What is a strong Acid An Acid that






- Slides: 20

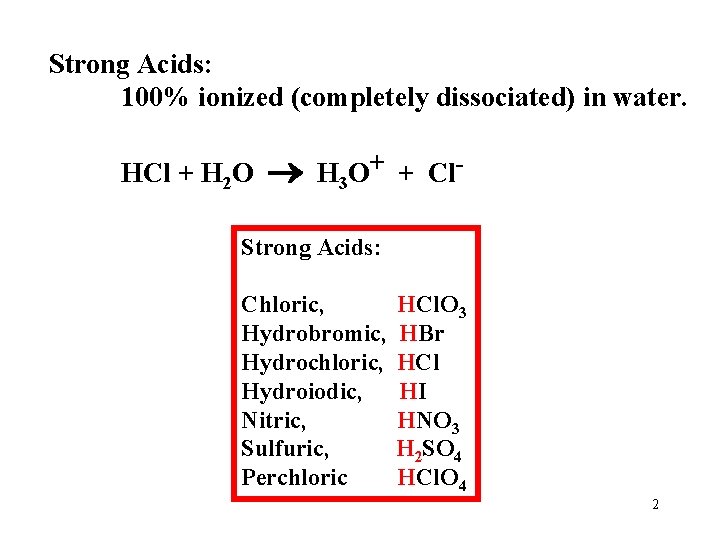
What is a strong Acid? An Acid that is 100% ionized in water. Strong Acids: 100% ionized (completely dissociated) in water. HCl + H 2 O H 3 O+ + Cl- often written as: HCl H+ + Cl 1

Strong Acids: 100% ionized (completely dissociated) in water. HCl + H 2 O H 3 O+ + Cl- Strong Acids: Chloric, Hydrobromic, Hydrochloric, Hydroiodic, Nitric, Sulfuric, Perchloric HCl. O 3 HBr HCl HI HNO 3 H 2 SO 4 HCl. O 4 2

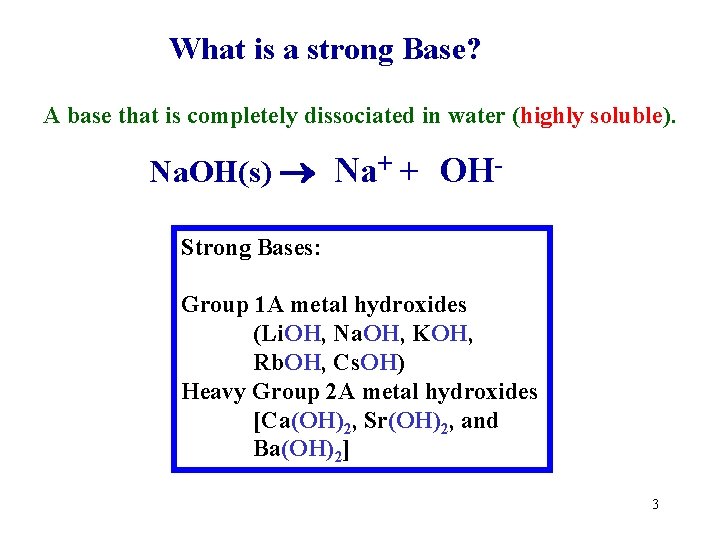
What is a strong Base? A base that is completely dissociated in water (highly soluble). Na. OH(s) Na+ + OHStrong Bases: Group 1 A metal hydroxides (Li. OH, Na. OH, KOH, Rb. OH, Cs. OH) Heavy Group 2 A metal hydroxides [Ca(OH)2, Sr(OH)2, and Ba(OH)2] 3

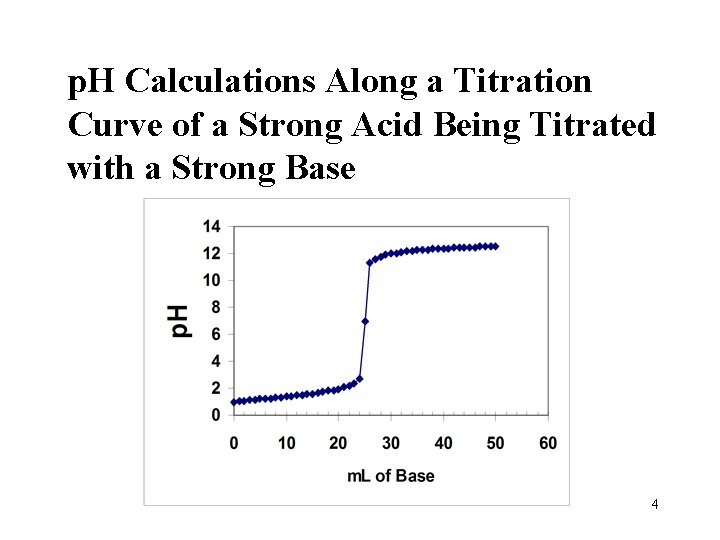
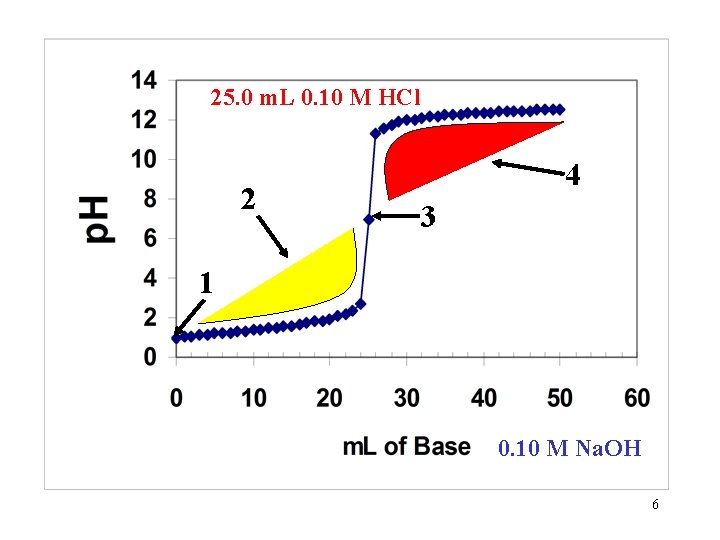
p. H Calculations Along a Titration Curve of a Strong Acid Being Titrated with a Strong Base 4

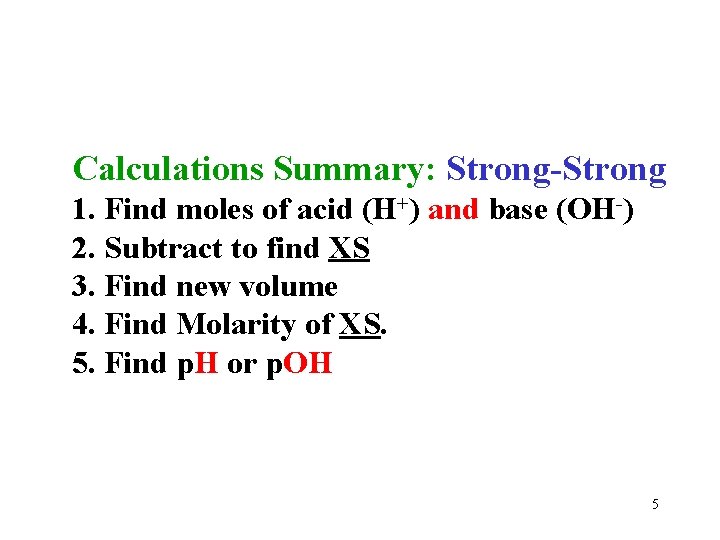
Calculations Summary: Strong-Strong 1. Find moles of acid (H+) and base (OH-) 2. Subtract to find XS 3. Find new volume 4. Find Molarity of XS. 5. Find p. H or p. OH 5

25. 0 m. L 0. 10 M HCl 2 4 3 1 0. 10 M Na. OH 6

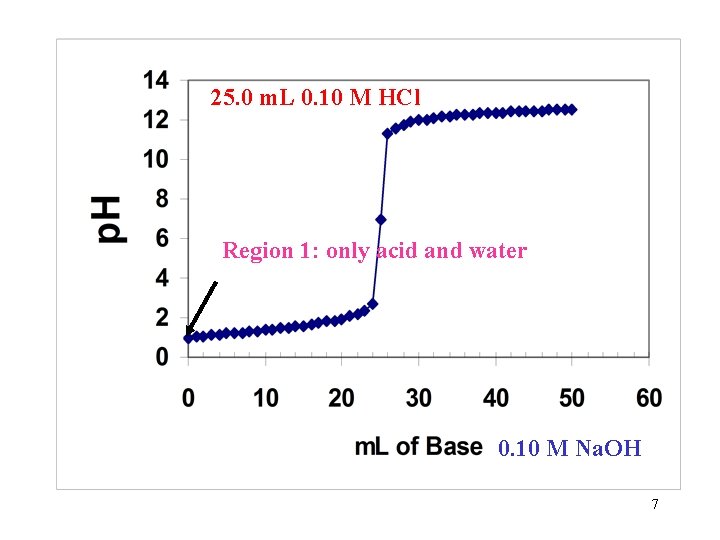
25. 0 m. L 0. 10 M HCl Region 1: only acid and water 0. 10 M Na. OH 7

Region 1: Only the Strong acid and water are present and no base has been added. General Solution: Since strong acid is completely ionized. The p. H is found by the expression: p. H = -Log[H+] but the total volume must be taken into account in calculating the Molarity of the hydronium ion. 8

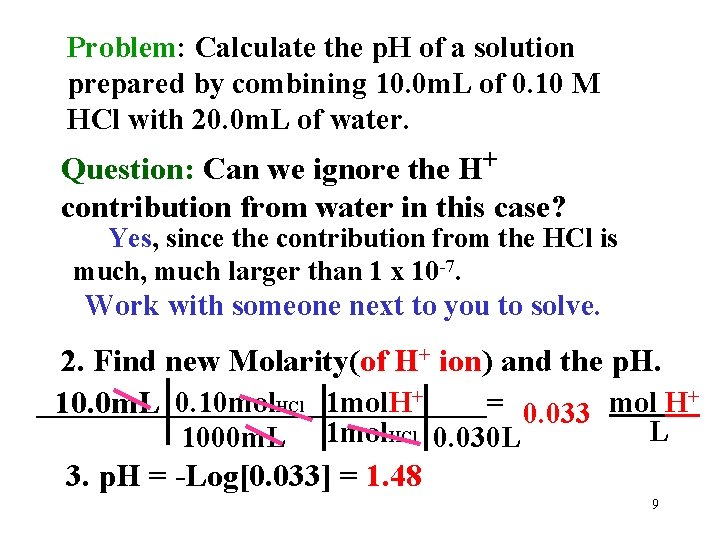
Problem: Calculate the p. H of a solution prepared by combining 10. 0 m. L of 0. 10 M HCl with 20. 0 m. L of water. Question: Can we ignore the H+ contribution from water in this case? Yes, since the contribution from the HCl is much, much larger than 1 x 10 -7. Work with someone next to you to solve. 2. Find new Molarity(of H+ ion) and the p. H. + _______________= mol H 10. 0 m. L 0. 10 mol. HCl 1 mol. H+ 0. 033 1000 m. L 1 mol. HCl 0. 030 L L 3. p. H = -Log[0. 033] = 1. 48 9

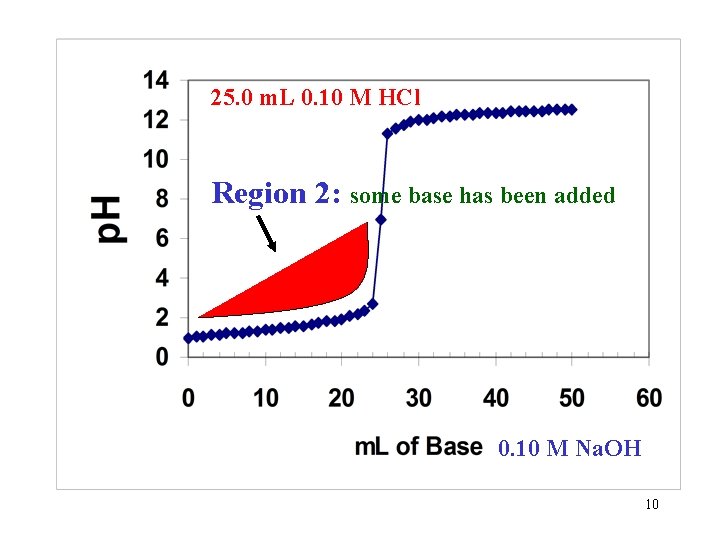
25. 0 m. L 0. 10 M HCl Region 2: some base has been added 0. 10 M Na. OH 10

Region 2: Some base has been added to the Strong acid solution. What has changed? 1. The number of moles of acid is reduced. HA + OH- salt + H 2 O ex. HCl + Na. OH Na. Cl + H 2 O or: H+ + OH- H 2 O 2. The total volume is also changed and must be taken into account when calculating the new Molarity of the acid. 11

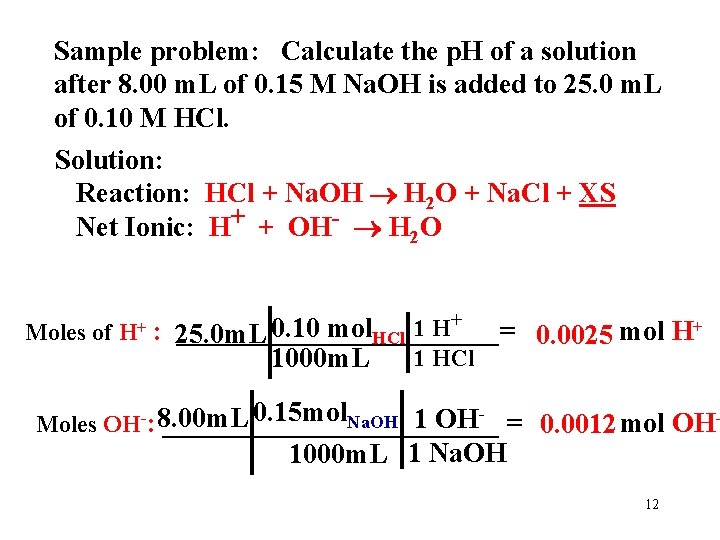
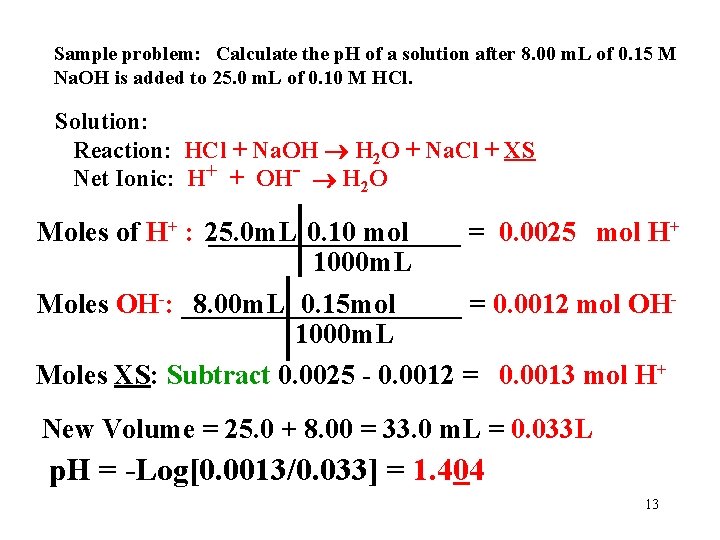
Sample problem: Calculate the p. H of a solution after 8. 00 m. L of 0. 15 M Na. OH is added to 25. 0 m. L of 0. 10 M HCl. Solution: Reaction: HCl + Na. OH H 2 O + Na. Cl + XS Net Ionic: H+ + OH- H 2 O Moles of H+ + 1 H 0. 10 mol : 25. 0 m. L ____________= HCl 0. 0025 mol H+ 1 HCl 1000 m. L 0. 15 mol. Na. OH 1 OH = 0. 0012 mol OHMoles OH-: 8. 00 m. L ____________ - 1000 m. L 1 Na. OH 12

Sample problem: Calculate the p. H of a solution after 8. 00 m. L of 0. 15 M Na. OH is added to 25. 0 m. L of 0. 10 M HCl. Solution: Reaction: HCl + Na. OH H 2 O + Na. Cl + XS Net Ionic: H+ + OH- H 2 O Moles of H+ : 25. 0 m. L _________ 0. 10 mol = 0. 0025 mol H+ 1000 m. L Moles OH-: __________ 8. 00 m. L 0. 15 mol = 0. 0012 mol OH 1000 m. L Moles XS: Subtract 0. 0025 - 0. 0012 = 0. 0013 mol H+ New Volume = 25. 0 + 8. 00 = 33. 0 m. L = 0. 033 L p. H = -Log[0. 0013/0. 033] = 1. 404 13

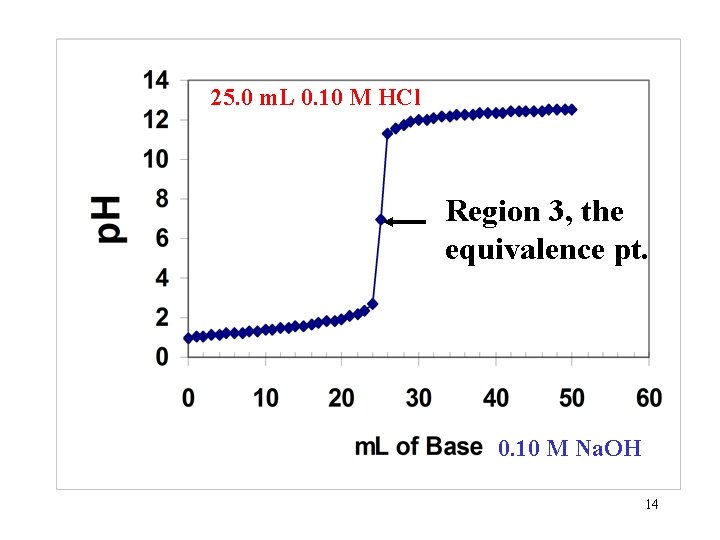
25. 0 m. L 0. 10 M HCl Region 3, the equivalence pt. 0. 10 M Na. OH 14

Region 3: The equivalence pt. All of the Strong acid has been neutralized and no XS base is present. Since the solution has a p. H, what furnishes the H+ ions? Only the ionization of water. Ions from strong acids and strong bases do not cause any ionization of water (no hydrolysis). So? @ 25 o. C [H+]= 1 x 10 -7 so p. H = 7 Question: What is conc. of OH- ion? 15

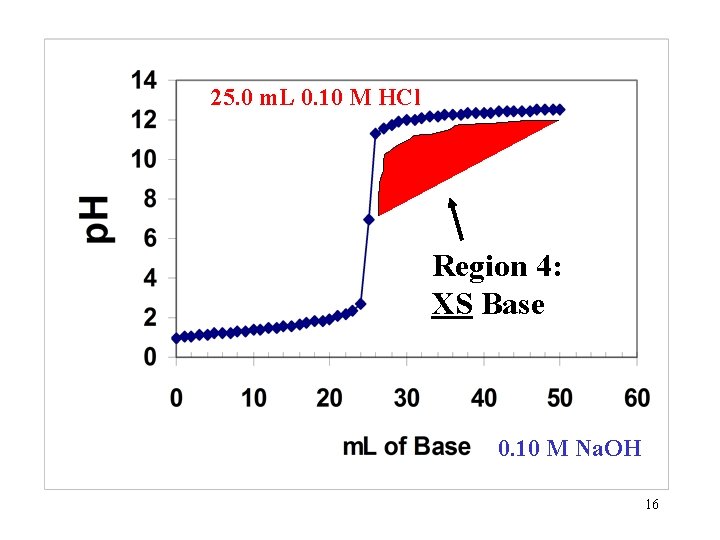
25. 0 m. L 0. 10 M HCl Region 4: XS Base 0. 10 M Na. OH 16

Region 4: All the Strong acid has been neutralized and the XS base is the dominating factor influencing p. H. General Solution: 1. Find moles of XS base (OH- ion). 2. Use total volume in Liters to find Molarity of XS base. 17

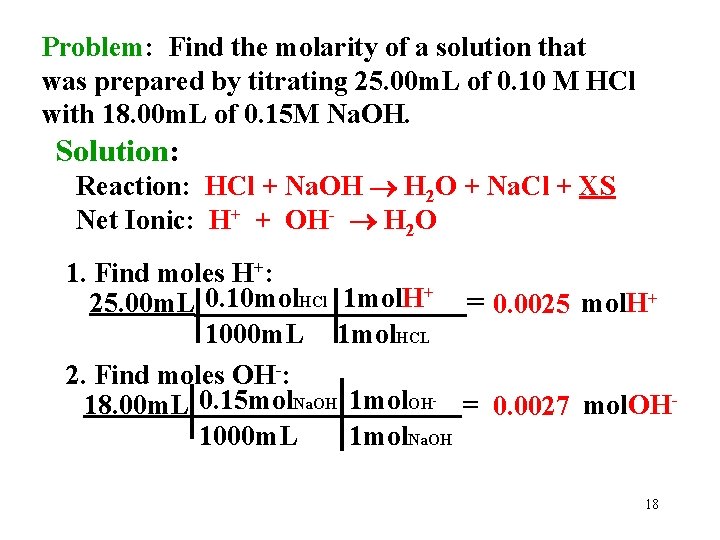
Problem: Find the molarity of a solution that was prepared by titrating 25. 00 m. L of 0. 10 M HCl with 18. 00 m. L of 0. 15 M Na. OH. Solution: Reaction: HCl + Na. OH H 2 O + Na. Cl + XS Net Ionic: H+ + OH- H 2 O 1. Find moles H+: 25. 00 m. L 0. 10 mol. HCl 1 mol. H+ = 0. 0025 mol. H+ 1000 m. L 1 mol. HCL 2. Find moles OH-: 18. 00 m. L 0. 15 mol. Na. OH 1 mol. OH = 0. 0027 mol. OH 1000 m. L 1 mol. Na. OH - 18

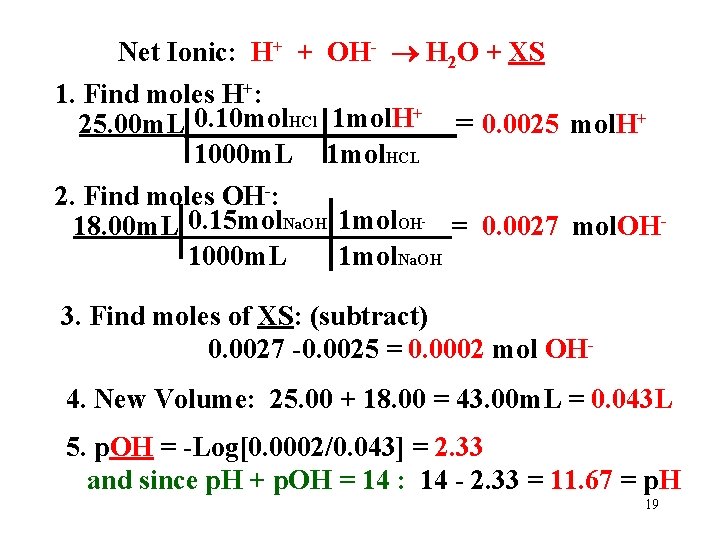
Net Ionic: H+ + OH- H 2 O + XS 1. Find moles H+: + 0. 10 mol HCl 1 mol. H 25. 00 m. L = 0. 0025 mol. H+ 1000 m. L 1 mol. HCL 2. Find moles OH-: 18. 00 m. L 0. 15 mol. Na. OH 1 mol. OH = 0. 0027 mol. OH 1000 m. L 1 mol. Na. OH - 3. Find moles of XS: (subtract) 0. 0027 -0. 0025 = 0. 0002 mol OH 4. New Volume: 25. 00 + 18. 00 = 43. 00 m. L = 0. 043 L 5. p. OH = -Log[0. 0002/0. 043] = 2. 33 and since p. H + p. OH = 14 : 14 - 2. 33 = 11. 67 = p. H 19

Any Questions? ? Summary: Strong-Strong 1. Find moles of acid and base 2. Subtract to find XS 3. Find new volume 4. Find Molarity of XS. 5. Find p. H and/or p. OH Quiz anyone? 20