 + H 2 O

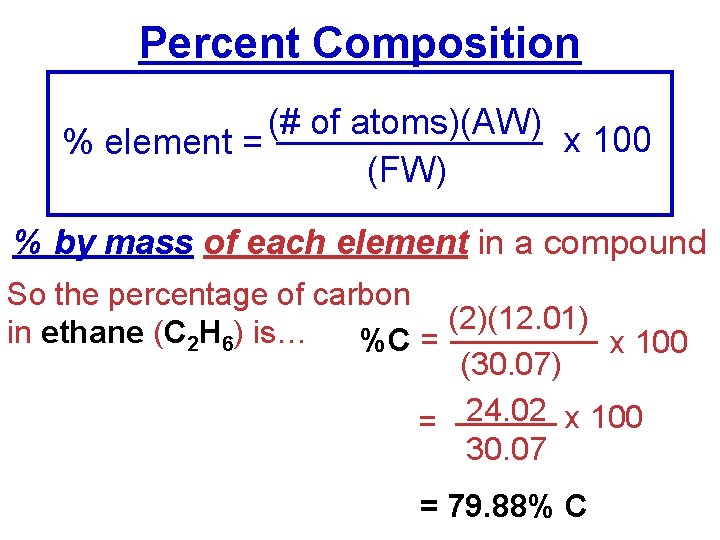
Percent Composition (# of atoms)(AW) x 100 % element = (FW) % by mass of each element in a compound So the percentage of carbon (2)(12. 01) in ethane (C 2 H 6) is… %C = x 100 (30. 07) = 24. 02 x 100 30. 07 = 79. 88% C

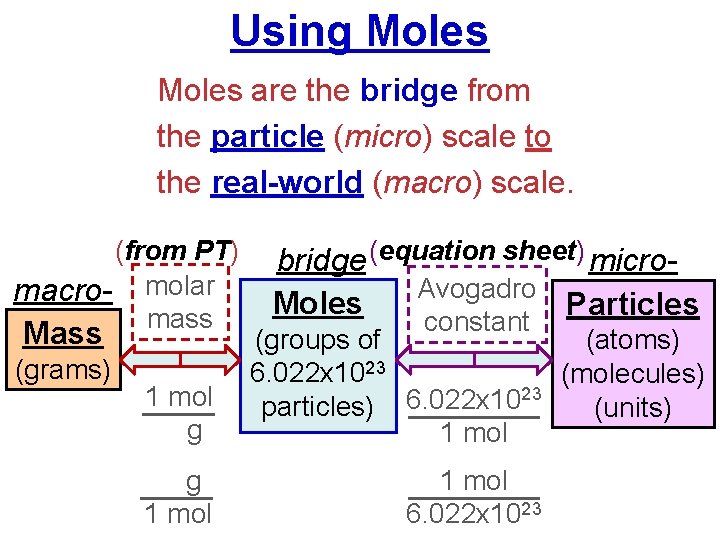
Using Moles are the bridge from the particle (micro) scale to the real-world (macro) scale. (from PT) macro- molar mass Mass (grams) bridge (equation sheet) micro. Avogadro Moles Particles constant 1 mol g (groups of (atoms) 6. 022 x 1023 (molecules) 23 particles) 6. 022 x 10 (units) 1 mol g 1 mol 6. 022 x 1023

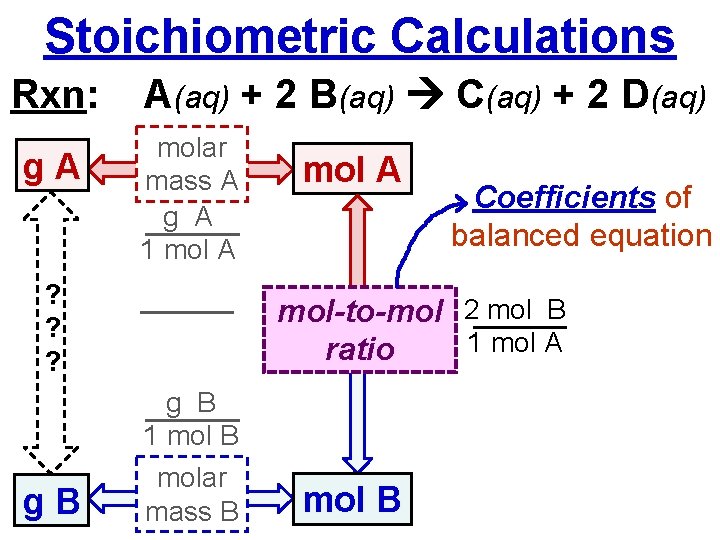
Stoichiometric Calculations Rxn: g. A A(aq) + 2 B(aq) C(aq) + 2 D(aq) molar mass A g A 1 mol A ? ? ? g. B mol A Coefficients of balanced equation mol-to-mol 2 mol B 1 mol A ratio g B 1 mol B molar mass B mol B

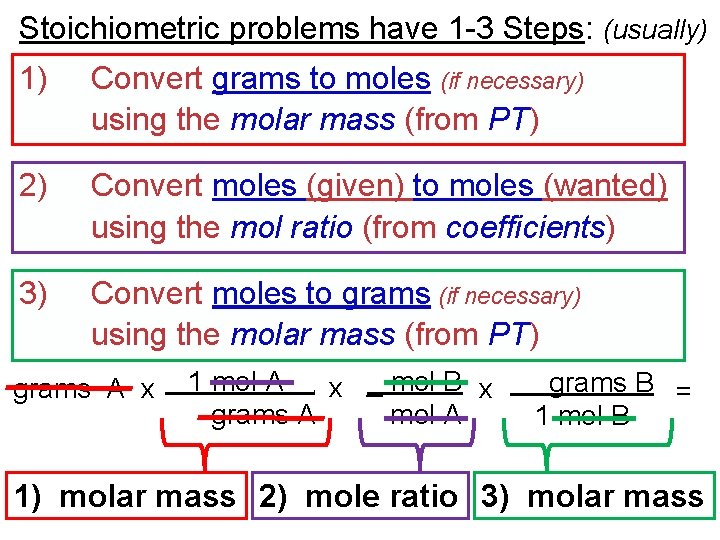
Stoichiometric problems have 1 -3 Steps: (usually) 1) Convert grams to moles (if necessary) using the molar mass (from PT) 2) Convert moles (given) to moles (wanted) using the mol ratio (from coefficients) 3) Convert moles to grams (if necessary) using the molar mass (from PT) grams A x 1 mol A x _ mol B x grams A mol A. grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass

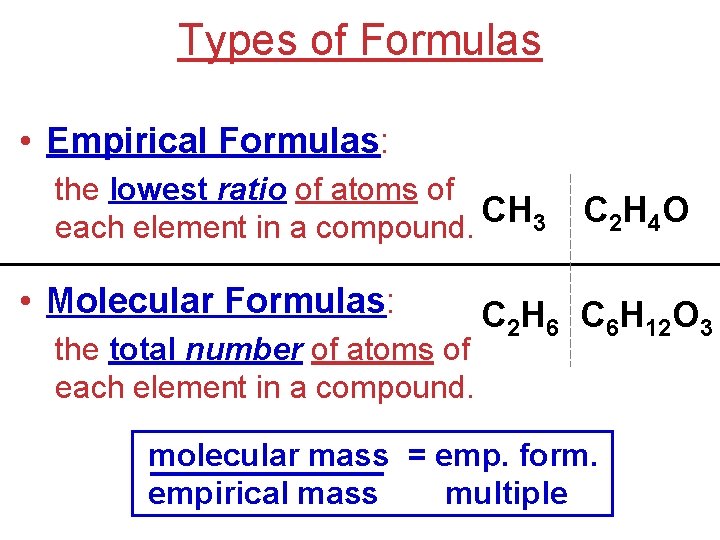
Types of Formulas • Empirical Formulas: the lowest ratio of atoms of CH 3 each element in a compound. • Molecular Formulas: the total number of atoms of each element in a compound. C 2 H 4 O C 2 H 6 C 6 H 12 O 3 molecular mass = emp. form. empirical mass multiple

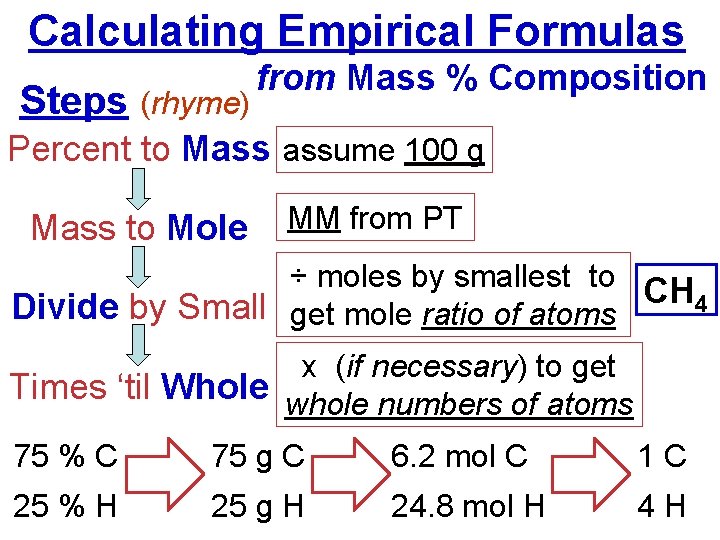
Calculating Empirical Formulas Steps (rhyme) from Mass % Composition Percent to Mass assume 100 g Mass to Mole MM from PT ÷ moles by smallest to CH 4 Divide by Small get mole ratio of atoms x (if necessary) to get Times ‘til Whole whole numbers of atoms 75 % C 75 g C 6. 2 mol C 1 C 25 % H 25 g H 24. 8 mol H 4 H

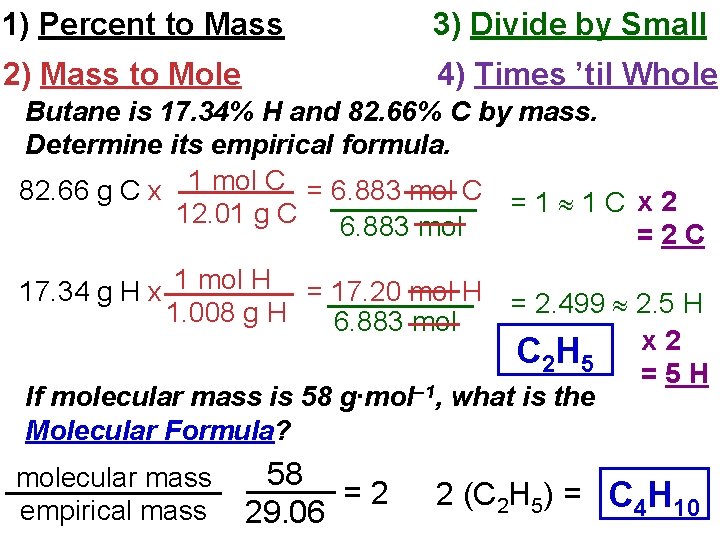
1) Percent to Mass 3) Divide by Small 2) Mass to Mole 4) Times ’til Whole Butane is 17. 34% H and 82. 66% C by mass. Determine its empirical formula. 82. 66 g C x 1 mol C = 6. 883 mol C = 1 1 C x 2 12. 01 g C 6. 883 mol =2 C 17. 34 g H x 1 mol H = 17. 20 mol H 1. 008 g H 6. 883 mol = 2. 499 2. 5 H C 2 H 5 x 2 =5 H If molecular mass is 58 g∙mol– 1, what is the Molecular Formula? molecular mass empirical mass 58 =2 29. 06 2 (C 2 H 5) = C 4 H 10

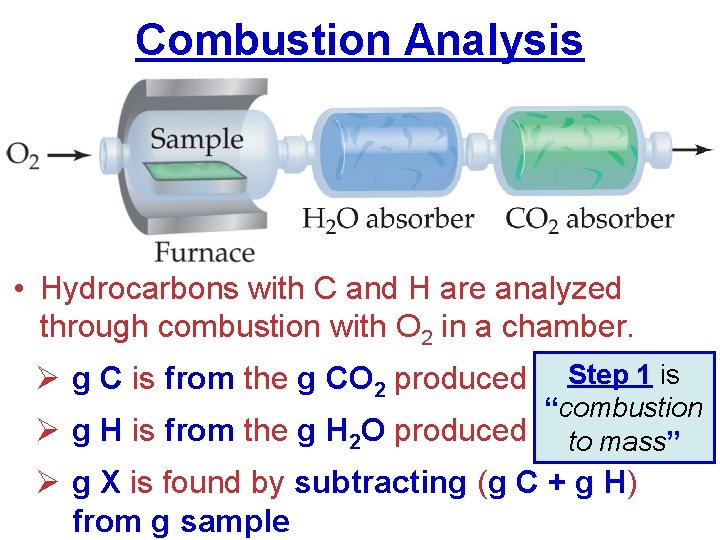
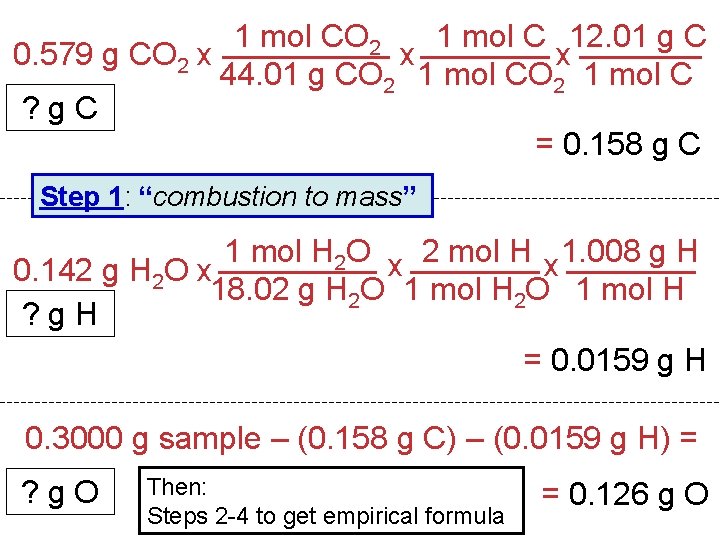
Combustion Analysis • Hydrocarbons with C and H are analyzed through combustion with O 2 in a chamber. Step 1 is “combustion Ø g H is from the g H 2 O produced to mass” Ø g C is from the g CO 2 produced Ø g X is found by subtracting (g C + g H) from g sample

1 mol CO 2 1 mol C 12. 01 g C 0. 579 g CO 2 x x x 44. 01 g CO 2 1 mol C ? g. C = 0. 158 g C Step 1: “combustion to mass” 1 mol H 2 O x 2 mol H x 1. 008 g H 0. 142 g H 2 O x 18. 02 g H 2 O 1 mol H ? g. H = 0. 0159 g H 0. 3000 g sample – (0. 158 g C) – (0. 0159 g H) = ? g. O Then: Steps 2 -4 to get empirical formula = 0. 126 g O

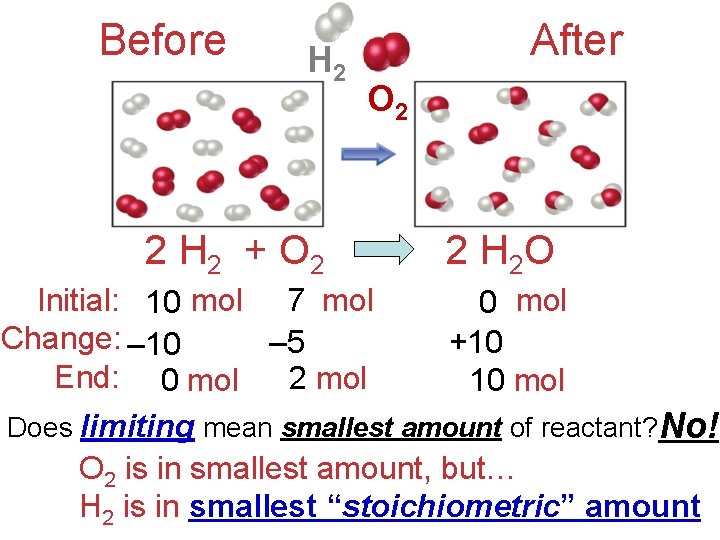
Before H 2 2 H 2 + O 2 After O 2 2 H 2 O 7 mol Initial: 10 ? mol ? ? 0 mol Change: – 10 – 5 +10 End: 0 mol 2 mol 10 mol Does limiting mean smallest amount of reactant? No! O 2 is in smallest amount, but… H 2 is in smallest “stoichiometric” amount

Find the Limiting Reactant 1) Convert reactant A to reactant B 2) Compare B available to B needed • If available < needed (limiting) • If available > needed (excess) Solid aluminum metal is reacted with aqueous copper(II) chloride in solution – 2 Al + 3 Cu. Cl 2 2 Al. Cl 3 + 3 Cu 54. 0 g Al 4. 50 mol Cu. Cl 2 (Which is limiting? ) 54. 0 g Al x 1 mol Al x 3 mol Cu. Cl 2 = 3. 00 mol Cu. Cl 2 26. 98 g Al 2 mol Al (4. 50 mol Cu. Cl 2) available > needed (3. 00 mol Cu. Cl 2) Al is limiting Cu. Cl 2 is excess

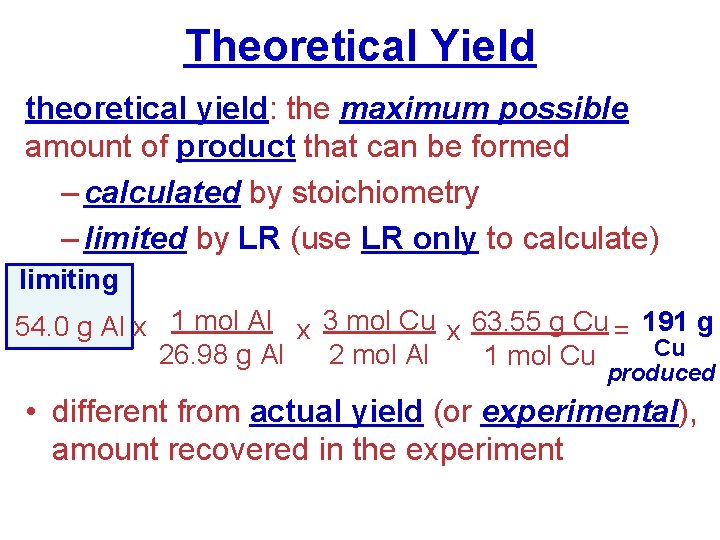
Theoretical Yield theoretical yield: the maximum possible amount of product that can be formed – calculated by stoichiometry – limited by LR (use LR only to calculate) limiting 54. 0 g Al x 1 mol Al x 3 mol Cu x 63. 55 g Cu = 191 g Cu 26. 98 g Al 2 mol Al 1 mol Cu produced • different from actual yield (or experimental), amount recovered in the experiment

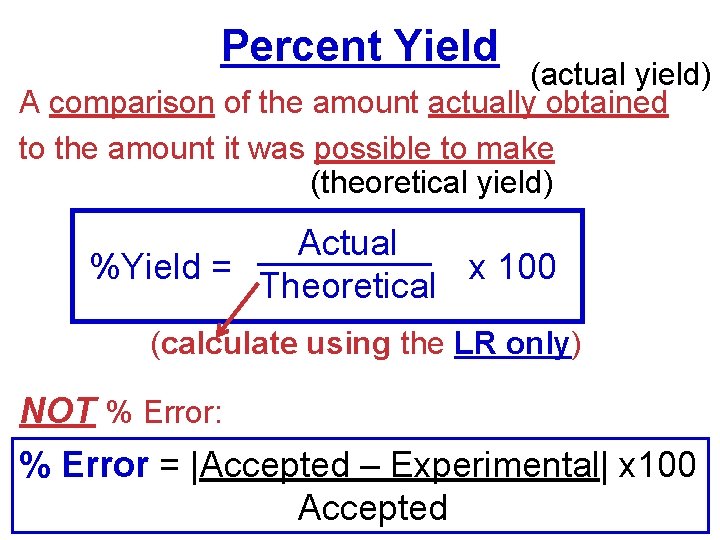
Percent Yield (actual yield) A comparison of the amount actually obtained to the amount it was possible to make (theoretical yield) Actual %Yield = x 100 Theoretical (calculate using the LR only) NOT % Error: % Error = |Accepted – Experimental| x 100 Accepted